Early Intensive Neurorehabilitation in Traumatic Peripheral Nerve Injury—State of the Art
Abstract
Simple Summary
Abstract
1. Introduction
2. Diagnostic Assessment of Nerve Repair
3. The Problem of Neuropathic Pain
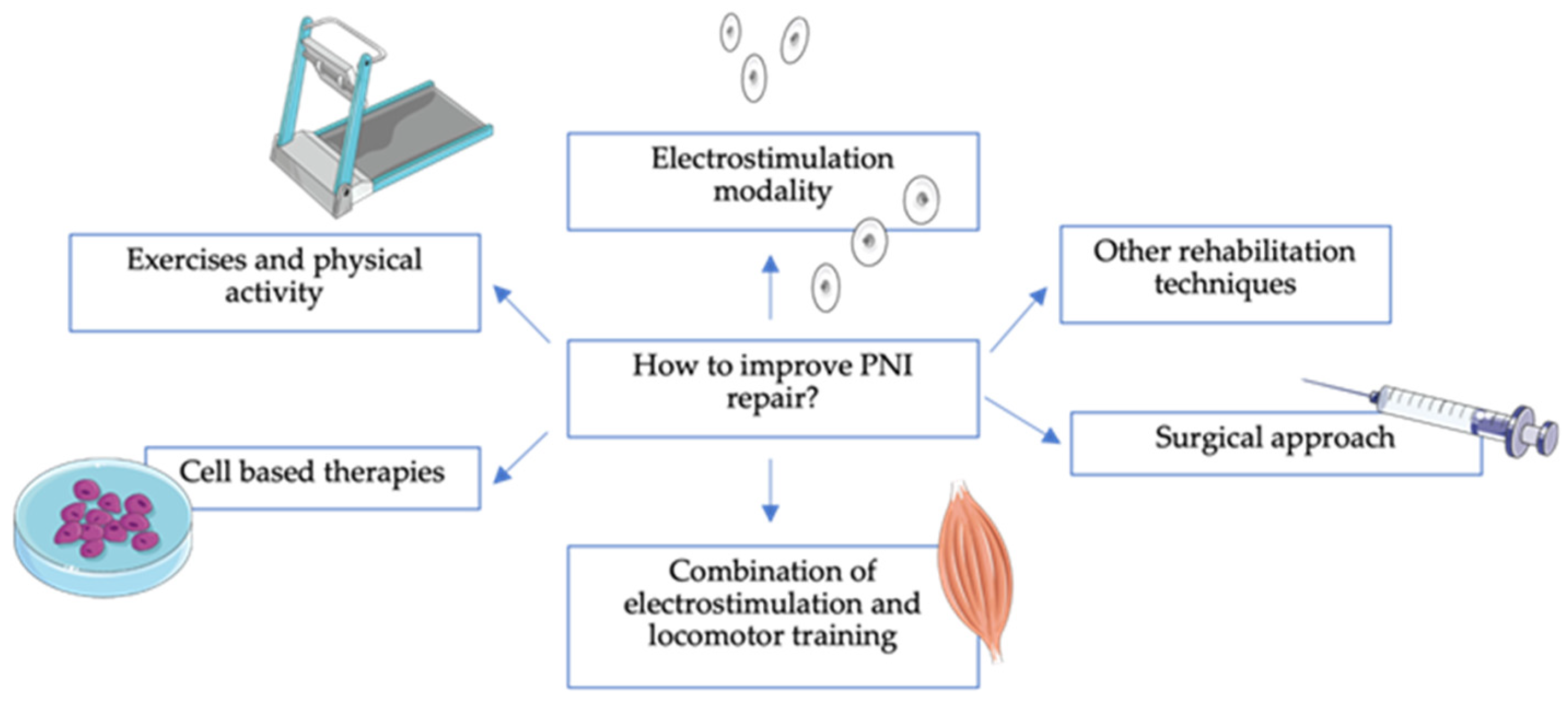
4. How to Improve PNI Repair?
4.1. Surgical Approach
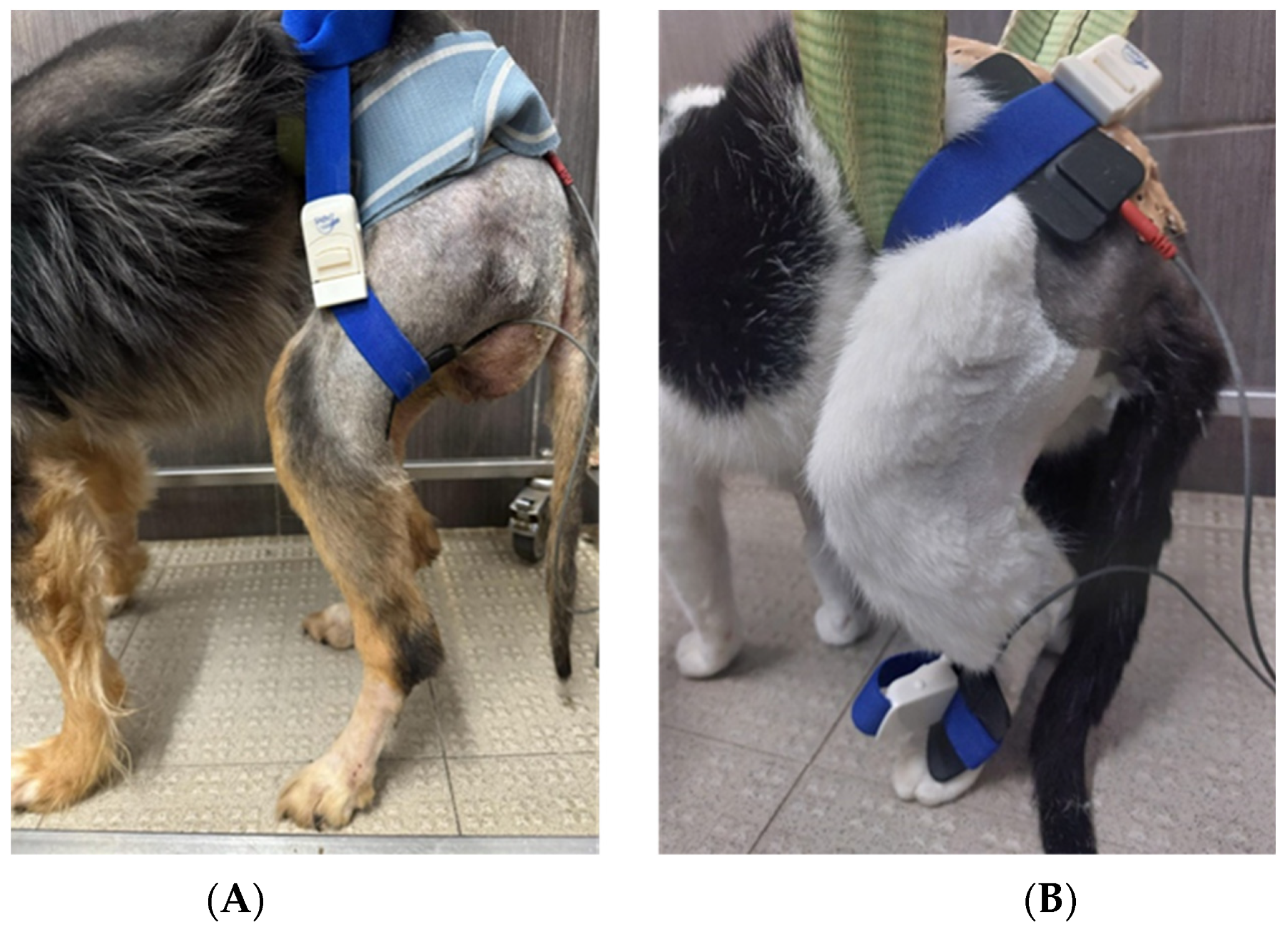
Electrostimulation Modality
4.2. Exercises and Physical Activity
4.3. Combination of Electrical Stimulation and Locomotor Training
4.4. Other Rehabilitation Modalities
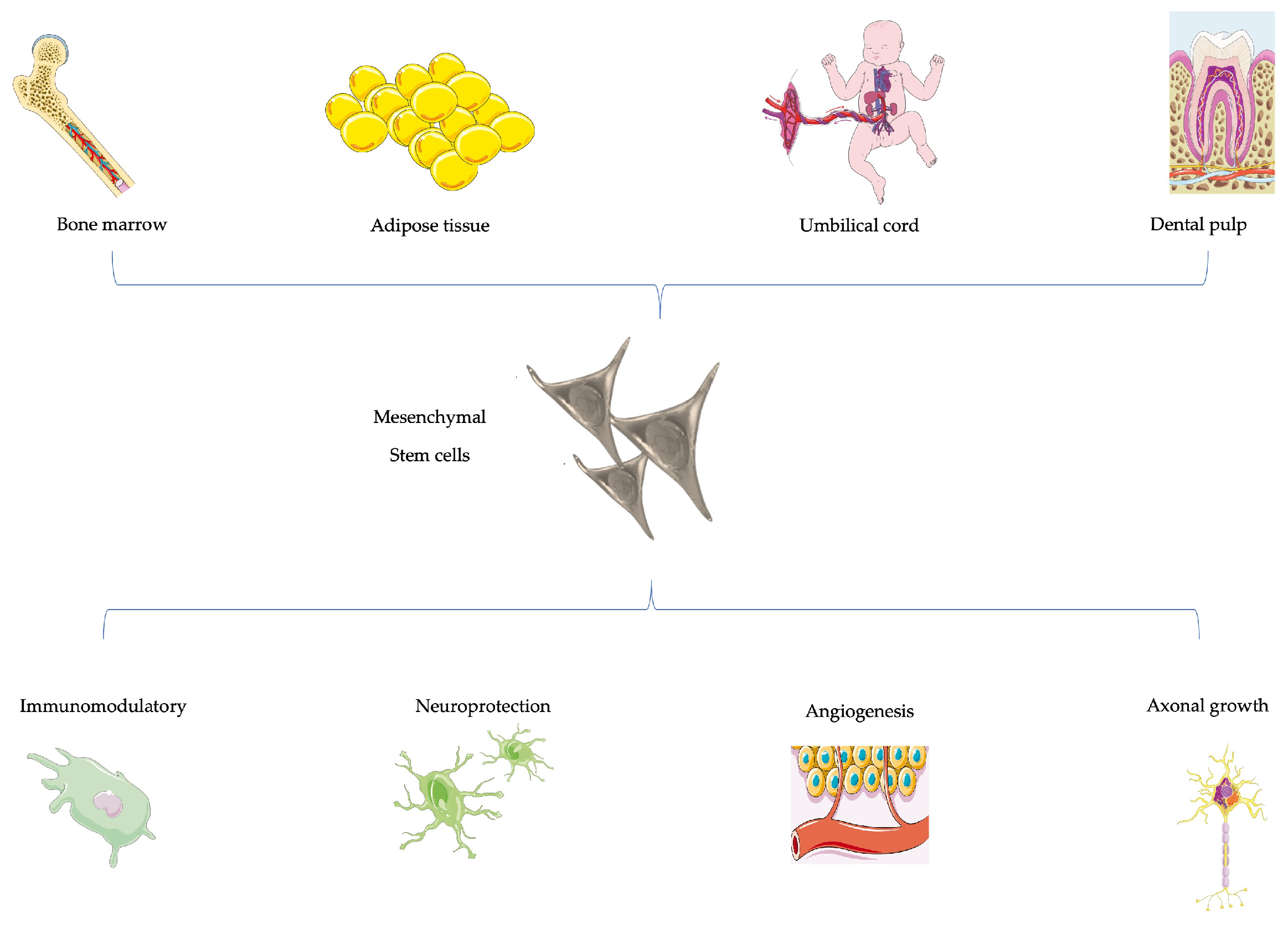
4.5. Cell-Based Therapies and PNI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biazar, E.; Khorasani, M.T.; Montazeri, N.; Pourshamsian, K.; Daliri, M.; Rezaei, T.M.; Jabarvand, B.M.; Khoshzaban, A.; Heidari, K.S.; Jafarpour, M.; et al. Types of neural guides and using nanotechnology for peripheral nerve reconstruction. Int. J. Nanomed. 2010, 5, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Lavorato, A.; Raimondo, S.; Boido, M.; Muratori, L.; Durante, G.; Cofano, F.; Vincitorio, F.; Petrone, S.; Titolo, P.; Tartara, F.; et al. Mesenchymal stem cell treatment perspectives in peripheral nerve regeneration: Systematic review. Int. J. Mol. Sci. 2021, 22, 572. [Google Scholar] [CrossRef] [PubMed]
- Tremp, M.; Zu Schwabedissen, M.M.; Kappos, E.A.; Engels, P.E.; Fischmann, A.; Scherberich, A.; Schaefer, D.J.; Kalbermatten, D.F. The Regeneration Potential after Human and Autologous Stem Cell Transplantation in a Rat Sciatic Nerve Injury Model can be Monitored by MRI. Cell Transplant. 2015, 24, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Matthes, S.M.; Reimers, K.; Janssen, I.; Liebsch, C.; Kocsis, J.D.; Vogt, P.M.; Radtke, C. Intravenous Transplantation of Mesenchymal Stromal Cells to Enhance Peripheral Nerve Regeneration. BioMed Res. Int. 2013, 2013, 573169. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, F.; Arab, F.L.; Nikkhah, K.; Amiri, H.; Mahmoudi, M. Novel approaches using mesenchymal stem cells for curing peripheral nerve injuries. Life Sci. 2019, 221, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Moattari, M.; Kouchesfehani, H.M.; Kaka, G.; Sadraie, S.H.; Naghdi, M. Evaluation of nerve growth factor (NGF) treated mesenchymal stem cells for recovery in neurotmesis model of peripheral nerve injury. J. Cranio-Maxillofac. Surg. 2018, 46, 898–904. [Google Scholar] [CrossRef]
- Menchetti, M.; Gandini, G.; Bravaccini, B.; Dondi, M.; Gagliardo, T.; Bianchi, E. Clinical and electrodiagnostic findings and quality of life of dogs and cats with brachial plexus injury. Vet. Sci. 2020, 7, 101. [Google Scholar] [CrossRef]
- Lopes, B.; Sousa, P.; Alvites, R.; Branquinho, M.; Sousa, A.C.; Mendonça, C.; Atayde, L.M.; Luís, A.L.; Varejão, A.S.P.; Maurício, A.C. Peripheral Nerve Injury Treatments and Advances: One Health Perspective. Int. J. Mol. Sci. 2022, 23, 918. [Google Scholar] [CrossRef]
- Ribitsch, I.; Baptista, P.M.; Lange-Consiglio, A.; Melotti, L.; Patruno, M.; Jenner, F.; Schnabl-Feichter, E.; Dutton, L.C.; Connolly, D.J.; van Steenbeek, F.G.; et al. Large Animal Models in Regenerative Medicine and Tissue Engineering: To Do or Not to Do. Front. Bioeng. Biotechnol. 2020, 8, 972. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; D’Agata, V.; Trovato, B.; Roggio, F.; Castorina, A.; Vecchio, M.; Di Rosa, M.; Musumeci, G. The role of exercise on peripheral nerve regeneration: From animal model to clinical application. Heliyon 2021, 7, e08281. [Google Scholar] [CrossRef] [PubMed]
- Khaled, M.M.; Ibrahium, A.M.; Abdelgalil, A.I.; El-Saied, M.A.; El-Bably, S.H. Regenerative strategies in treatment of peripheral nerve injuries in different animal models. Tissue Eng. Regen. Med. 2023, 20, 839–877. [Google Scholar] [CrossRef] [PubMed]
- Zack-Williams, S.D.; Butler, P.E.; Kalaskar, D.M. Current progress in use of adipose derived stem cells in peripheral nerve regeneration. World J. Stem Cells. 2015, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Al-Magsoosi, H.H.; Al-Bayati, H.S.; Al-Timmemi, H.A. Immuno-hematological response to radial nerve injury and human umbilical cord-mesenchymal stem cells (Huc-MSCS) therapy in dogs. IRAQ Biochem. Cell Arch. 2020, 20, 6447–6456. [Google Scholar]
- Seddon, H.J. Three types of nerve injury. Brain 1943, 66, 237. [Google Scholar] [CrossRef]
- Modrak, M.; Talukder, M.A.; Gurgenashvili, K.; Noble, M.; Elfar, J.C. Peripheral nerve injury and myelination: Potential therapeutic strategies. J. Neurosci. Res. 2020, 98, 780–795. [Google Scholar] [CrossRef] [PubMed]
- Campbell, W.W. Evaluation and management of peripheral nerve injury. Clin. Neurophysiol. 2008, 119, 1951–1965. [Google Scholar] [CrossRef] [PubMed]
- Missios, S.; Bekelis, K.; Spinner, R.J. Traumatic peripheral nerve injuries in children: Epidemiology and socioeconomics. J. Neurosurg. Pediatr. 2014, 14, 688–694. [Google Scholar] [CrossRef]
- De Albornoz, P.M.; Delgado, P.J.; Forriol, F.; Maffulli, N. Non-surgical therapies for peripheral nerve injury. Br. Med. Bull. 2011, 100, 73–100. [Google Scholar] [CrossRef]
- Javeed, S.; Faraji, A.H.; Dy, C.; Ray, W.Z.; MacEwan, M.R. Application of electrical stimulation for peripheral nerve regeneration: Stimulation parameters and future horizons. Interdiscip. Neurosurg. Adv. Tech. Case Manag. 2021, 24, 101117. [Google Scholar] [CrossRef]
- Hoke, A.; Brushart, T. Introduction to special issue: Challenges and opportunities for regeneration in the peripheral nervous system. Exp. Neurol. 2010, 223, 1–4. [Google Scholar] [CrossRef]
- Pestronk, A.; Drachman, D.B.; Griffin, J.W. Effects of aging on nerve sprouting and regeneration. Exp. Neurol. 1980, 70, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Grinsell, D.; Keating, C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. Biomed. Res. Int. 2014, 2014, 698256. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.W. Microsurgery of peripheral nerves. Plast. Rec. Surg. 1964, 33, 317–329. [Google Scholar] [CrossRef]
- Menorca, R.M.; Fussell, T.S.; Elfar, J.C. Peripheral nerve trauma: Mechanisms of injury and recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef]
- Hussain, G.; Wang, J.; Rasul, A.; Anwar, H.; Qasim, M.; Zafar, S.; Aziz, N.; Razzaq, A.; Hussain, R.; Aguilar, J.; et al. Current status of therapeutic approaches against peripheral nerve injuries: A detailed story from injury to recovery. Intern. J. Bio. Sci. 2020, 16, 116–134. [Google Scholar] [CrossRef] [PubMed]
- Houschyar, K.S.; Momeni, A.; Pyles, M.N.; Cha, J.Y.; Maan, Z.N.; Duscher, D.; Jew, O.S.; Siemers, F.; Schoonhoven, J. The role of current techniques and concepts in peripheral nerve repair. Plastic Surg. Intern. 2016, 2016, 4175293. [Google Scholar] [CrossRef]
- Noble, J.; Munro, C.A.; Prasad, V.S.; Midha, R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J. Trauma Acute Care Surg. 1998, 45, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, S. The anatomy and physiology of nerve injury. Muscle Nerve 1990, 13, 771–784. [Google Scholar] [CrossRef]
- Post, R.; de Boer, K.S.; Malessy, M.J. Outcome following nerve repair of high isolated clean sharp injuries of the ulnar nerve. PLoS ONE 2012, 7, e47928. [Google Scholar] [CrossRef]
- Jiang, L.; Jones, S.; Jia, X. Stem cell transplantation for peripheral nerve regeneration: Current options and opportunities. Int. J. Mol. Sci. 2017, 18, 94. [Google Scholar] [CrossRef]
- Burnett, M.G.; Zager, E.L. Pathophysiology of peripheral nerve injury: A brief review. Neurosurg. Focus. 2004, 16, E1. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Chiaramonte, R.; Pavone, V.; Testa, G.; Pesce, I.; Scaturro, D.; Musumeci, G.; Mauro, G.; Vecchio, M. The role of physical exercise and rehabilitative implication in the process of nerve repair in peripheral neuropathies: A systematic review. Diagnostics 2023, 13, 364. [Google Scholar] [CrossRef]
- Marqueste, T.; Alliez, J.-R.; Alluin, O.; Jammes, Y.; Decherchi, P. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J. Appl. Physiol. 2004, 96, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Asensio-Pinilla, E.; Udina, E.; Jaramillo, J.; Navarro, X. Electrical stimulation combined with exercise increase axonal regeneration after peripheral nerve injury. Exp. Neurol. 2009, 219, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-F.; Yang, T.-Y.; Chen, Y.-H.; Yao, C.-H.; Way, T.-D.; Chen, Y.-S. Effects of swimming exercise on nerve regeneration in a rat sciatic nerve transection model. Biomed. Pharmacother. 2017, 7, 3. [Google Scholar] [CrossRef]
