Retinal Structure of Poecilia sphenops: Photoreceptor Mosaics, Synaptic Ribbon Patterns, and Glial Cell Expressions
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
2.2. Histological and Histochemical Analysis
2.3. Immunohistochemistry
2.4. Semithin Sections and TEM Preparations
2.5. Morphometrical Analysis
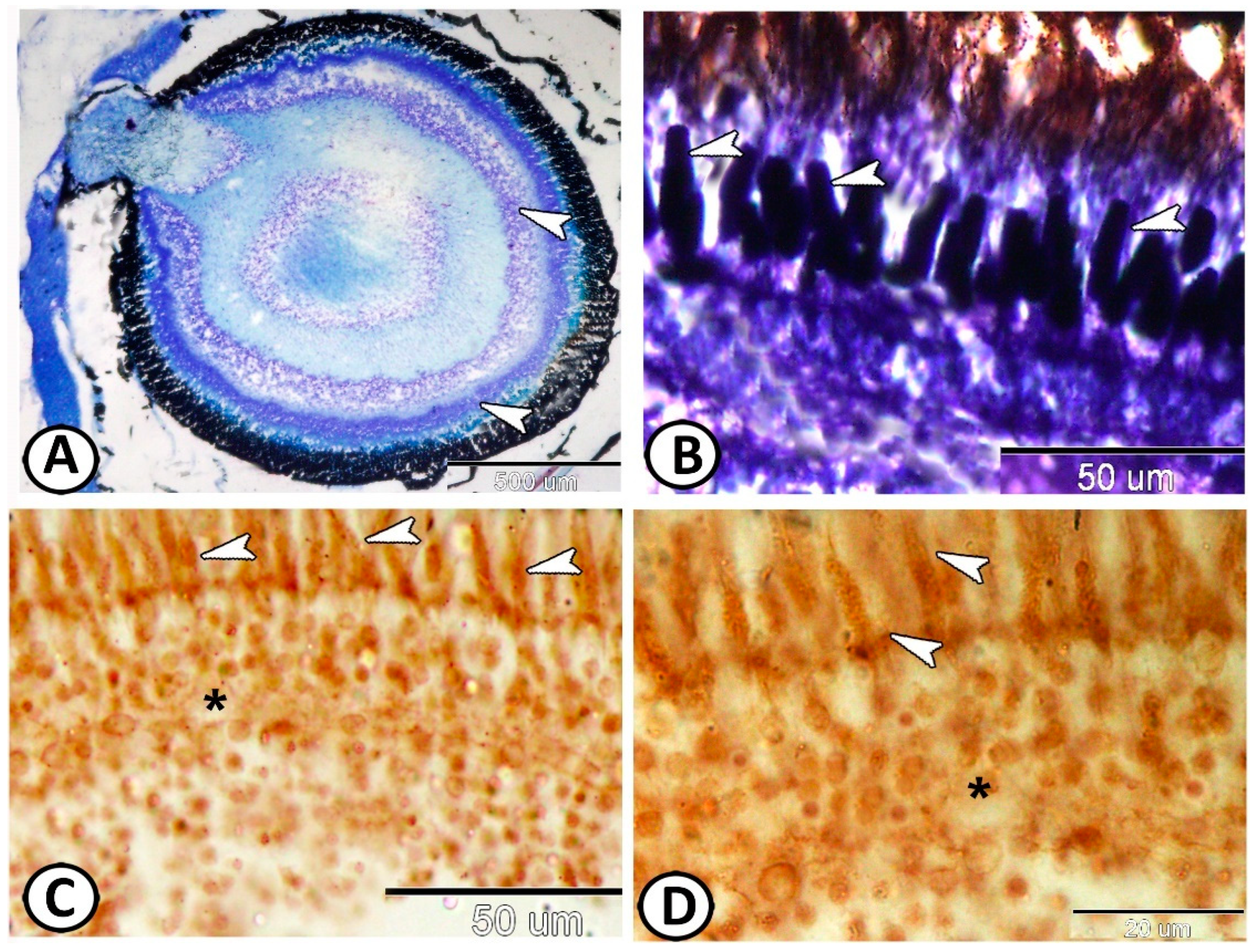
