Preliminary Study on Microplastic Contamination in Black Sea Cetaceans: Gastrointestinal Analysis of Phocoena phocoena relicta and Tursiops truncatus ponticus
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Post-Mortem Investigation
2.3. GIT Content Processing
2.4. Sample Analysis
2.5. Contamination Control and Procedural Blanks
2.6. Data Analysis
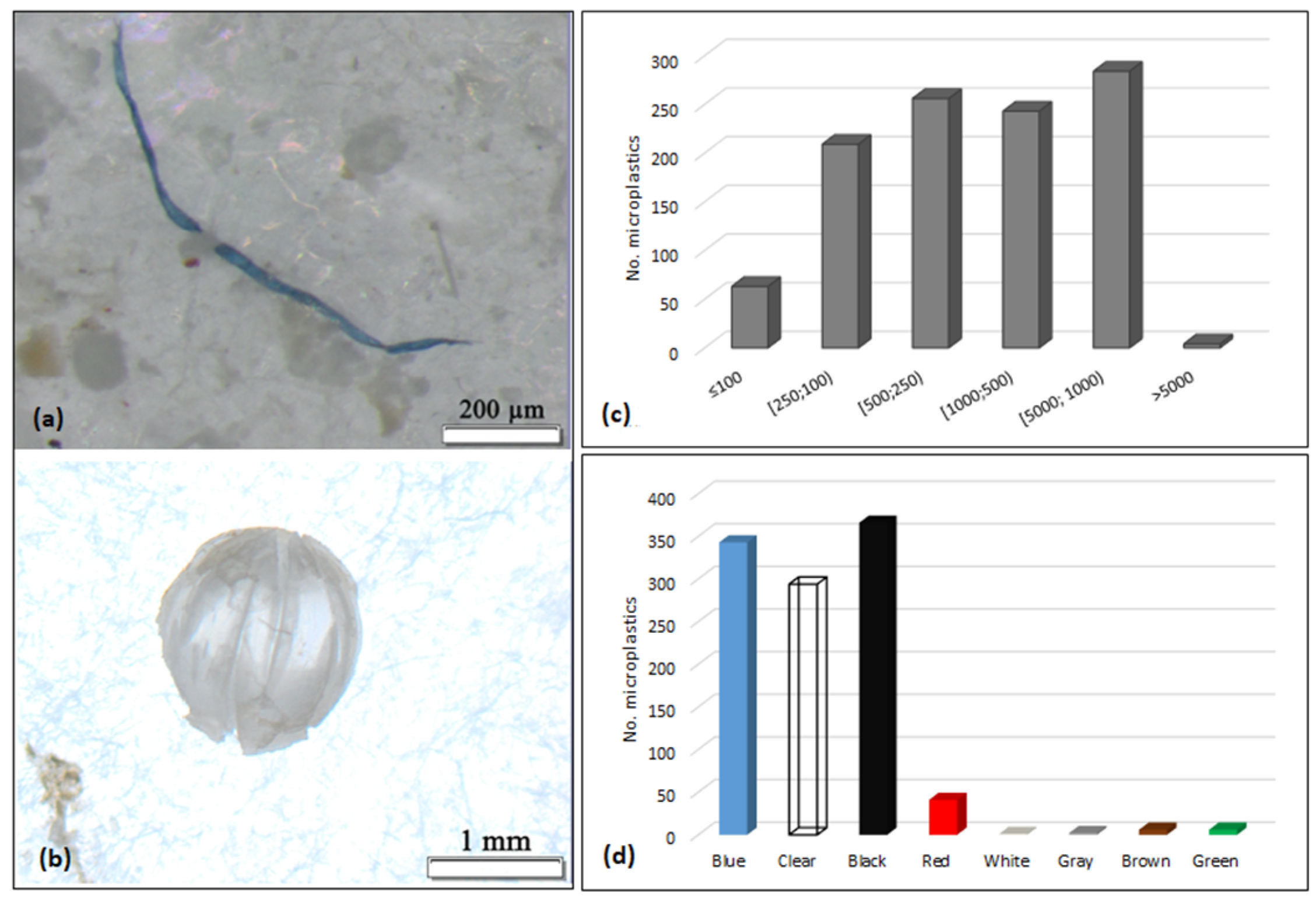
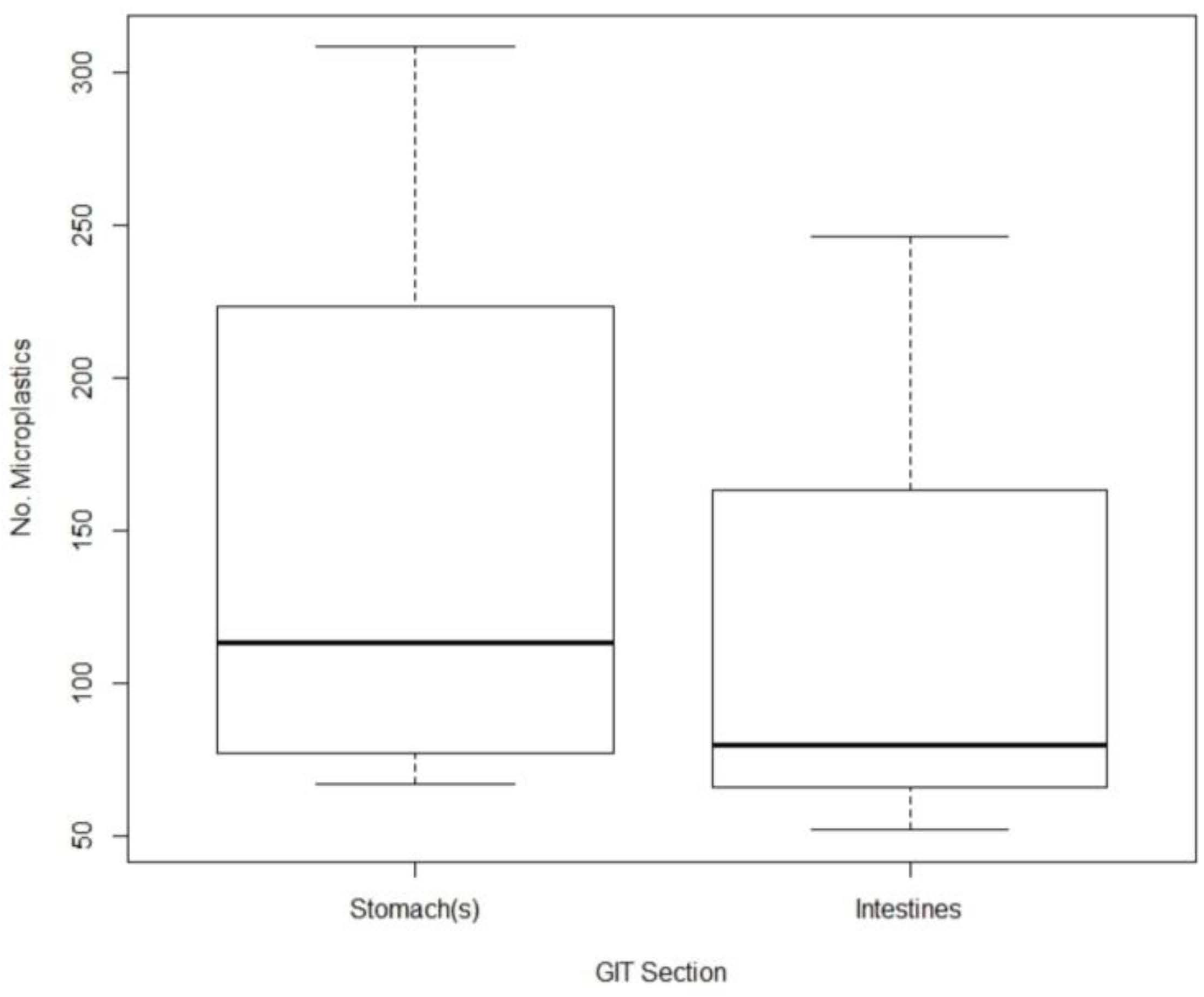
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrady, A.L. Microplastics in the Marine Environment. Mar. Pollut. Bull. 2011, 62, 1596–1605. [Google Scholar] [CrossRef]
- Almroth, B.C.; Eggert, H. Marine Plastic Pollution: Sources, Impacts, and Policy Issues. Rev. Environ. Econ. Policy 2019, 13, 317–326. [Google Scholar] [CrossRef]
- Bessa, F.; Frias, J.; Kögel, T.; Lusher, A.; Andrade, J.M.; Antunes, J.; Sobral, P.; Pagter, E.; Nash, R.; O’Connor, I.; et al. Harmonized Protocol for Monitoring Microplastics in Biota; JPI-Oceans BASEMAN Project: Brussels, Belgium, 2019. [Google Scholar]
- Hart, L.B.; Dziobak, M.; Wells, R.S.; Ertel, B.; Weinstein, J. Microplastics in Gastric Samples from Common Bottlenose Dolphins (Tursiops truncatus) Residing in Sarasota Bay FL (USA). Front. Mar. Sci. 2022, 9, 947124. [Google Scholar] [CrossRef]
- Directive, S.F. EC Directive 2008/56/EC of the European Parliament and of the Council of 17 June 2008 Establishing a Framework for Community Action in the Field of Marine Environmental Policy (Marine Strategy Framework Directive). Off. J. Eur. Union 2008, 164, 19–40. [Google Scholar]
- Rizzi, M.; Rodrigues, F.L.; Medeiros, L.; Ortega, I.; Rodrigues, L.; Monteiro, D.S.; Kessler, F.; Proietti, M.C. Ingestion of Plastic Marine Litter by Sea Turtles in Southern Brazil: Abundance, Characteristics and Potential Selectivity. Mar. Pollut. Bull. 2019, 140, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Yu, Y.; Wu, H.; Wu, W.; Wang, L.; An, L.; Cai, W. Microplastics in Spotted Seal Cubs (Phoca largha): Digestion after Ingestion? Sci. Total Environ. 2021, 785, 147426. [Google Scholar] [CrossRef]