- Boeltz, T.; Ireland, M.; Mathis, K.; Nicolini, J.; Poplavski, K.; Rose, S.J.; Wilson, E.; English, A.W. Effects of treadmill training on functional recovery following peripheral nerve injury in rats. J. Neurophysiol. 2013, 109, 2645–2657. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.W.; Kerr, R.G.; Turley, C.B.; Evans, P.J.; Jay, V.; Salter, R.B. The Effects of Postoperative Continuous Passive Motion on Peripheral Nerve Repair and Regeneration. J. Hand Surg. 1998, 23, 594–597. [Google Scholar] [CrossRef]
- van Meeteren, N.L.; Brakkee, J.H.; Hamers, F.P.; Helders, P.J.; Gispen, W.H. Exercise training improves functional recovery and motor nerve conduction velocity after sciatic nerve crush lesion in the rat. Arch. Phys. Med. Rehabil. 1997, 78, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Lu, L.; Zhang, J.; Hu, X.; Zhang, Y.; Liang, W.; Wu, S.; Luo, Z. Electrical Stimulation to Conductive Scaffold Promotes Axonal Regeneration and Remyelination in a Rat Model of Large Nerve Defect. PLoS ONE 2012, 7, e39526. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-H.; Chen, J.-J.J.; Hsu, Y.-M.; Bau, D.-T.; Yao, C.-H.; Chen, Y.-S. High-Frequency Electrical Stimulation Can Be a Complementary Therapy to Promote Nerve Regeneration in Diabetic Rats. PLoS ONE 2013, 8, e79078. [Google Scholar] [CrossRef]
- Udina, E.; Puigdemasa, A.; Navarro, X. Passive and active exercise improve regeneration and muscle reinnervation after peripheral nerve injury in the rat. Muscle Nerve 2011, 43, 500–509. [Google Scholar] [CrossRef]
- Dewey, C.W.; da Costa, R.C. Neurodiagnostics. In Practical Guide to Canine and Feline Neurology; Dewey, C.W., da Costa, R.C., Eds.; Wiley Blackweel: Oxford, UK, 2016; p. 76. [Google Scholar]
- Lorenz, M.D.; Coates, J.; Kent, M. Confirming a Diagnosis. In Handbook of Veterinary Neurology; Lorenz, M.D., Coates, J., Kent, M., Eds.; Elsevier Sauders: Philadelphia, PA, USA, 2011; pp. 85–86. [Google Scholar]
- Wood, M.D.; Kemp, S.W.; Weber, C.; Borschel, G.H.; Gordon, T. Outcome measures of peripheral nerve regeneration. Ann. Anatomy 2011, 193, 321–333. [Google Scholar] [CrossRef]
- Ikeda, M.; Oka, Y. The relationship between nerve conduction velocity and fiber morphology during peripheral nerve regeneration. Brain Behav. 2012, 2, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, S.; Marinelli, S.; Florenzano, F.; Pavone, F.; Luvisetto, S. Short- but not long-lasting treadmill running reduces allodynia and improves functional recovery after peripheral nerve injury. Neuroscience 2010, 168, 273–287. [Google Scholar] [CrossRef]
- Cobianchi, S.; de Cruz, J.; Navarro, X. Assessment of sensory thresholds and nociceptive fiber growth after sciatic nerve injury reveals the differential contribution of collateral reinnervation and nerve regeneration to neuropathic pain. Exp. Neurol. 2014, 255, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Herbison, G.J.; Jaweed, M.M.; Ditunno, J.F. Electrical stimulation of sciatic nerve of rats after partial denervation of soleus muscle. Arch. Phys. Med. Rehabil. 1986, 67, 79–83. [Google Scholar] [CrossRef]
- Jaweed, M.; Herbison, G.J.; Ditunno, J.F. Direct electrical stimulation of rat soleus during denervation-reinnervation. Exp. Neurol. 1982, 75, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Marqueste, T.; Decherchi, P.; Desplanches, D.; Favier, R.; Grelot, L.; Jammes, Y. Chronic electrostimulation after nerve repair by self-anastomosis: Effects on the size, the mechanical, histochemical and biochemical muscle properties. Acta Neuropathol. 2006, 111, 589–600. [Google Scholar] [CrossRef]
- Michel, R.N.; Gardiner, P.F. Influence of overload on recovery of rat plantaris from partial denervation. J. Appl. Physiol. 1989, 66, 732–740. [Google Scholar] [CrossRef]
- Eisen, A.A.; Carpenter, S.; Karpati, G.; Bellavance, A. The effect of muscle hyper- and hypoactivity upon fibre diameters of intact and regenerating nerves. J. Neurol. Sci. 1973, 20, 457–469. [Google Scholar] [CrossRef]
- Herbison, G.J.; Jaweed, M.; Ditunno, J.F.; Scott, C.M. Effect of overwork during reinnervation of rat muscle. Exp. Neurol. 1973, 41, 1–14. [Google Scholar] [CrossRef]
- Herbison, G.J.; Jaweed, M.M.; Ditunno, J.F. Effect of swimming on reinnervation of rat skeletal muscle. J. Neurol. Neurosurg. Psychiatry 1974, 37, 1247–1251. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sobral, L.L.; Oliviera, L.S.; Takeda, S.Y.M.; Somazz, M.C.; Montebelo, M.I.L.; Teodori, R.M. Immediate versus later exercises for rat sciatic nerve regeneration after axonotmesis: Histomorphometric and functional analyses. Rev. Bras. Fisioter. 2008, 12, 311–316. [Google Scholar] [CrossRef]
- Teodori, R.M.; Betini, J.; de Oliveira, L.S.; Sobral, L.L.; Takeda, S.Y.M.; Montebelo, M.I.D.L. Swimming Exercise in the Acute or Late Phase after Sciatic Nerve Crush Accelerates Nerve Regeneration. Neural Plast. 2011, 2011, 783901. [Google Scholar] [CrossRef] [PubMed]
- Skouras, E.; Merkel, D.; Grosheva, M.; Angelova, S.K.; Schiffer, G.; Thelen, U.; Kaidoglou, K.; Sinis, N.; Igelmund, P.; Dunlop, S.A.; et al. Manual stimulation, but not acute electrical stimulation prior to reconstructive surgery, improves functional recovery after facial nerve injury in rats. Restor. Neurol. Neurosci. 2009, 27, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.M.; Simões, M.J.; Maurício, A.C.; Varejão, A.S. Methods and protocols in peripheral nerve regeneration experimental research: Part IV—Kinematic gait analysis to quantify peripheral nerve regeneration in the rat. Int. Rev. Neurobiol. 2009, 87, 127–139. [Google Scholar] [PubMed]
- Bozkurt, A.; Deumens, R.; ScheVel, J.; O’Dey, D.M.; Weis, J.; Joosten, E.A.; Führmann, T.; Brook, G.A.; Pallua, N. CatWalk gait analysis in assessment of functional recovery after sciatic nerve injury. J. Neurosci. Methods 2008, 173, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, R.; Schnider, J.T.; Fanzio, P.M.; Tsuji, W.; Kostereva, N.; Solari, M.G.; Plock, J.A.; Gorantla, V.S. Effect of systemic adipose-derived stem cell therapy on functional nerve regeneration in a rodent model. PRS Global Open. 2020, 8, e2953. [Google Scholar] [CrossRef]
- Targosinksi, S.; Henzi, A.; Engmann, A.K.; Rushing, E.J.; Barth, A.A.; Klein, H.J.; Kim, B.; Giovanoli, P.; Schwab, M.E.; Plock, J.A.; et al. A swim test for functional assessment of rodent peripheral nerve regeneration. J. Neurosci. Met. 2022, 379, 109663. [Google Scholar] [CrossRef]
- Senger, J.B.; Rabey, K.N.; Morhart, M.J.; Chan, K.M.; Webber, C.A. Conditioning electrical stimulation accelerates regeneration in nerve transfers. Ann. Neurol. 2020, 88, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Senger, J.L.B.; Verge, V.M.K.; Macandili, H.S.J.; Olson, J.L.; Chan, K.M.; Webber, C.A. Electrical stimulation as a conditioning strategy for promoting and accelerating peripheral nerve regeneration. Expl. Neurol. 2018, 302, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Molteni, R.; Zheng, J.-Q.; Ying, Z.; Gomez-Pinilla, F.; Twiss, J.L. Voluntary exercise increases axonal regeneration from sensory neurons. Proc. Natl. Acad. Sci. USA 2004, 101, 8473–8478. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.; Brushart, T.M.; Gordon, T. Electrical stimulation accelerates and increases expression of BDNF and trkB mRNA in regenerating rat femoral motoneurons. Eur. J. Neurosci. 2000, 12, 4381–4390. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Schwartz, G.; Meador, W.; Sabatier, M.J.; Mulligan, A. Electrical stimulation promotes peripheral axon regeneration by enhanced neuronal neurotrophin signaling. Dev. Neurobiol. 2007, 67, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.F.; Mazzardo-Martins, L.; Gadotti, V.M.; Nascimento, F.P.; Lima, D.A.; Speckhann, B.; Favretto, G.A.; Bobinski, F.; Cargnin-Ferreira, E.; Bressan, E.; et al. Ankle joint mobilization reduces axonotmesis-induced neuropathic pain and glial activation in the spinal cord and enhances nerve regeneration in rats. Pain 2011, 152, 2653–2661. [Google Scholar] [CrossRef] [PubMed]
- Ying, Z.; Roy, R.R.; Edgerton, V.; Gómez-Pinilla, F. Voluntary exercise increases neurotrophin-3 and its receptor TrkC in the spinal cord. Brain Res. 2003, 987, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.B.; Oh, M.-J.; You, B.-G.; Kwon, K.-B.; Chang, I.-A.; Yoon, J.-H.; Lee, C.Y.; Namgung, U. ERK1/2-Mediated Schwann Cell Proliferation in the Regenerating Sciatic Nerve by Treadmill Training. J. Neurotrauma 2009, 26, 1733–1744. [Google Scholar] [CrossRef]
- Gómez-Pinilla, F.; Ying, Z.; Roy, R.R.; Molteni, R.; Edgerton, V.R. Voluntary Exercise Induces a BDNF-Mediated Mechanism That Promotes Neuroplasticity. J. Neurophysiol. 2002, 88, 2187–2195. [Google Scholar] [CrossRef]