3. Results
3.1. Histological and Histochemical Analysis
Immunohistochemistry
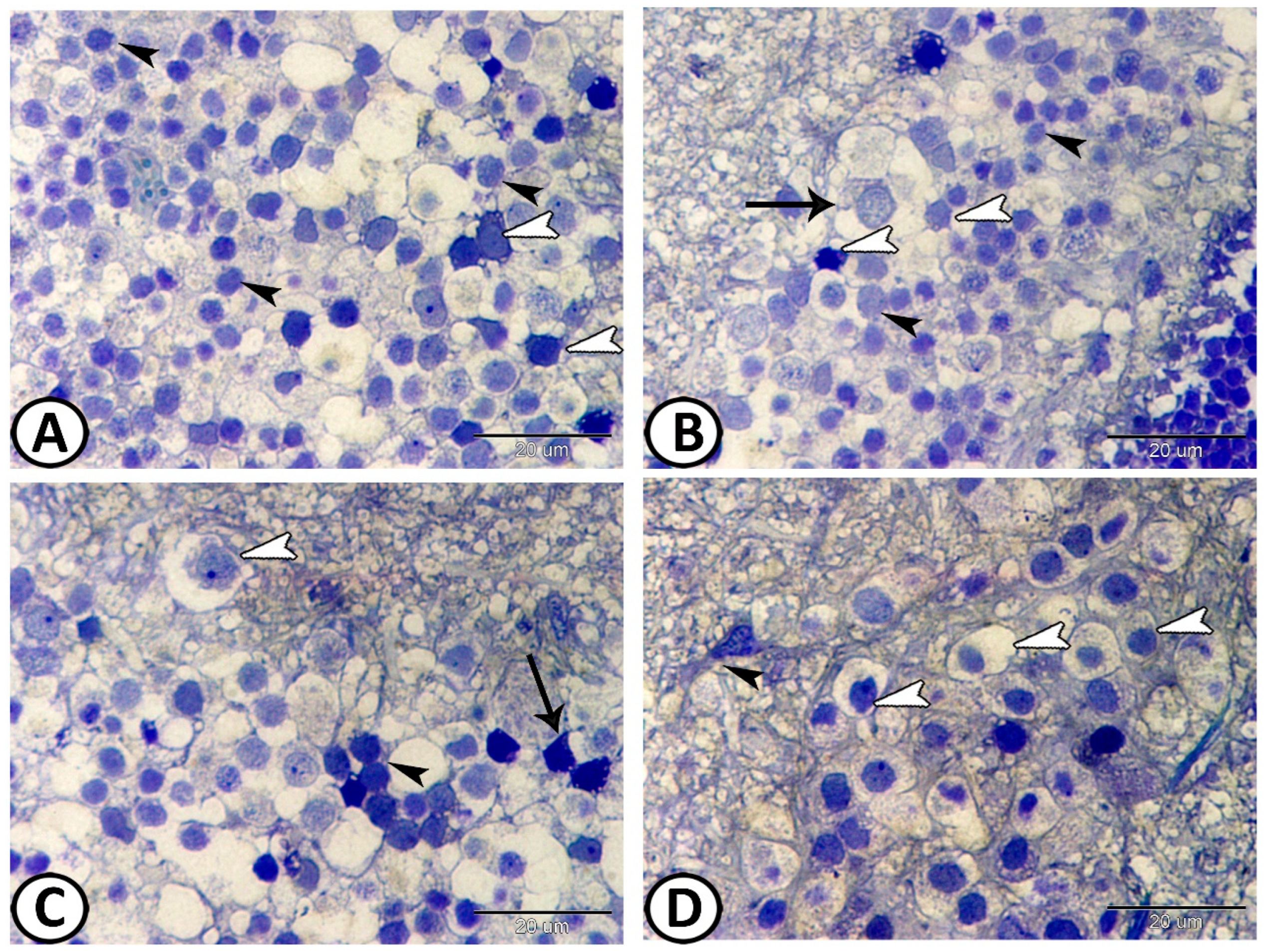
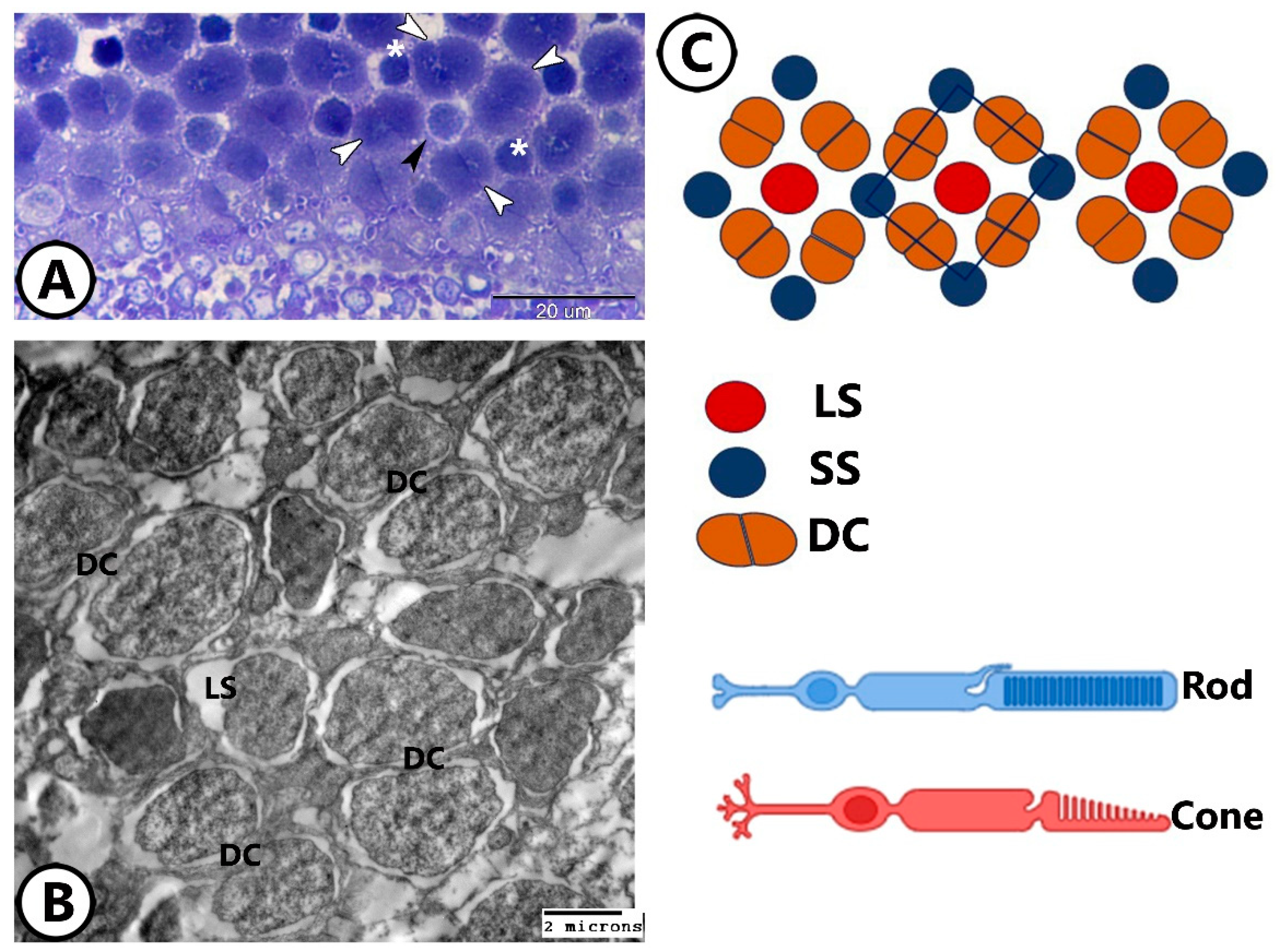
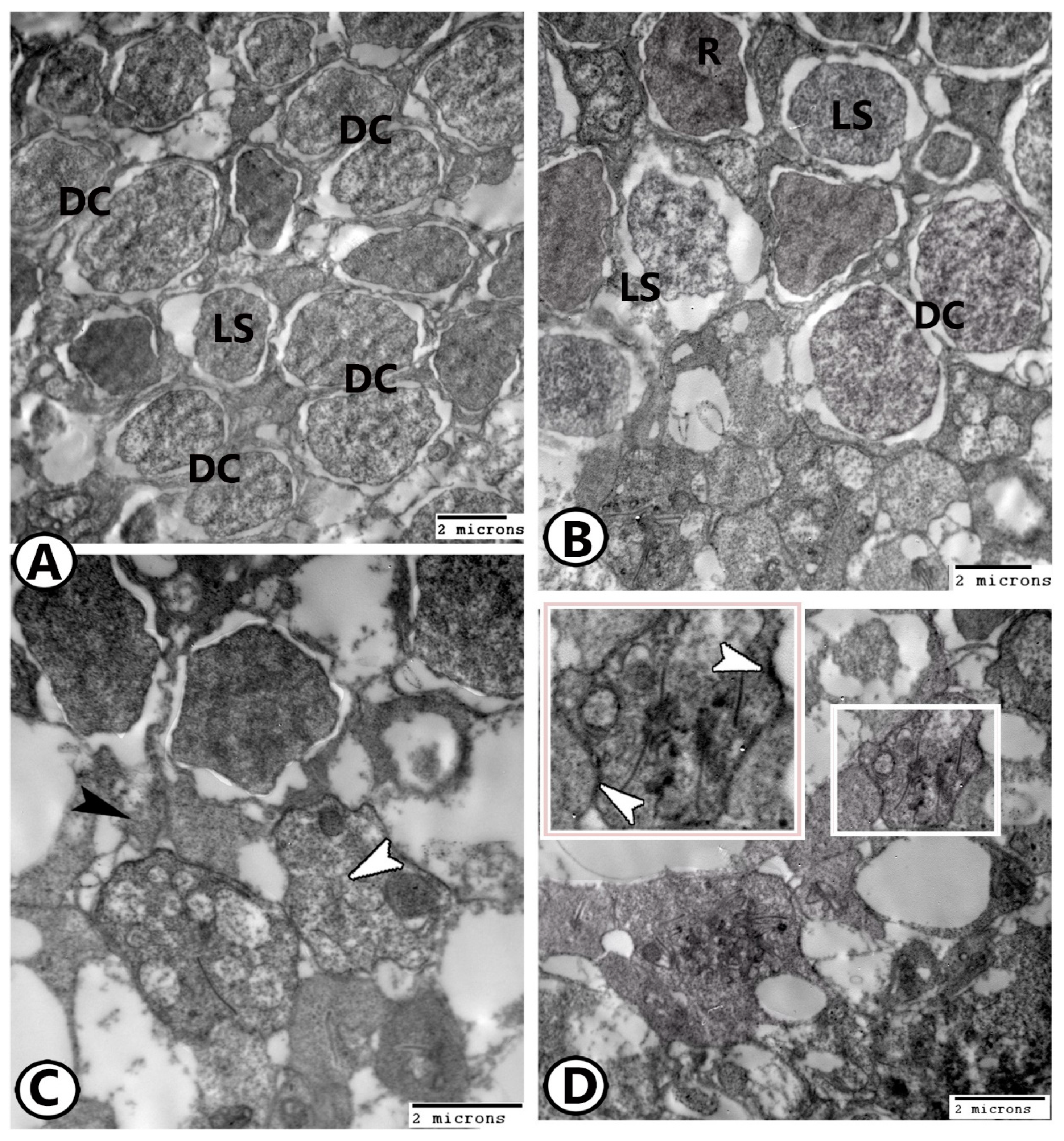
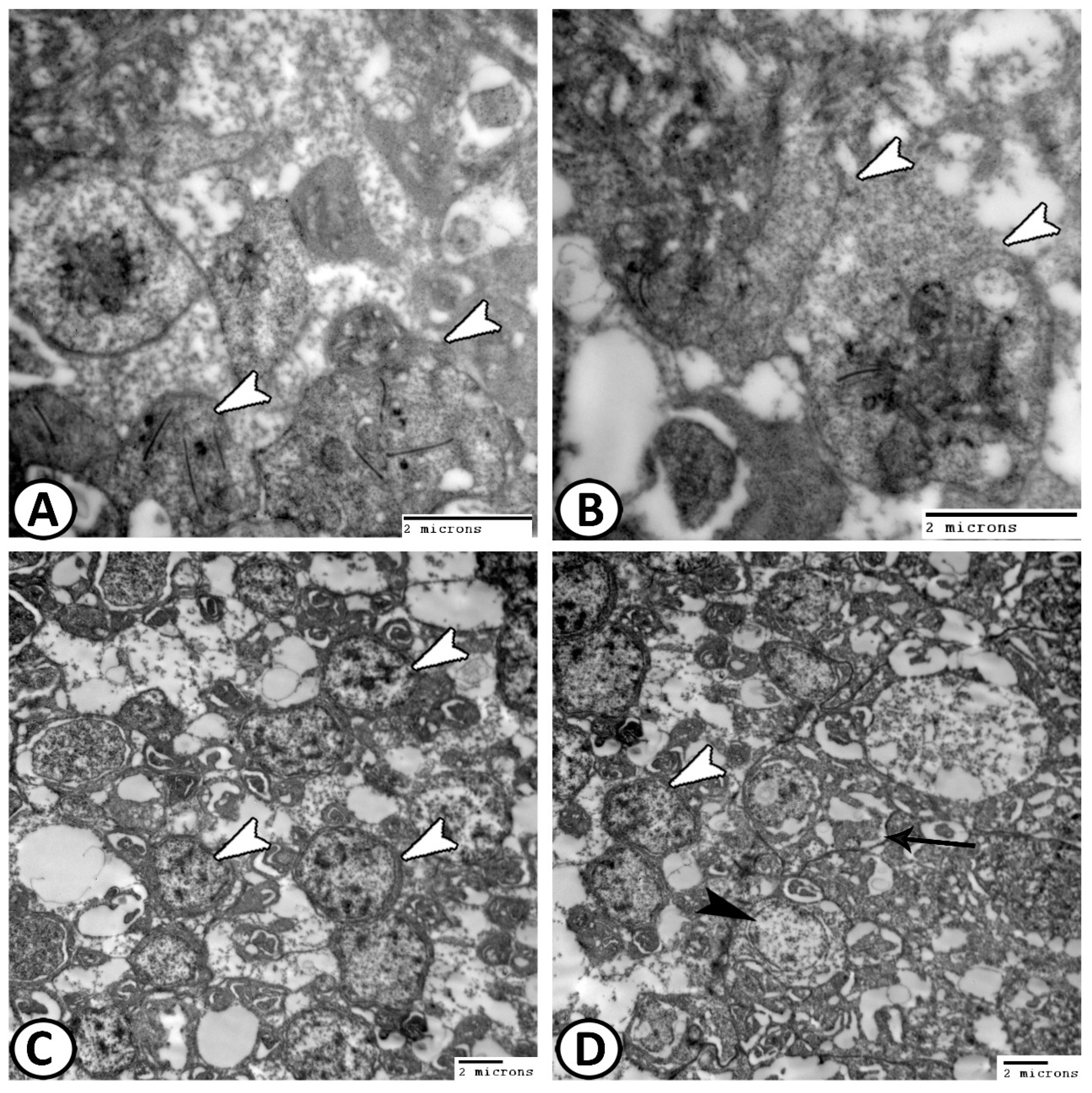
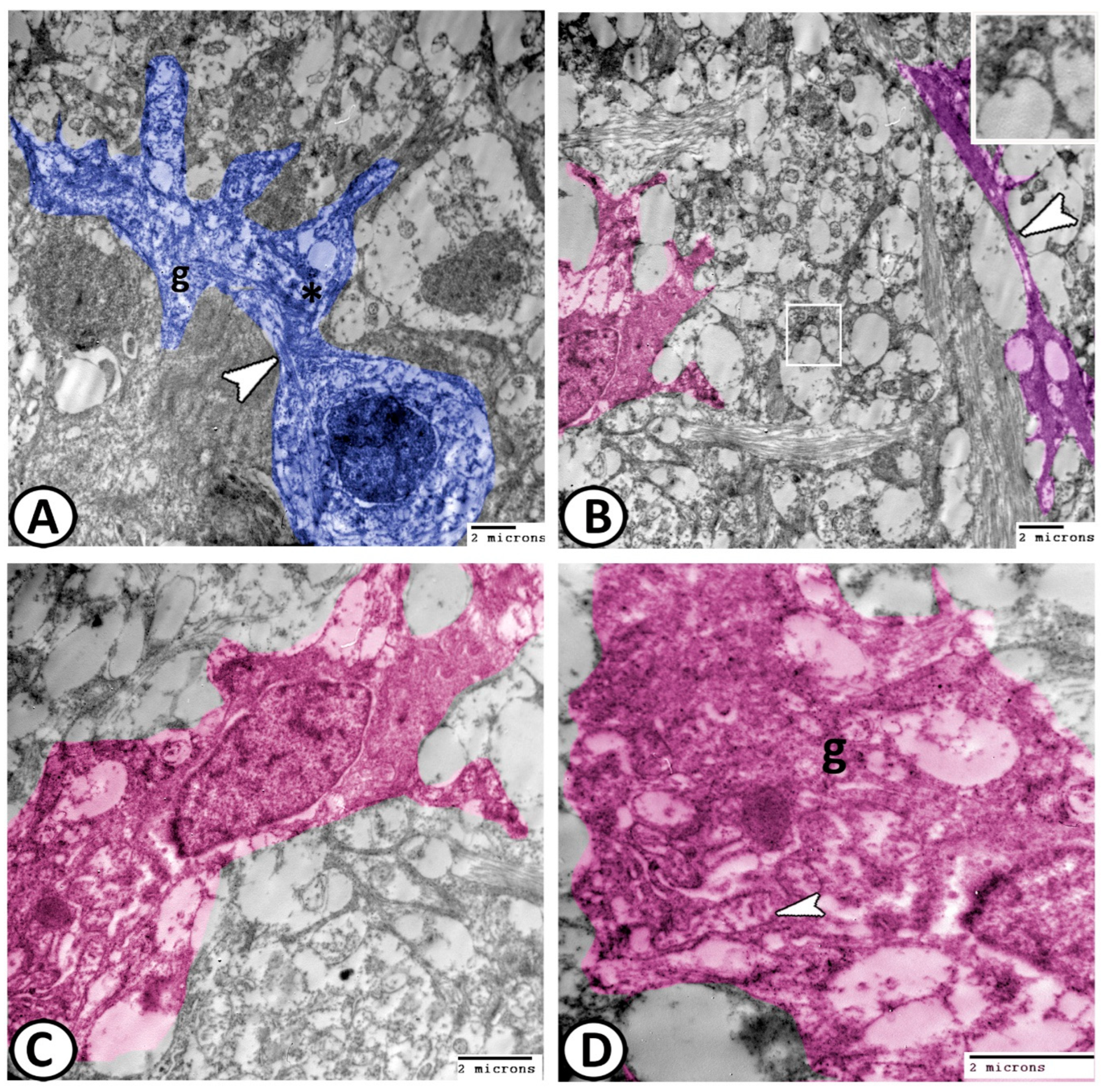
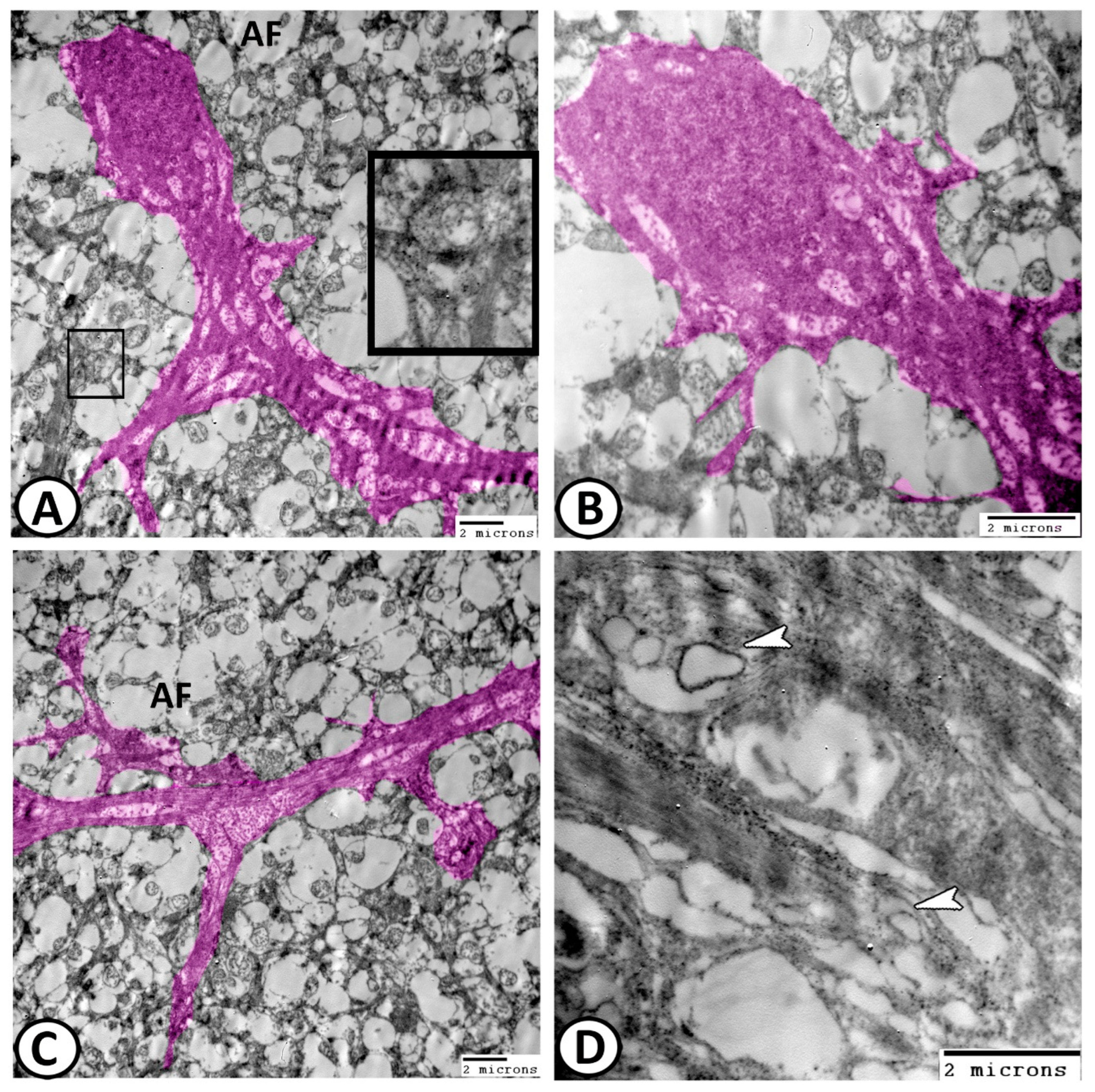
3.2. Transmission Electron Microscopy