- Franco-Trecu, V.; Drago, M.; Katz, H.; Machín, E.; Marín, Y. With the Noose around the Neck: Marine Debris Entangling Otariid Species. Environ. Pollut. 2017, 220, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Thiel, M.; Luna-Jorquera, G.; Álvarez-Varas, R.; Gallardo, C.; Hinojosa, I.A.; Luna, N.; Miranda-Urbina, D.; Morales, N.; Ory, N.; Pacheco, A.S.; et al. Impacts of Marine Plastic Pollution from Continental Coasts to Subtropical Gyres—Fish, Seabirds, and Other Vertebrates in the SE Pacific. Front. Mar. Sci. 2018, 5, 238. [Google Scholar] [CrossRef]
- Jepsen, E.M.; de Bruyn, P.J.N. Pinniped Entanglement in Oceanic Plastic Pollution: A Global Review. Mar. Pollut. Bull. 2019, 145, 295–305. [Google Scholar] [CrossRef]
- Weideman, E.A.; Munro, C.; Perold, V.; Omardien, A.; Ryan, P.G. Ingestion of Plastic Litter by the Sandy Anemone Bunodactis reynaudi. Environ. Pollut. 2020, 267, 115543. [Google Scholar] [CrossRef]
- Marino, L.; Gulland, F.; Parsons, E.C.M. Protecting Wild Dolphins and Whales: Current Crises, Strategies, and Future Projections. J. Mar. Sci. 2012, 2012, 934048. [Google Scholar] [CrossRef]
- Simeonova, A.; Chuturkova, R.; Yaneva, V. Seasonal Dynamics of Marine Litter along the Bulgarian Black Sea Coast. Mar. Pollut. Bull. 2017, 119, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Suaria, G.; Avio, C.G.; Mineo, A.; Lattin, G.L.; Magaldi, M.G.; Belmonte, G.; Moore, C.J.; Regoli, F.; Aliani, S. The Mediterranean Plastic Soup: Synthetic Polymers in Mediterranean Surface Waters. Sci. Rep. 2016, 6, 37551. [Google Scholar] [CrossRef]
- Moncheva, S.; Stefanova, K.; Krastev, A.; Apostolov, A.; Bat, L.; Sezgin, M.; Sahin, F.; Timofte, F. Marine Litter Quantification in the Black Sea: A Pilot Assessment. Turk. J. Fish. Aquat. Sci. 2016, 16, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Topçu, E.N.; Öztürk, B. Abundance and Composition of Solid Waste Materials on the Western Part of the Turkish Black Sea Seabed. Aquat. Ecosyst. Health Manag. 2010, 13, 301–306. [Google Scholar] [CrossRef]
- Oztekin, A.; Bat, L. Microlitter Pollution in Sea Water: A Preliminary Study from Sinop Coast of the Southern Black Sea. Turk. J. Fish. Aquat. Sci. 2017, 17, 1431–1440. [Google Scholar] [CrossRef]
- Terzi, Y.; Seyhan, K. Marine Plastics in the Fishing Grounds in the Black Sea. In Marine Litter in the Black Sea; Aytan, Ü., Pogojeva, M., Simeonova, A., Eds.; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2020; Volume 56, pp. 151–160. [Google Scholar]