- Ahlborn, P.; Schachner, M.; Irintchev, A. One hour electrical stimulation accelerates functional recovery after femoral nerve repair. Exp. Neurol. 2007, 208, 137–144. [Google Scholar] [CrossRef]
- Troupel, T.; Caenegem, N.V.; Jeandel, A.; Thibaud, J.; Nicolle, A.; Blot, S. Epidemiological, clinical, and electrophysiological findings in dogs and cats with traumatic brachial plexus injury: A retrospectove study of 226 cases. J. Vet. Intern. Med. 2021, 35, 2837–2845. [Google Scholar] [CrossRef]
- Griffiths, I.R.; Duncan, I.D.; Lawson, D.D. Avulsion of the brachial plexus-2. Clinical aspects. J. Small Anim. Pract. 1974, 15, 177–183. [Google Scholar] [CrossRef]
- Belviso, I.; Palermi, S.; Sacco, A.M.; Romano, V.; Corrado, B.; Zappia, M.; Sirico, F. Brachial plexus injuries in sport medicine: Clinical evaluation, diagnostic approaches, treatment options and rehabilitative interventions. J. Funct. Morphol. Kinesiol. 2020, 5, 22. [Google Scholar] [CrossRef]
- Preston, D.C.; Shapiro, B.E. Needle electromyography. Fundamentals, normal and abnormal patterns. Neurol. Clin. 2002, 20, 361–396. [Google Scholar] [CrossRef]
- Anson, A.; Gil, F.; Laredo, F.G.; Soler, M.; Belda, E.; Ayala, M.D.; Agut, A. Correlative ultrasound anatomy of the feline brachial plexus and major nerves of the thoracic limb. Vet. Radiol. Ultrasound 2013, 54, 185–193. [Google Scholar] [CrossRef]
- Benecke, R.; Berthold, A.; Conrad, B. Denervation activity in the EMG of patients with upper motor neuron lesions: Time course, local distribution and pathogenetic aspects. J. Neurol. 1983, 230, 143–151. [Google Scholar] [CrossRef]
- Effron, C.R.; Beasley, R.W. Compression neuropathies in the upper limb and electrophysiological studies. In Grabb and Smith’s Plastic Surgery; Thorne, C.H., Bartlett, S.P., Beasley, R.W., Aston, S.J., Gurtner, G.C., Spear, S.L., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2006; p. 86. [Google Scholar]
- Robinson, L.R. Traumatic injury to peripheral nerves. Muscle Nerve 2000, 23, 863–887. [Google Scholar] [CrossRef]
- Teixeira, M.J.; da Paz, M.G.D.S.; Bina, M.T.; Santos, S.N.; Raicher, I.; Galhardoni, R.; Fernandes, D.T.; Yeng, L.T.; Baptista, A.F.; de Andrade, D.C. Neuropathic pain after brachial plexus avulsion-central and peripheral mechanisms. BMC Neurol. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Sadosky, A.; McDermott, A. A review of the epidemiology of painful diabetic peripheral neuropathy, postherpetic neuralgia, and less commonly studied neuropathic pain conditions. Pain Pract. 2008, 8, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Nikolajsen, L.; Staehelin, T. Phantom limb pain: A case of maladaptive CNS plasticity? Nat. Rev. Neurosci. 2006, 7, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Flor, H.; Elbert, T.; Knecht, S.; Wienbruch, C.; Pantev, C.; Birbaumer, N.; Larbig, W.; Taub, E. Phantom-limb pain as a perceptual correlate of cortical reorganization following arm amputation. Nature 1995, 375, 482–484. [Google Scholar] [CrossRef]
- Menchetti, M.; Gandini, G.; Gallucci, A.; Della Rocca, G.; Matiasek, L.; Matiasek, K.; Gentilini, F.; Rosati, M. Approaching phantom complex after limb amputation in the canine species. J. Vet. Behav. 2017, 22, 24–28. [Google Scholar] [CrossRef]
- Melzack, R. Phantom limbs. Sci. Am. 1992, 266, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Ehde, D.M.; Czerniecki, J.M.; Smith, D.G.; Campbell, K.M.; Edwards, W.T.; Jensen, M.P.; Robinson, L.R. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch. Phys. Med. Rehabil. 2000, 81, 1039–1044. [Google Scholar] [CrossRef] [PubMed]
- Probstner, D.; Thuler, L.C.; Ishikawa, N.M.; Alvarenga, R.M. Phantom limb phenomena in cancer amputees. Pain Pract. 2010, 10, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Varejão, A.S.P.; Cabrita, A.M.; Geuna, S.; Melo-Pinto, P.; Filipe, V.M.; Gramsbergen, A.; Meek, M.F. Toe out angle: A functional index for the evaluation of sciatic nerve recovery in the rat model. Exp. Neurol. 2003, 183, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Cashmore, R.G.; Harcourt-Brown, T.R.; Freeman, P.M.; Jeffery, N.D.; Granger, N. Clinical diagnosis and treatment of suspected neuropathic pain in three dogs. Aust. Vet. J. 2009, 87, 45–50. [Google Scholar] [CrossRef]
- Gilron, I.; Baron, R.; Jensen, T. Neuropathic pain: Principles of diagnosis and treatment. Mayo Clin. Proc. 2015, 90, 532–545. [Google Scholar] [CrossRef]
- Moore, S.A. Managing Neuropathic Pain in Dogs. Front. Vet. Sci. 2016, 3, 12. [Google Scholar] [CrossRef]
- Radulovic, L.L.; Turck, D.; Hodenberg, A.; Vollmer, K.O.; McNally, W.P.; DeHart, P.D.; Hanson, B.J.; Bockbrader, H.N.; Chang, T. Disposition of gabapentin (neurontin) in mice, rats, dogs, and monkeys. Drug Metab. Dipos. 1995, 23, 441–448. [Google Scholar]
- Kukanich, B.; Cohen, R.L. Pharmacokinetics of oral gabapentin in greyhound dogs. Vet. J. 2011, 187, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Salazar, V.; Dewey, C.W.; Schwark, W.; Badgley, B.L.; Gleed, R.D.; Horne, W.; Ludders, J.W. Pharmacokinetics of single-dose oral pregabalin administration in normal dogs. Vet. Anaesth. Analg. 2009, 36, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Kukes, V.G.; Kondratenko, S.N.; Savelyeva, M.I.; Starodubtev, A.K.; Gneushev, T.E. Experimental and clinical pharmacokinetics of amitryptiline: Comparative analysis. Bull. Exp. Biol. Med. 2009, 147, 434–437. [Google Scholar] [CrossRef] [PubMed]
- Kukanich, B. Outpatient oral analgesics in dogs and cats beyond nonsteroidal antiinflammatory drugs: An evidence-based approach. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Norkus, C.; Rankin, D.; Warner, M.; Kukanich, B. Pharmacokinetics of oral amantadine in greyhound dogs. J. Vet. Pharmacol. Ther. 2015, 38, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Vivo, A.M.; Puigdemasa, L.; Casals, E.; Asensio, E.; Udina, X. Navarro, Immediate electrical stimulation enhances regeneration and reinnervation and modulates spinal plastic changes after sciatic nerve injury and repair. Exp. Neurol. 2008, 211, 180–193. [Google Scholar] [CrossRef] [PubMed]
- Woolf, C.J. Evidence for a central component of post-injury pain hypersensitivity. Nature 1983, 306, 686–688. [Google Scholar] [CrossRef]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- Campero, M.; Serra, J.; Marchettini, P.; Ochoa, J.L. Ectopic impulse generation and autoex-citation in single myelinated afferent fibers in patients with peripheral neuropathy and positive sensory symptoms. Muscle Nerve 1998, 21, 1661–1667. [Google Scholar] [CrossRef]
- Nordin, M.; Nyström, B.; Wallin, U.; Hagbarth, K.E. Ectopic sensory discharges and pares-thesiae in patients with disorders of peripheral nerves, dorsal roots and dorsal columns. Pain 1984, 20, 231–245. [Google Scholar] [CrossRef]
- Ochoa, J.; Torebjörk, E. Sensations evoked by intraneural microstimulation of C noci-ceptor fibres in human skin nerves. J. Physiol. 1989, 415, 583–599. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, N.P.; Vægter, C.B.; Andersen, H.; Østergaard, L.; Calcutt, N.A.; Jensen, T.S. Schwann cell interactions with axons and microvessels in diabetic neuropathy. Nat. Rev. Neurol. 2017, 13, 135–147. [Google Scholar] [CrossRef] [PubMed]
- De Logu, F.; Li Puma, S.; Landini, L.; Portelli, F.; Innocenti, A.; de Araujo, D.S.M.; Janal, M.N.; Patacchini, R.; Bunnett, N.W.; Geppetti, P.; et al. Schwann cells expressing nociceptivechannel TRPA1 orchestrate ethanol-evoked neuropathic pain in mice. J. Clin. Investig. 2019, 129, 5424–5441. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Beggs, S. Sublime microglia: Expanding roles for the guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.W.; Daunter, A.K.; Yang, L.J.S.; Wilson, T.J. An Update on the Management of Neonatal Brachial Plexus Palsy—Replacing Old Paradigms: A Review. JAMA Pediatr. 2018, 172, 585. [Google Scholar] [CrossRef] [PubMed]
- Rich, J.A.; Newell, A.; Williams, T. Traumatic brachial plexus injury rehabilitation using neuromuscular electrical muscle stimulation in a polytrauma patient. BMJ Case Rep. 2019, 12, e232107. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, G.A.; Nikkhah, G.; Matthies, C.; Penkert, G.; Samii, M. Diagnosis of root avulsions in traumatic brachial plexus injuries: Value of computerized tomography myelography and magnetic resonance imaging. J. Neurosurg. 1997, 86, 69–76. [Google Scholar] [CrossRef]
- Abdel-Aziz, S.; Ghaleb, A.H. Cervical Spinal Cord Stimulation for the Management of Pain from Brachial Plexus Avulsion. Pain Med. 2014, 15, 712–714. [Google Scholar] [CrossRef][Green Version]