- Aytan, Ü.; Esensoy, F.B.; Şentürk, Y.; Ağırbaş, E.; Valente, A. Presence of Microplastics in Zooplankton and Planktivorous Fish in the Southeastern Black Sea. In Marine Litter in the Black Sea; Aytan, Ü., Pogojeva, M., Simeonova, A., Eds.; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2020; Volume 56, pp. 314–325. [Google Scholar]
- Gomerčić, M.Đ.; Galov, A.; Gomerčić, T.; Škrtić, D.; Ćurković, S.; Lucić, H.; Vuković, S.; Arbanasić, H.; Gomerčić, H. Bottlenose Dolphin (Tursiops truncatus) Depredation Resulting in Larynx Strangulation with Gill-Net Parts. Mar. Mamm. Sci. 2009, 25, 392–401. [Google Scholar] [CrossRef]
- Bilgin, S.; Onay, H.; Kose, O.; Yesilcicek, T. Karadeniz’de Karaya Vuran ve Kazara Yakalanan Yunuslar (Cetacea) Hakkında: Ölüm Nedenleri, Beslenme Özellikleri ve Gebelik Durumu. Turk Tarım Doga Bilim. Derg. 2018, 5, 447–454. [Google Scholar] [CrossRef]
- Tonay, M.; Dede, A.; Öztürk, A.; Oztürk, B. Stomach Content of Harbour Porpoises (Phocoena phocoena) from the Turkish Western Black Sea in Spring and Early Summer. Rapp. Comm. Int. Mer. Medit. 2007, 38, 616. [Google Scholar]
- Tonay, M.; Dede, A.; Öztürk, A. Stomach Contents of Bycaught Harbour Porpoises (Phocoena phocoena) from the Marmara Sea. Rapp. Comm. Int. Mer. Medit. 2007, 38, 617. [Google Scholar]
- Gladilina, E.V.; Gol’din, P.E. New Prey Fishes in Diet of Black Sea Bottlenose Dolphins, Tursiops truncatus (Mammalia, Cetacea). Vestn. Zool. 2014, 48, 83–92. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; O’Brien, J.; Berrow, S.; O’Connor, I.; Officer, R. Microplastic and Macroplastic Ingestion by a Deep Diving, Oceanic Cetacean: The True’s Beaked Whale Mesoplodon mirus. Environ. Pollut. 2015, 199, 185–191. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, X.; Zhang, Q.; Li, Y.; Tan, S.; Li, D.; Yang, Z.; Wang, J. Cetaceans and Microplastics: First Report of Microplastic Ingestion by a Coastal Delphinid, Sousa chinensis. Sci. Total Environ. 2019, 659, 649–654. [Google Scholar] [CrossRef]
- Battaglia, F.M.; Beckingham, B.A.; McFee, W.E. First Report from North America of Microplastics in the Gastrointestinal Tract of Stranded Bottlenose Dolphins (Tursiops truncatus). Mar. Pollut. Bull. 2020, 160, 111677. [Google Scholar] [CrossRef] [PubMed]
- Philipp, C.; Unger, B.; Ehlers, S.M.; Koop, J.H.E.; Siebert, U. First Evidence of Retrospective Findings of Microplastics in Harbour Porpoises (Phocoena phocoena) From German Waters. Front. Mar. Sci. 2021, 8, 682532. [Google Scholar] [CrossRef]
- Yücel, N.; Kılıç, E.; Turan, C.; Demirhan, S. Microplastic Occurrence in the Gastrointestinal Tract of a Risso’s Dolphin Grampus Griseus in the Northeastern Mediterranean Sea. Aquat. Sci. Eng. 2022, 37, 235–239. [Google Scholar] [CrossRef]
- Sá, S.; Torres-Pereira, A.; Ferreira, M.; Monteiro, S.S.; Fradoca, R.; Sequeira, M.; Vingada, J.; Eira, C. Microplastics in Cetaceans Stranded on the Portuguese Coast. Animals 2023, 13, 3263. [Google Scholar] [CrossRef] [PubMed]
- Corazzola, G.; Baini, M.; Grattarola, C.; Panti, C.; Marcer, F.; Garibaldi, F.; Berio, E.; Mancusi, C.; Galli, M.; Mazzariol, S.; et al. Analysis of the Gastro-Intestinal Tract of Marine Mammals: A Multidisciplinary Approach with a New Multi-Sieves Tool. Animals 2021, 11, 1824. [Google Scholar] [CrossRef] [PubMed]
- Panin, N.; Jipa, D. Danube River Sediment Input and Its Interaction with the North-Western Black Sea. Estuar. Coast. Shelf Sci. 2002, 54, 551–562. [Google Scholar] [CrossRef]
- Ijsseldijk, L.L.; Brownlow, A.C.; Mazzariol, S. Best Practice on Cetacean Post Mortem Investigation and Tissue Sampling. In Proceedings of the Seventh Meeting of the Parties to ACCOBAMS, Istanbul, Turkey, 5–8 November 2019. [Google Scholar]
- Van Franeker, J.A.; Bravo Rebolledo, E.L.; Hesse, E.; IJsseldijk, L.L.; Kühn, S.; Leopold, M.; Mielke, L. Plastic Ingestion by Harbour Porpoises Phocoena phocoena in The Netherlands: Establishing a Standardised Method. Ambio 2018, 47, 387–397. [Google Scholar] [CrossRef]
- Lusher, A.L.; Hernandez-Milian, G.; Berrow, S.; Rogan, E.; O’Connor, I. Incidence of Marine Debris in Cetaceans Stranded and Bycaught in Ireland: Recent Findings and a Review of Historical Knowledge. Environ. Pollut. 2018, 232, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Lusher, A.L.; Bråte, I.L.N.; Munno, K.; Hurley, R.R.; Welden, N.A. Is It or Isn’t It: The Importance of Visual Classification in Microplastic Characterization. Appl. Spectrosc. 2020, 74, 1139–1153. [Google Scholar] [CrossRef]