- Zhou, Y.; Liu, P.; Rui, J.; Zhao, X.; Lao, J. The clinical characteristics of neuropathic pain in patients with total brachial plexus avulsion: A 30-case study. Injury 2016, 47, 1719–1724. [Google Scholar] [CrossRef]
- Wang, L.; Yuzhou, L.; Yingjie, Z.; Jie, L.; Xin, Z. A new rat model of neuropathic pain: Complete brachial plexus avulsion. Neurosci. Lett. 2015, 589, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Lunden, L.K.; Kleggetveit, I.P.; Schmelz, M.; Jorum, E. Cold allodynia is correlated to paroxysmal and evoked mechanical pain in complex regional pain Syndrome (CRPS). Scand. J. Pain 2022, 22, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Jensen, T.S.; Finnerup, N.B. Allodynia and hyperalgesia in neuropathic pain: Clinical manifestations and mechanisms. Lancet Neurol. 2014, 13, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged denervation. J. Neurosci. 1995, 15, 3886–3895. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.R.; Meek, M.; Robinson, P.H.; Gramsbergen, A. Methods to evaluate functional nerve recovery in adult rats: Walking track analysis, video analysis and the withdrawal reflex. J. Neurosci. Met. 2000, 96, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Meek, M.F.; Van Der Werff, J.F.A.; Nicolai, J.P.A.; Gramsbergen, A. Biodegradable p (DLLA-e-CL) Nerve guides versus autologous nerve grafts: Electromyographic amd video analysis. Muscle Nerve 2001, 24, 753–759. [Google Scholar] [CrossRef]
- Watson, N.C.; Jejurikar, S.; Kalliainen, L.K.; Calderon, M.S.; URbanchek, M.G.; Eguchi, T.; Kuzon, J.R. Range of motion physiotherapy reduces the force deficit in antagonists to denervated rat muscles. J. Surg. Res. 2001, 99, 156–160. [Google Scholar] [CrossRef]
- Millis, D.; Levine, D. Exercises for Proprioception and Balance. In Canine Rehabilitation and Physical Therapy; Millis, D., Levine, D., Eds.; Elsevier Sauders: Philadelphia, PA, USA, 2014; pp. 490–493. [Google Scholar]
- Du, Z.; Zhang, J.; Han, X.; Yu, W.; Gu, X. Potential novel therapeutic strategies for neuropathic pain. Front. Mol. Neurosci. 2023, 16, 1138798. [Google Scholar] [CrossRef]
- Chandrashekhar, R.; Wang, H.; Dionne, C.; James, S.; and Burzycki, J. Wearable focal muscle vibration on pain, balance, mobility, and sensation in individuals with diabetic peripheral neuropathy: A pilot study. Int. J. Environ. Res. Public. Health 2021, 18, 2415. [Google Scholar] [CrossRef]
- Heo, J.; Jo, J.; Lee, J.; Kang, H.; Choi, T.; Lee, M.; Kim, J. Electroacupuncture for the treatment of frozen shoulder. Medicine 2021, 100, e28179. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.I.; Paley, C.A.; Jones, G.; Mulvey, M.R.; and Wittkopf, P.G. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: A systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open. 2022, 12, e051073. [Google Scholar] [CrossRef] [PubMed]
- Martins, Â.; Gouveia, D.; Cardoso, A.; Viegas, I.; Gamboa, Ó.; Ferreira, A. A comparison between body weight-supported treadmill training and conventional over-ground training in dogs with incomplete spinal cord injury. Front. Vet. Sci. 2021, 8, 597949. [Google Scholar] [CrossRef]
- Lovaglio, A.; Socolovsky, M.; Di Masi, G.; Bonilla, G. Treatment of neuropathic pain after peripheral nerve and brachial plexus traumatic injury. Neurol. India 2019, 67, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Zhou, J.; Gu, L.; Zuo, Y. The change of HCN1/HCN2 mRNA expression in peripheral nerve after chronic constriction injury induced neuropathy followed by pulsed electromagnetic field therapy. Oncotarget 2017, 8, 1110–1116. [Google Scholar] [CrossRef] [PubMed]
- Basbaum, A.L.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and Molecular Mechanisms of Pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Emery, E.C.; Young, G.T.; McNaughton, P.A. HCN2 ion channels: An emerging role as the pacemakers of pain. Trends Pharmacol. Sci. 2012, 33, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, M.I.; Cole, S.P. Pulsed magnetic field therapy in refractory neuropathic pain secondary to peripheral neuropathy: Electrodiagnostic parameters-pilot study. Neurorehabil. Neural Repair. 2004, 18, 42–46. [Google Scholar] [CrossRef]
- Xu, Q.; Niu, C.; Li, J.; Hu, C.; He, M.; Qiu, X.; Yao, Q.; Tian, W.; Zhang, M. Electroacupuncture alleviates neuropathic pain caused by spared nerve injury by promoting AMPK/mTOR-mediated autophagy in dorsal root ganglion macrophage. Ann. Transl. Med. 2022, 10, 1341. [Google Scholar] [CrossRef]
- Tian, M.-Y.; Yan, Y.-D.; Qin, W.-T.; Liu, B.-N.; Mou, F.-F.; Zhu, J.; Guo, H.-D.; Shao, S.-J. Electroacupuncture Promotes Nerve Regeneration and Functional Recovery Through Regulating IncRNA GAS5 Targeting miR-21 After Sciatic Nerve Injury. Mol. Neurobiol. 2024, 61, 935–949. [Google Scholar] [CrossRef]
- Du, J.; Fang, J.; Xiang, X.; Yu, J.; Le, X.; Liang, Y.; Jin, X.; Fang, J. Effects of low- and high-frequency electroacupuncture on protein expression and distribution of TRPV1 and P2X3 in rats with peripheral nerve injury. Acupunct. Med. 2021, 39, 478–490. [Google Scholar] [CrossRef]
- Dragomir, M.F.; Pestean, C.P.; Melega, L.; Danciu, C.G.; Purdoiu, R.C.; Oana, L. Current Aspects Regarding the Clinical Relevance of Electroacupuncture in Dogs with Spinal Cord Injury: A Literature Review. Animal 2021, 11, 219. [Google Scholar] [CrossRef]
- Laim, A.; Jaggy, A.; Forterre, F.; Doherr, M.; Aeschbacher, G.; Glardon, O. Effects of adjunct electroacupuncture on severity of postoperative pain in dogs undergoing hemilaminectomy because of acute thoracolumbar intervertebral disk disease. J. Am. Vet. Med. Assoc. 2009, 234, 1141–1146. [Google Scholar] [CrossRef]
- Zhang, S.; Tang, H.; Zhou, J.; Gu, Y. Electroacupuncture attenuates neuropathic pain after brachial plexus injury. Neural Regen. Res. 2014, 9, 1365–1370. [Google Scholar] [PubMed]
- Memar, M.Y.; Yekani, M.; Alizadeh, N.; Baghi, H.B. Hyperbaric oxygen therapy: Antimicrobial mechanisms and clinical application for infections. Biomed. Pharmacother. 2019, 109, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Hengel, T.V.; Haar, G.T.; Kirpensteijn, J. Wound management: A new protocol for dogs and cats. In Reconstructive Surgery and Wound Management of the Dog and Cat; Manson Publishing: London, UK, 2013; pp. 21–48. [Google Scholar]
- Abdullah, M.S.; Al-Waili, N.; Butler, G.; Baban, N.K. Hyperbaric oxygen as an adjunctive therapy for bilateral compartment syndrome, rhabdomyolysis and acute renal failure after heroin intake. Arch. Med. Res. 2006, 37, 559–562. [Google Scholar] [CrossRef]
- Gouveia, D.; Bimbarra, S.; Carvalho, C.; Cardoso, A.; Gamboa, O.; Teixeira, R.; Ferreira, A.; Martins, A. Effects of hyperbaric oxygen therapy on wound healing in veterinary medicine: A pilot study. Open Vet. J. 2021, 11, 544. [Google Scholar] [PubMed]
- Brenna, C.T.; Khan, S.; Katznelson, R.; Brull, R. The role of hyperbaric oxygen therapy in the management of perioperative peripheral nerve injury: A scoping review of the literature. Reg. Anesth. Pain Med. 2023, 48, 443–453. [Google Scholar] [CrossRef]
- Sullivan, R.; Dailey, T.; Duncan, K.; Abel, N.; Borlongan, C.V. Peripheral Nerve Injury: Stem Cell Therapy and Peripheral Nerve Transfer. Int. J. Mol. Sci. 2016, 17, 2101. [Google Scholar] [CrossRef]
- Blits, B.; Boer, G.J.; Verhaagen, J. Pharmacological, cell, and gene therapy strategies to promote spinal cord regeneration. Cell Transplant. 2002, 11, 593–613. [Google Scholar] [CrossRef]
- Faroni, A.; Mobasseri, S.A.; Kingham, P.J.; Reid, A.J. Peripheral nerve regeneration: Experimental strategies and future perspectives. Adv. Drug Deliv. Rev. 2015, 82–83, 160–167. [Google Scholar] [CrossRef]
- Magnaghi, V.; Procacci, P.; Tata, A.M. Chapter 15: Novel pharmacological approaches to Schwann cells as neuroprotective agents for peripheral nerve regeneration. Int. Rev. Neurobiol. 2009, 87, 295–315. [Google Scholar]
- Vijayavenkataraman, S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020, 106, 54–69. [Google Scholar] [CrossRef] [PubMed]
- Raza, C.; Riaz, H.A.; Anjum, R.; Shakeel, N.U.A. Repair strategies for injured peripheral nerve: Review. Life Sci. 2020, 243, 117308. [Google Scholar] [CrossRef] [PubMed]
- Isvoranu, G.; Manole, E.; Neagu, M. Gait Analysis Using Animal Models of Peripheral Nerve and Spinal Cord Injuries. Biomedicines 2021, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.P.S.; Pinto, C.G.; Tiburcio, F.C.; Sartori, A.A.; de Castro Rodrigues, A.; Barraviera, B.; Ferreira, R.S.J.; Filadelpho, A.L.; Matheus, S.M.M. Heterologous fibrin sealant potentiates axonal regeneration after peripheral nerve injury with reduction in the number of suture points. Injury 2019, 50, 834–847. [Google Scholar] [CrossRef]