- Hanke, G. Guidance on Monitoring of Marine Litter in European Seas; A Guidance Document within the Common Implementation Strategy for the Marine Strategy Framework Directive. J.R.C. MSDF Technical Subgroup on Marine Litter, Report No. EUR 26113 EN; Publications Office: Ispra, Italy, 2013. [Google Scholar]
- Nelms, S.E.; Barnett, J.; Brownlow, A.; Davison, N.J.; Deaville, R.; Galloway, T.S.; Lindeque, P.K.; Santillo, D.; Godley, B.J. Microplastics in Marine Mammals Stranded around the British Coast: Ubiquitous but Transitory? Sci. Rep. 2019, 9, 1075. [Google Scholar] [CrossRef]
- Hernandez-Gonzalez, A.; Saavedra, C.; Gago, J.; Covelo, P.; Santos, M.B.; Pierce, G.J. Microplastics in the Stomach Contents of Common Dolphin (Delphinus delphis) Stranded on the Galician Coasts (NW Spain, 2005–2010). Mar. Pollut. Bull. 2018, 137, 526–532. [Google Scholar] [CrossRef]
- Montoto-Martínez, T.; De la Fuente, J.; Puig-Lozano, R.; Marques, N.; Arbelo, M.; Hernández-Brito, J.J.; Fernández, A.; Gelado-Caballero, M.D. Microplastics, Bisphenols, Phthalates and Pesticides in Odontocete Species in the Macaronesian Region (Eastern North Atlantic). Mar. Pollut. Bull. 2021, 173, 113105. [Google Scholar] [CrossRef]
- Novillo, O.; Raga, J.A.; Tomás, J. Evaluating the Presence of Microplastics in Striped Dolphins (Stenella coeruleoalba) Stranded in the Western Mediterranean Sea. Mar. Pollut. Bull. 2020, 160, 111557. [Google Scholar] [CrossRef] [PubMed]
- Mihova, S.; Doncheva, V.; Stefanova, K.; Stefanova, E.; Popov, D.; Panayotova, M. Plastic Ingestion by Phocoena phocoena and Tursiops truncatus from the Black Sea. In Environmental Protection and Disaster Risks; Dobrinkova, N., Nikolov, O., Eds.; Springer: Cham, Switzerland, 2022; pp. 295–307. [Google Scholar]
- Suaria, G.; Achtypi, A.; Perold, V.; Lee, J.R.; Pierucci, A.; Bornman, T.G.; Aliani, S.; Ryan, P.G. Microfibers in Oceanic Surface Waters: A Global Characterization. Sci. Adv. 2020, 6, eaay8493. [Google Scholar] [CrossRef]
- Merrill, G.B.; Hermabessiere, L.; Rochman, C.M.; Nowacek, D.P. Microplastics in Marine Mammal Blubber, Melon, & Other Tissues: Evidence of Translocation. Environ. Pollut. 2023, 335, 122252. [Google Scholar] [CrossRef]
- Şentürk, Y.; Esensoy, F.B.; Öztekin, A.; Aytan, Ü. Microplastics in Bivalves in the Southern Black Sea. In Marine Litter in the Black Sea; Ülgen, A., Pogojeva, M., Simeonova, A., Eds.; Turkish Marine Research Foundation (TUDAV): Istanbul, Turkey, 2020; Volume 56, pp. 303–313. [Google Scholar]
- Cincinelli, A.; Scopetani, C.; Chelazzi, D.; Martellini, T.; Pogojeva, M.; Slobodnik, J. Microplastics in the Black Sea Sediments. Sci. Total Environ. 2021, 760, 143898. [Google Scholar] [CrossRef]
- Aytan, U.; Esensoy, F.B.; Senturk, Y.; Arifoglu, E.; Karaoglu, K.; Ceylan, Y.; Valente, A. Plastic Occurrence in Commercial Fish Species of the Black Sea. Turk. J. Fish. Aquat. Sci. 2022, 22, TRJFAS20504. [Google Scholar] [CrossRef]
- Karlsson, T.M.; Arneborg, L.; Broström, G.; Almroth, B.C.; Gipperth, L.; Hassellöv, M. The Unaccountability Case of Plastic Pellet Pollution. Mar. Pollut. Bull. 2018, 129, 52–60. [Google Scholar] [CrossRef]
- Kittner, M.; Kerndorff, A.; Ricking, M.; Bednarz, M.; Obermaier, N.; Lukas, M.; Asenova, M.; Bordós, G.; Eisentraut, P.; Hohenblum, P.; et al. Microplastics in the Danube River Basin: A First Comprehensive Screening with a Harmonized Analytical Approach. ACS ES&T Water 2022, 2, 1174–1181. [Google Scholar] [CrossRef]
- United Nations Environment Programme. From Pollution to Solution: A Global Assessment of Marine Litter and Plastic Pollution; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2021. [Google Scholar]
- Wang, C.; O’Connor, D.; Wang, L.; Wu, W.-M.; Luo, J.; Hou, D. Microplastics in Urban Runoff: Global Occurrence and Fate. Water Res. 2022, 225, 119129. [Google Scholar] [CrossRef]
- Welden, N.A.; Cowie, P.R. Degradation of Common Polymer Ropes in a Sublittoral Marine Environment. Mar. Pollut. Bull. 2017, 118, 248–253. [Google Scholar] [CrossRef]
- Ugwu, K.; Herrera, A.; Gómez, M. Microplastics in Marine Biota: A Review. Mar. Pollut. Bull. 2021, 169, 112540. [Google Scholar] [CrossRef]
- Aydın, İ.; Terzi, Y.; Gündoğdu, S.; Aytan, Ü.; Öztürk, R.Ç.; Atamanalp, M.; Alak, G.; Sivri, N.; Akarsu, C.; Atıcı, A.A.; et al. Microplastic Pollution in Turkish Aquatic Ecosystems: Sources, Characteristics, Implications, and Mitigation Strategies. Turk. J. Fish. Aquat. Sci. 2023, 23, TRJFAS24773. [Google Scholar] [CrossRef]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe Scad Decapterus Muroadsi (Carangidae) Fish Ingest Blue Microplastics Resembling Their Copepod Prey along the Coast of Rapa Nui (Easter Island) in the South Pacific Subtropical Gyre. Sci. Total Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Zantis, L.J.; Carroll, E.L.; Nelms, S.E.; Bosker, T. Marine Mammals and Microplastics: A Systematic Review and Call for Standardisation. Environ. Pollut. 2021, 269, 116142. [Google Scholar] [CrossRef] [PubMed]
- Donohue, M.J.; Masura, J.; Gelatt, T.; Ream, R.; Baker, J.D.; Faulhaber, K.; Lerner, D.T. Evaluating Exposure of Northern Fur Seals, Callorhinus Ursinus, to Microplastic Pollution through Fecal Analysis. Mar. Pollut. Bull. 2019, 138, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, C.; Burton, H. Origins and Biological Accumulation of Small Plastic Particles in Fur Seals from Macquarie Island. AMBIO J. Hum. Environ. 2003, 32, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Desclos-Dukes, L.; Butterworth, A.; Cogan, T. Using a Non-invasive Technique to Identify Suspected Microplastics in Grey Seals (Halichoerus grypus) Living in the Western North Sea. Vet. Rec. 2022, 190, e1484. [Google Scholar] [CrossRef] [PubMed]
- Hocking, D.P.; Marx, F.G.; Park, T.; Fitzgerald, E.M.G.; Evans, A.R. A Behavioural Framework for the Evolution of Feeding in Predatory Aquatic Mammals. Proc. R. Soc. B Biol. Sci. 2017, 284, 20162750. [Google Scholar] [CrossRef] [PubMed]
- Ioakeimidis, C.; Zeri, C.; Kaberi, H.; Galatchi, M.; Antoniadis, K.; Streftaris, N.; Galgani, F.; Papathanassiou, E.; Papatheodorou, G. A Comparative Study of Marine Litter on the Seafloor of Coastal Areas in the Eastern Mediterranean and Black Seas. Mar. Pollut. Bull. 2014, 89, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Stanev, E.V.; Ricker, M. The Fate of Marine Litter in Semi-Enclosed Seas: A Case Study of the Black Sea. Front. Mar. Sci. 2019, 6, 660. [Google Scholar] [CrossRef]
- Aydın, R.B.; Yozukmaz, A.; Şener, İ.; Temiz, F.; Giannetto, D. Occurrence of Microplastics in Most Consumed Fruits and Vegetables from Turkey and Public Risk Assessment for Consumers. Life 2023, 13, 1686. [Google Scholar] [CrossRef]