- Morris, M.; Brogan, D.M.; Boyer, M.I.; Dy, C.J. Trends in nerve transfer procedures among board-eligible orthopedic hand surgeons. J. Hand Surg. Global 2021, 3, 24–29. [Google Scholar] [CrossRef]
- Kobayashi, J.; Mackinnon, S.E.; Watanabe, O.; Ball, D.J.; Ming Gu, X.; Hunter, D.A.; Kuzon, W.M., Jr. The effect of duration of muscle denervation on functional recovery in the rat model. Muscle Nerve 1997, 20, 858–866. [Google Scholar] [CrossRef]
- Brown, J.M.; Tung, T.H.; Mackinnon, S.E. Median to radial nerve transfer to restore wrist and finger extension: Technical nuances. Neurosurgery 2010, 66 (Suppl. S3), ons75–ons83. [Google Scholar] [CrossRef]
- Mackinnon, S.E.; Roque, B.; Tung, T.H. Median to radial nerve transfer for treatment of radial nerve palsy: Case report. J. Neurosurg. 2007, 107, 666–671. [Google Scholar] [CrossRef]
- Dy, C.J.; Aunins, B.; Brogan, D.M. Barriers to epineural scarring: Role in treatment of traumatic nerve injury and chronic compressive neuropathy. J. Hand Surg. Am. 2018, 43, 360–367. [Google Scholar] [CrossRef]
- Kokkalis, Z.T.; Mavrogenis, A.F.; Ballas, E.G.; Papagelopoulos, P.J.; Soucacos, P.N. Collagen nerve wrap for median nerve scarring. Orthopedics 2015, 38, 117–121. [Google Scholar] [CrossRef][Green Version]
- Soltani, A.M.; Allan, B.J.; Best, M.J.; Mir, H.S.; Panthaki, Z.J. Revision decompression and collagen nerve wrap for recurrent and persistent compression neuropathies of the upper extremity. Ann. Plast. Surg. 2014, 72, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, L.; Adam, C.; Legagneux, J.; Bruneval, P.; Masmejean, E. Reduction of neural scarring after peripheral nerve suture: An experimental study about collagen membrane and autologous vein wrapping. Chir. Main. 2012, 31, 311–317. [Google Scholar] [CrossRef]
- Papatheodorou, L.K.; Williams, B.G.; Sotereanos, D.G. Preliminary results of recurrent cubital tunnel syndrome treated with neurolysis and porcine extracellular matrix nerve wrap. J. Hand Surg. Am. 2015, 40, 987–992. [Google Scholar] [CrossRef]
- Magill, C.K.; Tuffaha, S.H.; Yee, A.; Luciano, J.P.; Hunter, D.A.; Mackinnon, S.E.; Borschel, G.H. The short- and long-term effects of Seprafilm on peripheral nerves: A histological and functional study. J. Reconstr. Microsurg. 2009, 25, 345–354. [Google Scholar] [CrossRef]
- Ozgenel, G.Y.; Filiz, G. Effects of human amniotic fluid on peripheral nerve scarring and regeneration in rats. J. Neurosurg. 2003, 98, 371–377. [Google Scholar] [CrossRef]
- Gaspar, M.P.; Abdelfattah, H.M.; Welch, I.W.; Vosbikian, M.M.; Kane, P.M.; Rekant, M.S. Recurrent cubital tunnel syndrome treated with revision neurolysis and amniotic membrane nerve wrapping. J. Shoulder Elb. Surg. 2016, 25, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xu, J.; Pan, X.; Ding, Z.; Xie, H.; Wang, X.; Xie, H. Advances of adipose-derived mesenchymal stem cells-based biomaterial scaffolds for oral and maxillofacial tissue engineering. Bioact. Mater. 2021, 6, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Zorko, B.; Rozman, J.; Seliškar, A. Influence of electrical stimulation on regeneration of the radial nerve in dogs. Acta Vet. Hung. 2000, 48, 99–105. [Google Scholar] [CrossRef]
- Foecking, E.M.; Fargo, K.N.; Coughlin, L.M.; Kim, J.M.; Sam, J.; Jones, K.J. Crush Injury Enhances Functional Recovery of Rat Facial Nerve. J. Rehabil. Res. Dev. 2012, 49, 451–458. [Google Scholar] [CrossRef]
- Brushart, T.M.; Hoffman, P.N.; Royall, R.M.; Murinson, B.B.; Witzel, T. Gordon Electrical stimulation promotes motoneuron regeneration without increasing its speed or conditioning the neuron. J. Neurosci. 2002, 22, 6631–6638. [Google Scholar] [CrossRef]
- Geremia, N.M.; Gordon, T.; Brushart, T.M.; Al-Majed, A.A.; Verge, V.M.K. Electrical stimulation promotes sensory neuron regeneration and growth-associated gene expression. Exp. Neurol. 2007, 205, 347–359. [Google Scholar] [CrossRef]
- Huang, J.; Lu, L.; Hu, X.; Ye, Z.; Peng, Y.; Yan, X.; Geng, D.; Luo, Z. Electrical stimulation accelerates motor functional recovery in the rat model of 15-mm sciatic nerve gap bridged by scaffolds with longitudinally oriented microchannels. Neurorehabil. Neural Repair. 2010, 24, 736–745. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Yao, Z.; Zhao, Y.; Zhang, T.; Wang, J.; Li, S.; Chen, Z. Electrical stimulation therapy for peripheral nerve injury. Front. Neurol. 2023, 14, 1081458. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, Y.; Lu, L.; Hu, X.; Luo, Z. Electrical stimulation accelerates nerve regeneration and functional recovery in delayed peripheral nerve injury in rats. Eur. J. Neurosci. 2013, 38, 3691–3701. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; McConnell, K.W.; Amores, D.; Levinson, A.; Vogel, H.; Quarta, M.; Rando, T.A.; George, P.M. Electrical stimulation of human neural stem cells via conductive polymer nerve guides enhances peripheral nerve recovery. Biomaterials 2021, 275, 120982. [Google Scholar] [CrossRef] [PubMed]
- Cobianchi, S.; Casals-Diaz, L.; Jaramillo, J.; Navarro, X. Differential effects of activity dependent treatments on axonal regeneration and neuropathic pain after peripheral nerve injury. Exp. Neurol. 2013, 240, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Al-Majed, A.A.; Tam, S.L.; Gordon, T. Electrical stimulation accelerates and enhances expression of regeneration-associated genes in regenerating rat femoral motoneurons. Cell Mol. Neurobiol. 2004, 24, 379–402. [Google Scholar] [CrossRef] [PubMed]
- Eberhardt, K.A.; Irintchev, A.; Al-Majed, A.A.; Simova, O.; Brushart, T.M.; Gordon, T.; Schachner, M. BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery after peripheral nerve repair. Exp. Neurol. 2006, 198, 500–510. [Google Scholar] [CrossRef]
- Batty, N.J.; Torres-Espín, A.; Vavrek, R.; Raposo, P.; Fouad, K. Single-session cortical electrical stimulation enhances the efficacy of rehabilitative motor training after spinal cord injury in rats. Exp. Neurol. 2020, 324, 113136. [Google Scholar] [CrossRef]
- Martins, A.; Gouveia, D.; Cardoso, A.; Gamboa, Ó.; Millis, D.; Ferreira, A. Nervous system modulation through electrical stimulation in companion animals. Acta Vet. Scand. 2021, 63, 22. [Google Scholar] [CrossRef]
- Boyd, J.G.; Gordon, T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Mol Neurobiol. 2003, 27, 277–324. [Google Scholar] [CrossRef]
- Lu, B.; Pang, P.T.; Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef]
- Richner, M.; Ulrichsen, M.; Elmegaard, S.L.; Dieu, R.; Pallesen, L.T.; Vaegter, C.B. Peripheral nerve injury modulates neurotrophin signaling in the peripheral and central nervous system. Mol. Neurobiol. 2014, 50, 945–970. [Google Scholar] [CrossRef]
- Gordon, T. Electrical Stimulation to Enhance Axon Regeneration After Peripheral Nerve Injuries in Animal Models and Humans. Neurotherapeutics 2016, 13, 295–310. [Google Scholar] [CrossRef] [PubMed]
- Dow, D.E.; Dennis, R.G.; Faulkner, J.A. Electrical stimulation attenuates denervation and age-related atrophy in extensor digitorum longus muscles of old rats. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Eberstein, A.; Eberstein, S. Electrical stimulation of denervated muscle: Is it worthwhile? Med. Sci. Sports Exerc. 1996, 28, 1463–1469. [Google Scholar] [CrossRef]
- Brushart, T.M.; Jari, R.; Verge, V.; Rohde, C.; Gordon, T. Electrical stimulation restores the specificity of sensory axon regeneration. Exp. Neurol. 2005, 194, 221–229. [Google Scholar] [CrossRef]
- Tam, S.L.; Archibald, V.; Jassar, B.; Tyreman, N.; Gordon, T. Increased Neuromuscular Activity Reduces Sprouting in Partially Denervated Muscles. J. Neurosci. 2001, 21, 654–667. [Google Scholar] [CrossRef]
- Love, F.M.; Son, Y.-J.; Thompson, W.J. Activity alters muscle reinnervation and terminal sprouting by reducing the number of schwann cell pathways that grow to link synaptic sites. J. Neurobiol. 2003, 54, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Alvites, R.; Rita Caseiro, A.; Santos Pedrosa, S.; Vieira Branquinho, M.; Ronchi, G.; Geuna, S.; Varejão, A.S.P.; Colette Maurício, A.; Spurkland, A. Peripheral nerve injury and axonotmesis: State of the art and recent advances. Cogent. Med. 2018, 5, 1466404. [Google Scholar] [CrossRef]
- Bula-Oyola, E.; Belda-Lois, J.; Porcar-Seder, R.; Page, A. Effectiveness of electrophysical modalities in the sensorimotor rehabilitation of radial, ulnar, and median neuropathies: A meta-analysis. PLoS ONE 2021, 16, e0248484. [Google Scholar] [CrossRef]
- Colbert, A.P.; Markov, M.S.; Carlson, N.; Gregory, W.L.; Carlson, H.; Elmer, P.J. Static Magnetic Field Therapy for Carpal Tunnel Syndrome: A Feasibility Study. Arch. Phys. Med. Rehabil. 2010, 91, 1098–1104. [Google Scholar] [CrossRef]
- Ozkan, F.U.; Saygı, E.K.; Senol, S.; Kapcı, S.; Aydeniz, B.; Aktaş, İ.; Gozke, E. New treatment alternatives in the ulnar neuropathy at the elbow: Ultrasound and low-level laser therapy. Acta Neurol. Belg. 2015, 115, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Bilgin Badur, N.; Unlu Ozkan, F.; Aktas, I. Efficacy of shortwave diathermy in ulnar nerve entrapment at the elbow: A double-blind randomized controlled clinical trial. Clin. Rehabil. 2020, 34, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Oshima, C.; Nakazawa, H.; Izukura, H.; Miyagi, M.; Mizutani, A.; Harada, T.; Ohshiro, T.; Ebihara, S. Low Level Laser Therapy for Radial Nerve Palsy Patients: Our Experience. LASER Ther. 2018, 27, 56–60. [Google Scholar] [CrossRef]
- Kim, J.K.; Jeon, S.H. Minimal clinically important differences in the Carpal Tunnel Questionnaire after carpal tunnel release. J. Hand Surg. 2013, 38, 75–79. [Google Scholar] [CrossRef]
- Gunter, C.; Delbeke, J.; Ortiz-Catalan, M. Safety of long-term electrical peripheral nerve stimulation: Review of the state of the art. J. NeuroEng Rehab. 2019, 16, 13. [Google Scholar] [CrossRef]
- Agnew, W.F.; McCreery, D.B. Considerations for safety with chronically implanted nerve electrodes. Epilepsia 1990, 31, S27–S32. [Google Scholar] [CrossRef] [PubMed]
- McCreery, D.B.; Agnew, W.F.; Yuen, T.G.H.; Bullara, L.A. Relationship between stimulus amplitude, stimulus frequency and neural damage during electrical stimulation of sciatic nerve of cat. Med. Biol. Eng. Comput. 1995, 33, 426–429. [Google Scholar] [CrossRef]
- Waters, R.L.; McNeal, D.R.; Faloon, W.; Clifford, B. Functional electrical stimulation of the peroneal nerve for hemiplegia. Long-term clinical follow-up. J. Bone Jt. Surg. 1985, 67, 792–793. [Google Scholar] [CrossRef]
- Agnew, W.F.; McCreery, D.B.; Yuen, T.G.H.; Bullara, L.A. Histologic and physiologic evaluation of electrically stimulated peripheral nerve: Considerations for the selection of parameters. Ann. Biomed. Eng. 1989, 17, 39–60. [Google Scholar] [CrossRef]
- Hasiba-Pappas, S.; Kamolz, L.; Luze, H.; Nischwitz, S.; Holzer-Geissler, J.; Tuca, A.; Rienmuller, T.; Polz, M.; Ziesel, D.; Winter, R. Does electrical stimulation through nerve conduits improve peripheral nerve regeneration?—A systematic review. J. Pers. Med. 2023, 13, 414. [Google Scholar] [CrossRef]
- Kubiak, C.A.; Kung, T.A.; Brown, D.L.; Cederna, P.S.; Kemp, S.W.P. State-of-The-Art techniques in treating peripheral nerve injury. Plast. Reconstr. Surg. 2018, 141, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.M.; Curran, M.W.T.; Gordon, T. Neuroscience The use of brief post-surgical low frequency electrical stimulation to enhance nerve regeneration in clinical practice. J. Physiol. 2016, 594, 3553–3559. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Kao, C.H.; Chen, C.C.; Ke, C.J.; Yao, C.H.; Chen, Y.S. Time-course effect of electrical stimulation on nerve regeneration of diabetic rats. PLoS ONE 2015, 10, e0116711. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Sun, B.; Liu, S.; Chen, W.; Zhang, Y.; Wang, C.; Mo, X.; Che, J.; Ouyang, Y.; Yuan, W.; et al. Polymerizing pyrrole coated poly (l-lactic acid-co-ε-caprolactone) (PLCL) conductive nanofibrous conduit combined with electric stimulation for long-range peripheral nerve regeneration. Front. Mol. Neurosci. 2016, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Dorrian, R.M.; Berryman, C.F.; Lauto, A.; Leonard, A.V. Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements. Front. Cell Neurosci. 2023, 17, 1095259. [Google Scholar] [CrossRef] [PubMed]
- Griffin, L.; Decker, M.J.; Hwang, J.Y.; Wang, B.; Kitchen, K.; Ding, Z.; Ivy, J.L. Functional electrical stimulation cycling improves body composition, metabolic and neural factors in persons with spinal cord injury. J. Electromyogr. Kinesiol. 2009, 19, 614–622. [Google Scholar] [CrossRef]
- Bakkum, A.J.; Paulson, T.A.; Bishop, N.C.; Goosey-Tolfrey, V.L.; Stolwijk-Swuste, J.M.; van Kuppevelt, D.J.; de Groot, S.; Janssen, T.W. Effects of hybrid cycle and handcycle exercise on cardiovascular disease risk factors in people with spinal cord injury: A randomized controlled trial. J. Rehabil. Med. 2015, 47, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Ayanwuyi, L.; Tokarska, N.; McLean, N.A.; Johnston, J.M.; Verge, V.M.K. Brief electrical nerve stimulation enhances intrinsic repair capacity of the focally demyelinated central nervous system. Neural Regen. Res. 2022, 17, 1042–1050. [Google Scholar]
- Hahm, S.-C.; Yoon, Y.W.; Kim, J. High-Frequency transcutaneous electrical nerve stimulation alleviates spasticity after spinal contusion by inhibiting activated microglia in rats. Neurorehabil. Neural Rep. 2014, 29, 370–381. [Google Scholar] [CrossRef]
- Chu, X.; Song, X.; Li, Q.; Li, Y.; He, F.; Gu, X.; Ming, D. Basic mechanisms of peripheral nerve injury and treatment via electrical stimulation. Neural Regen. Res. 2022, 17, 2185–2193. [Google Scholar]
- Martins, D.F.; Martins, T.C.; Batisti, A.P.; Leonel, L.D.S.; Bobinski, F.; Belmonte, L.A.O.; Mazzardo-Martins, L.; Cargnin-Ferreira, E.; Santos, A.R.S. Long-Term Regular Eccentric Exercise Decreases Neuropathic Pain-like Behavior and Improves Motor Functional Recovery in an Axonotmesis Mouse Model: The Role of Insulin-like Growth Factor-1. Mol. Neurobiol. 2017, 55, 6155–6168. [Google Scholar] [CrossRef]
- López-Álvarez, V.M.; Modol, L.; Navarro, X.; Cobianchi, S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain 2015, 156, 1812–1825. [Google Scholar] [CrossRef]
- de Moraes, A.A.; de Almeida, C.A.S.; Lucas, G.; Thomazini, J.A.; DeMaman, A.S. Effect of swimming training on nerve morphological recovery after compressive injury. Neurol. Res. 2018, 40, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Ilha, J.; Araujo, R.T.; Malysz, T.; Hermel, E.E.S.; Rigon, P.; Xavier, L.L.; Achaval, M. Endurance and Resistance Exercise Training Programs Elicit Specific Effects on Sciatic Nerve Regeneration After Experimental Traumatic Lesion in Rats. Neurorehabilit. Neural Repair. 2007, 22, 355–366. [Google Scholar] [CrossRef]
- Pachter, B.R.; Eberstein, A. Passive Exercise and Reinnervation of the Rat Denervated Extensor Digitorum Longus Muscle after Nerve Crush. Am. J. Phys. Med. Rehabil. 1989, 68, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Florence, S.L.; Boydston, L.A.; Hackett, T.A.; Lachoff, H.T.; Strata, F.; Niblock, M.M. Sensory enrichment after peripheral nerve injury restores cortical, not thalamic, receptive field organization. Eur. J. Neurosci. 2001, 13, 1755–1766. [Google Scholar] [CrossRef]
- Sinis, N.; Guntinas-Lichius, O.; Irintchev, A.; Skouras, E.; Kuerten, S.; Pavlov, S.P.; Schaller, H.E.; Dunlop, S.A.; Angelov, D.N. Manual stimulation of forearm muscles does not improve recovery of motor function after injury to a mixed peripheral nerve. Med. Sci. Sports Exerc. 2006, 38, 1267–1276. [Google Scholar] [CrossRef]
- Pan, B.; Fromholt, S.E.; Hess, E.J.; Crawford, T.O.; Griffin, J.W.; Sheikh, K.A.; Schnaar, R.L. Myelin-associated glycoprotein and complementary axonal ligands, gangliosides, mediate axon stability in the CNS and PNS: Neuropathology and behavioral deficits in single- and double-null mice. Exp. Neurol. 2005, 195, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Trapp, B.D.; Andrews, S.B.; Cootauco, C.; Quarles, R. The myelin-associated glycoprotein is enriched in multivesicular bodies and periaxonal membranes of actively myelinating oligodendrocytes. J. Cell Biol. 1989, 109, 2417–2426. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Taylor, R.S. Guillain-Barre Syndrome; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sabatier, M.J.; Redmon, N.; Schwartz, G.; English, A.W. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp. Neurol. 2008, 211, 489–493. [Google Scholar] [CrossRef] [PubMed]
- English, A.W.; Cucoranu, D.; Mulligan, A.; Sabatier, M. Treadmill training enhances axon regeneration in injured mouse peripheral nerves without increased loss of topographic specificity. J. Comp. Neurol. 2009, 517, 245–255. [Google Scholar] [CrossRef]
- Hunter, G.; Sarvestany, A.A.; Roche, S.; Symes, R.; Gillinwater, T.H. SMN-dependent intrinsic defects in Schwann cells in mouse models of spinal muscular atrophy. Hum. Mol. Genet. 2014, 23, 2235–2250. [Google Scholar] [CrossRef]