- González Fernández, D.; Pogojeva, M.; Hanke, G.; Machitadze, N.; Kotelnikova, Y.; Tretiak, I.; Savenko, O.; Gelashvili, N.; Bilashvili, K.; Kulagin, D.; et al. Anthropogenic Litter Input through Rivers in the Black Sea. In Marine Litter in the Black Sea; Turkish Marine Research Foundation: Istanbul, Turkey, 2020; Volume 56, pp. 183–191. ISBN 978-975-8825-48-6. [Google Scholar]
- Black Sea Twice as Polluted by Marine Litter as Mediterranean Sea—EU Project’s Survey. Available online: https://www.undp.org/ukraine/press-releases/black-sea-twice-polluted-marine-litter-mediterranean-sea-%E2%80%93-eu-project%E2%80%99s-survey (accessed on 29 December 2023).

| Animal ID | Species | Coordinates | Found | Estimated Age | Sex | DCC | Organ |
|---|---|---|---|---|---|---|---|
| PCETSTR040423#1 | T. t. ponticus | 44.0390 28.6515 | Stranded | Adult | Female | 2 | Stomach Intestine |
| PCETSTR180423#2 | T. t. ponticus | 44.4323 28.6455 | Stranded | Adult | Male | 3 | Stomach Intestine |
| PCETGN140223#1 | T. t. ponticus | 44.4153 29.4459 | By-caught | Adult | Male | 2 | Stomach Intestine |
| PCETGN090423#2 | P. p. relicta | 44.3527 29.1578 | By-caught | Adult | Male | 2 | Stomach Intestine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filimon, A.; Ciucă, A.-M.; Harcotă, G.-E.; Stoica, E. Preliminary Study on Microplastic Contamination in Black Sea Cetaceans: Gastrointestinal Analysis of Phocoena phocoena relicta and Tursiops truncatus ponticus. Animals 2024, 14, 886. https://doi.org/10.3390/ani14060886
Filimon A, Ciucă A-M, Harcotă G-E, Stoica E. Preliminary Study on Microplastic Contamination in Black Sea Cetaceans: Gastrointestinal Analysis of Phocoena phocoena relicta and Tursiops truncatus ponticus. Animals. 2024; 14(6):886. https://doi.org/10.3390/ani14060886
Chicago/Turabian StyleFilimon, Adrian, Andreea-Mădălina Ciucă, George-Emanuel Harcotă, and Elena Stoica. 2024. "Preliminary Study on Microplastic Contamination in Black Sea Cetaceans: Gastrointestinal Analysis of Phocoena phocoena relicta and Tursiops truncatus ponticus" Animals 14, no. 6: 886. https://doi.org/10.3390/ani14060886
APA StyleFilimon, A., Ciucă, A.-M., Harcotă, G.-E., & Stoica, E. (2024). Preliminary Study on Microplastic Contamination in Black Sea Cetaceans: Gastrointestinal Analysis of Phocoena phocoena relicta and Tursiops truncatus ponticus. Animals, 14(6), 886. https://doi.org/10.3390/ani14060886