- Armada-da-Silva, P.; Pereira, C.; Amado, S.; Veloso, A. Role of physical exercise for improving posttraumatic nerve regeneration. Int. Rev. Neurobiol. 2013, 109, 125–149. [Google Scholar] [PubMed]
- Gouveia, D.; Cardoso, A.; Carvalho, C.; Almeida, A.; Gamboa, O.; Ferreira, A.; Martins, A. Approach to Small Animal Neurorehabilitation by Locomotor Training: Na Update. Animals 2022, 12, 3582. [Google Scholar] [CrossRef]
- Willand, M.; Nguyen, M.; Borschel, G.; Gordon, T. Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil. Neural Repair 2016, 30, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.J.; Sengelaub, D.R.; English, A.W. Enhancement of peripheral nerve regeneration due to treadmill training and electrical stimulation is dependent on androgen receptor signaling. Dev. Neurobiol. 2014, 74, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Elzinga, K.; Tyreman, N.; Ladak, A.; Savaryn, B.; Olson, J.; Gordon, T. Brief electrical stimulation improves nerve regeneration after delayed repair in Sprague Dawley rats. Exp. Neurol. 2015, 269, 142–153. [Google Scholar] [CrossRef]
- Gordon, T.; Amirjani, N.; Edwards, D.C.; Chan, K.M. Brief post-surgical electrical stimulation accelerates axon regeneration and muscle reinnervation without affecting the functional measures in carpal tunnel syndrome patients. Exp. Neurol. 2010, 223, 192–202. [Google Scholar] [CrossRef]
- Wong, J.N.; Olson, J.L.; Morhart, M.J.; Chan, K.M. Electrical stimulation enhances sensory recovery: A randomized control trial. Ann. Neurol. 2015, 77, 996–1006. [Google Scholar] [CrossRef] [PubMed]
- Juckett, L.; Saffari, T.M.; Ormseth, B.; Senger, J.; Moore, A. The effect of electrical stimulation on nerve regeneration following peripheral nerve injury. Biomolecules 2022, 12, 1856. [Google Scholar] [CrossRef] [PubMed]
- Bal-Price, A.; Brown, G.C. Nitric-oxide-induced necrosis and apoptosis in PC12 cells mediated bu mitochondria. J. Neurochem. 2000, 75, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Khalil, Z.; Khodr, B. A role for free radicals and nitric oxide in delayed recovery in aged rats with chronic constriction nerve injury. Free Rad. Biol. Med. 2001, 31, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Kono, T.; Kasai, S.; Sakamoto, T.; Mito, M. Cord dorsum potentials suppressed by low power laser irradiation on a peripheral nerve in the cat. J. Clin. Laser Med. Surg. 1993, 11, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Rochkind, S.; Ouaknine, G.E. New trend in neuroscience: Low-power laser effect on peripheral and central nervous system (basic science, preclinical and clinical studies). Neurol. Res. 1992, 14, 2–11. [Google Scholar] [CrossRef]
- Robinson, N.G. Beyond the Laboratory, Into the Clinic: What Dogs with Disk Disease Have Taught Us About Photobiomodulation for Spinal Cord Injury. Photomed. Laser Surg. 2017, 35, 589–594. [Google Scholar] [CrossRef]
- Geng, X.; Sun, T.; Li, J.-h.; Zhao, N.; Wang, Y.; Yu, H.-l. Electroacupuncture in the repair of spinal cord injury: Inhibiting the Notch signaling pathway and promoting neural stem sell proliferation. Neural Regen. Res. 2015, 10, 394–403. [Google Scholar]
- Tsai, S.-R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef]
- Salazar, T.E.; Richardson, M.R.; Beli, E.; Ripsch, M.S.; George, J.; Kim, Y.; Duan, Y.; Moldovan, L.; Yan, Y.; Bhatwadekar, A.; et al. Electroacupuncture Promotes Central Nervous System-Dependent Release of mesenchymal Stem Cells. Stem Cells 2017, 35, 1303–1315. [Google Scholar] [CrossRef]
- Salehpour, F.; Ahmadian, N.; Rasta, S.H.; Farhoudi, M.; Karimi, P.; Sadigh-Eteghad, S. Transcranial low-level laser therapy improves brain mitochondrial function and cognitive impairment in D-galactose-induced aging mice. Neurobiol. Aging 2017, 58, 140–150. [Google Scholar] [CrossRef]
- Salehpour, F.; Farajdokht, F.; Erfani, M.; Sadigh-Eteghad, S.; Shotorbani, S.S.; Hamblin, M.R.; Karimi, P.; Rasta, S.H.; Mahmoudi, J. Transcranial near-infrared photobiomodulation attenuates memory impairment and hippocampal oxidative stress in sleep-deprived mice. Brain Res. 2018, 1682, 36–43. [Google Scholar] [CrossRef]
- Barbosa, R.I.; Marcolino, A.M.; de Jesus Guirro, R.R.; Mazzer, N.; Barbieri, C.H.; de Cássia Registro Fonseca, M. Comparative effects of wavelengths of low-power laser in regeneration of sciatic nerve in rats following crushing lesion. Lasers Med. Sci. 2010, 25, 423–430. [Google Scholar] [CrossRef]
- Gigo-Benato, D.; Geuna, S.; de Castro Rodrigues, A.; Tos, P.; Fornaro, M.; Boux, E.; Battiston, B.; Giacobini-Robecchi, M.G. Low-power laser biostimulation enhances nerve repair after end-to-side neurorrhaphy: A double-blind randomized study in the rat median nerve model. Lasers Med. Sci. 2004, 19, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.C.; Yang, Y.C.; Liu, B.S. Large-area irradiated low-level laser effect in a biodegradable nerve guide conduit on neural regeneration of peripheral nerve injury in rats. Injury 2011, 42, 803–813. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; OConnor, D.; Pitt, V.; Massy-Westropp, N. Therapeutic ultrasound for carpal tunnel syndrome. Cochrane Database Syst. Rev. 2013, 3, CD009601. [Google Scholar] [CrossRef] [PubMed]
- Piao, D.; Sypniewski, L.A.; Dugat, D.; Bailey, C.; Burba, D.J.; De Taboada, L. Transcutaneos transmission of photobiomodulation light to the spinal canal of dog as measured from cadaver dogs using a multi-channel intra-spinal probe. Lasers Med. Sci. 2019, 34, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Meireles, A.; Rocha, B.P.; Rosa, C.T.; Silva, L.I.; Bonfleur, M.L.; Bertolini, G.R.F. Avaliação do papel de opioides endógenos na analgesia do laser de baixa potência, 820 nm, em joelhos de ratos Wistar. Rev. Dor. 2012, 13, 152–155. [Google Scholar] [CrossRef][Green Version]
- Serra, A.; Ashmawi, H.A. Influência da Naloxona e Metisergida sobre o Efeito Analgésico do Laser em Baixa Intensidade em Modelo Experimental de Dor. Rev. Bras. Anestesiol. 2010, 60, 302–310. [Google Scholar] [CrossRef][Green Version]
- Hawkins, D.; Abrahamse, H. Phototherapy—A treatment modality for wound healing and pain relief. Afri. J. Biomed. Res. 2007, 10, 99–109. [Google Scholar] [CrossRef]
- Ramer, L.M.; Ramer, M.S.; Bradbury, E.J. Restoring function after spinal cord injury: Towards clinical translation of experimental strategies. Lancet Neurol. 2014, 13, 1241–1256. [Google Scholar] [CrossRef]
- Bekhet, A.; Ragab, B.; Abushouk, A.; Elgebaly, A.; Ali, O. Efficacy of low-level laser therapy in carpal tunnel syndrome management: A systematic review and meta-analysis. Lasers Med. Sci. 2017, 32, 1439–1448. [Google Scholar] [CrossRef]
- Keyan, Z.; Liqian, Z.; Xinzhong, X.; Juehua, J.; Chungui, X. Pulsed electromagnetic fields improved peripheral nerve regeneration after delayed repair of one month. Bioelectromagnetics 2023, 44, 133–143. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Kim, B.; Lee, S.-H.; Ju, K.; Kim, S.-M.; Lee, J.-H.; Pang, K. Biomechanical microenvironmental stimulating effect of pulsed electromagnet field on the regeneration of crush injured rat sciatic nerve. Biomed. Eng. Lett. 2023, 13, 235–243. [Google Scholar] [CrossRef]
- Orgel, M.G.; O’Brien, W.J.; Murray, H.M. Pulsing electromagnetic field therapy in nerve regeneration: An experimental study in the cat. Plast. Reconstr. Surg. 1984, 73, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.R.; Bowden, R.E. Effects of high-peak pulsed electromagnetic field on the degeneration and regeneration of the common peroneal nerve in rats. J. Bone Jt. Surg. Br. 1983, 65, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Zienowicz, R.J.; Thomas, B.A.; Kurtz, W.H.; Orgel, M.G. A multivariate approach to the treatment of peripheral nerve transection injury: The role of electromagnetic field therapy. Plast. Reconstr. Surg. 1991, 87, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Bademoglu, G.; Erdal, N.; Uzun, C.; Tasdelen, B. The effects of pulsed electromagnetic field on experimentally induced sciatic nerve injury in rats. Electromagn. Biol. Med. 2021, 40, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.L.; Kryscio, R.; Smith, J.; Pilla, A.; Sisken, B.F. Electromagnetic field treatment of nerve crush injury in a rat model: Effect of signal configuration on functional recovery. Bioelectromagnetics 2007, 28, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.; Fratini, P.; Matias, G.; Bocabello, R.; Monteriro, J.; Santos, C., Jr.; Joaquim, J.; Giglio, R.; Possebon, F.; Stella, S.; et al. Combination of stem cells from deciduous teeth and electroacupuncture for therapy in dogs with chronic spinal cord injury: A pilot study. Res. Vet. Sci. 2019, 123, 247–251. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Luo, Z.-R.; Wang, C.; Cai, H.; Zhao, T.-T.; Li, H.; Shao, S.-J.; Guo, H.-D. Electroacupuncture Promoted Nerve Repair After Peripheral Nerve Injury by Regulating miR-1b and Its Target Brain-Derived Neurotrophic Factor. Front. Neurosci. 2020, 14, 525144. [Google Scholar] [CrossRef]
- Hu, L.-N.; Tian, J.-X.; Gao, W.; Zhu, J.; Mou, F.-F.; Ye, X.-C.; Liu, Y.-P.; Lu, P.-P.; Shao, S.-J.; Guo, H.-D. Electroacupuncture and moxibustion promote regeneration of injured sciatic nerve through Schwann cell proliferation and nerve growth factor secretion. Neural Regen. Res. 2018, 13, 477–483. [Google Scholar] [PubMed]
- Strickland, L.T.; Richards, L.; Holmes, F.E.; Wynick, D.; Uney, J.B.; Wong, L.-F. Axotomy-induced miR-21 promotes axon growth in adult dorsal root ganglion neurons. PLoS ONE 2011, 6, e23423. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Yang, Y.-D.; Mou, F.-F.; Zhu, J.; Li, H.; Zhao, T.-T.; Zhao, Y.; Shao, S.-J.; Cui, G.-H.; Guo, H.-D.; et al. Exosome-Mediated miR-21 Was Involved in the Promotion of Structural and Functional Recovery Effect Produced by Electroacupuncture in Sciatic Nerve Injury. Oxid. Med. Cell Longev. 2022, 2022, 7530101. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, F.J. Turning 21: Induction of miR-21 as a key switch in the inflammatory response. Front. Immunol. 2015, 29, 19. [Google Scholar] [CrossRef] [PubMed]
- Nasci, V.L.; Chuppa, S.; Griswold, L.; Goodreau, K.A.; Dash, R.K.; Kriegel, A.J. MiR-21-5p regulates mitochondrial respiration and lipid content in H9C2 cells. Am. J. Heart Circ. Physiol. 2019, 316, H710–H721. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; He, Y.; Wang, X.; Peng, D.; Chen, X.; Li, X.; Wang, Q. Overexpression of miR-21 in stem cells improves ovarian structure and function in rats with chemotherapy-induced ovarian damage bu targeting PDCD4 and PTEN to inhibit granulosa cell apoptosis. Stem Cell Res. Ther. 2017, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, L.; Liu, Y.; Wang, S.; Hou, Z.; Zhou, J. miR-21-5p promotes cell proliferation by targeting BCL11B in Thp-1 cells. Oncol. Lett. 2021, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Cen, Y.; Chen, J.; Lei, L.; Zhang, L.; Qin, X.; Gao, X.; Wang, Y.; Zeng, C. Effects of electroacupuncture on functional indices and pS6 expression following acute sciatic nerve crush injury in rats. Acupunct. Med. 2020, 38, 181–187. [Google Scholar] [CrossRef]
- Chen, X.D.; Gu, Y.D.; Yang, Y. Effect of electroacupuncture on mRNA expression of NGF and IGF-1 injured nerve. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2000, 14, 328–331. [Google Scholar]
- Wang, Y.; Zheng, L.; Zhu, L.; Chen, H.; Zhang, L.; Pei, F.; Liu, B.; Zhao, B.; Jin, L. Effect of the neurodynamic mobilization technique combined with electroacupuncture on functinal recovery and GAP-43 expression after sciatic nerve injury in rabbits. J. Clin. Electroneurophys 2007, 16, 268270. [Google Scholar]
- Liu, Y.-L.; Li, Y.; Ren, L.; Dai, L.-L.; Bai, Z.-H.; Bai, R.; Ma, T.-M. Effect of deep electroacupuncture stimulation of “Huantiao” (GB 30) on changes of function and nerve grouwth factor expression of the injured sciatic nerve in rats. Zhen Ci Yan Jiu. 2014, 39, 93–99. [Google Scholar]
- Huang, Z.Y.; Zou, X.; Wang, K.F. Effects of electroacupuncture on mRNA expression of NGF and IGF-1 in sciatic nerve of diabetic rat. Res. Int. Tradit. Chin. West. Med. 2010, 2, 57–60. [Google Scholar]
- Cheng, Y.-C.; Lin, J.-L.; Su, S.; Shih, P.-C.; Chen, K.-S.; Wang, H.-C.; Lee, W.-M. Case report: Efficacy of Combination of Electroacupuncture and Aquapuncture Using Vitamin B Complex on Promotion of Ambulation Perception in 15 Dogs with Hansen Type I Intervertebral Disc Disease Undergoing Hemilaminectomy. Thai J. Vet. Medicine. 2015, 45, 463–468. [Google Scholar] [CrossRef]
- Han, H.-J.; Yoon, H.-Y.; Kim, J.-Y.; Jang, H.-Y.; Lee, B.; Choi, S.H.; Jeong, S.-W. Clinical effect of additional electroacupuncture on thoracolumbar intervertebral disc herniation in 80 paraplegic dogs. Am. J. Chin. Med. 2010, 38, 1015–1025. [Google Scholar] [CrossRef]
- Hayashi, A.M.; Matera, J.M.; Pinto, A. Evaluation of electroacupuncture treatment for thoracolumbar intervertebral disk disease in dogs. J. Am. Vet. Med. Assoc. 2007, 231, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yan, Q.; Ruan, J.-W.; Zhang, Y.-Q.; Li, W.-J.; Zeng, X.; Huang, S.-F.; Zhang, Y.-J.; Wang, S.; Dong, H.; et al. Bone marrow mesenchymal stem cells and electroacupuncture downregulate the inhibitor molecules and promote the axonal regeneration in the transected spinal cord of rats. Cell Transplant. 2011, 20, 475–491. [Google Scholar] [CrossRef]
- Zhang, B.-L.; Guo, X.-L. Electroacupuncture promotes nerve regeneration and functional recovery in rats with spinal cord contusion through the coordinate interaction of CD4 and BDNF. Ibrain 2022, 8, 285–301. [Google Scholar] [CrossRef]
- Fei, J.; Gao, L.; Li, H.-H.; Yuan, Q.-L.; Li, L.-J. Electroacupuncture promotes peripheral nerve regeneration after facial nerve crush injury and upregulates the expression of glial cell-derived neurotrophic factor. Neural Regen. Res. 2019, 14, 673–682. [Google Scholar] [PubMed]
- Ho, C.Y.; Yao, C.H.; Chen, W.C.; Shen, W.C.; Bau, D.T. Electroacupuncture and Acupuncture Promote the Rat's Transected Median Nerve Regeneration. Evid. Based Complement. Alternat Med. 2013, 2013, 514610. [Google Scholar] [CrossRef]
- Yao, C.-H.; Chang, R.-L.; Chang, S.-L.; Tsai, C.-C.; Tsai, F.-J.; Chen, Y.-S. Electrical stimulation improves peripheral nerve regeneration in sptreptozotocin-induced diabetic rats. J. Trauma. Acute Care Surg. 2012, 72, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, J.A.; Mira, J.-A. Behavioral evaluating methods in the objective clinical assessment of motor function after experimental brachial plexus reconstruction in the rat. J. Neurosci. Methods 1993, 46, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Yao, C.-H.; Chen, T.-H.; Lin, J.-G.; Hsieh, C.-L.; Lin, C.-C.; Lao, C.-J.; Tsai, C.-C. Effect of acupuncture stimulation on peripheral nerve regeneration using silicone rubber chambers. Am. J. Chin. Med. 2001, 29, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Chen, R.; Cooper, E.L. Neuroendocrine mechanisms of acupuncture. Evid. Based Complement. Alternat Med. 2012, 2012, 792793. [Google Scholar] [CrossRef] [PubMed]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal Stem Cells for Spinal Cord Injury: Current Options, Limitations, and Future of Cell Therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef]
- Rowland, J.W.; Hawryluk, G.W.; Kwon, B.; Fehlings, M.G. Current status of acute spinal cord injury pathophysiology and emerging therapies: Promise on the horizon. Neurosurg. Focus. 2008, 25, E2. [Google Scholar] [CrossRef]
- Gazdic, M.; Volarevic, V.; Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Arsenijevic, N.; Stojkovic, M. Stem Cells Therapy for Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 1039. [Google Scholar] [CrossRef]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor. Rev. 2019, 46, 1–9. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouveia, D.; Cardoso, A.; Carvalho, C.; Oliveira, A.C.; Almeida, A.; Gamboa, Ó.; Lopes, B.; Coelho, A.; Alvites, R.; Varejão, A.S.; et al. Early Intensive Neurorehabilitation in Traumatic Peripheral Nerve Injury—State of the Art. Animals 2024, 14, 884. https://doi.org/10.3390/ani14060884
Gouveia D, Cardoso A, Carvalho C, Oliveira AC, Almeida A, Gamboa Ó, Lopes B, Coelho A, Alvites R, Varejão AS, et al. Early Intensive Neurorehabilitation in Traumatic Peripheral Nerve Injury—State of the Art. Animals. 2024; 14(6):884. https://doi.org/10.3390/ani14060884
Chicago/Turabian StyleGouveia, Débora, Ana Cardoso, Carla Carvalho, Ana Catarina Oliveira, António Almeida, Óscar Gamboa, Bruna Lopes, André Coelho, Rui Alvites, Artur Severo Varejão, and et al. 2024. "Early Intensive Neurorehabilitation in Traumatic Peripheral Nerve Injury—State of the Art" Animals 14, no. 6: 884. https://doi.org/10.3390/ani14060884
APA StyleGouveia, D., Cardoso, A., Carvalho, C., Oliveira, A. C., Almeida, A., Gamboa, Ó., Lopes, B., Coelho, A., Alvites, R., Varejão, A. S., Maurício, A. C., Ferreira, A., & Martins, Â. (2024). Early Intensive Neurorehabilitation in Traumatic Peripheral Nerve Injury—State of the Art. Animals, 14(6), 884. https://doi.org/10.3390/ani14060884