The Cellular Constituents of the Retina Include
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Genten, F.; Terwinghe, E.; Danguy, A. Atlas of Fish Histology; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Collin, S.P.; Hart, N.S.; Shand, J.; Potter, I.C. Morphology and spectral absorption characteristics of retinal photoreceptors in the southern hemisphere lamprey (Geotria australis). Vis. Neurosci. 2003, 20, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M. Fish Histology: From Cells to Organs; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Donatti, L.; Fanta, E. Morphology of the retina in the freshwater fish Metynnis roosevelti Eigenmann (Characidae, Serrasalminae) and the effects of monochromatic red light. Rev. Bras. Zool. 1999, 16, 151–173. [Google Scholar] [CrossRef][Green Version]
- Reckel, F.; Hoffmann, B.; Melzer, R.; Horppila, J.; Smola, U. Photoreceptors and cone patterns in the retina of the smelt Osmerus eperlanus (L.) (Osmeridae: Teleostei). Acta Zool. 2003, 84, 161–170. [Google Scholar] [CrossRef]
- Nag, T.C.; Bhattacharjee, J. Retinal cytoarchitecture in some mountain-stream teleosts of India. Environ. Biol. Fishes 2002, 63, 435–449. [Google Scholar] [CrossRef]
- De Busserolles, F.; Fitzpatrick, J.L.; Marshall, N.J.; Collin, S.P. The influence of photoreceptor size and distribution on optical sensitivity in the eyes of lanternfishes (Myctophidae). PLoS ONE 2014, 9, e99957. [Google Scholar] [CrossRef]
- Al-Adhami, M.A.; Qar, J.; AlKhdour, M. Ultrastructure of the outer retina in the Killifish, Aphanius sirhani (Cyprinodontidae, Teleostei). In Anales de Biología; Universidad de Murcia, Servicio de Publicaciones: Madrid, Spain, 2010. [Google Scholar]
- Kunz, Y.W. Review of development and aging in the eye of teleost fish. Neuroembryol. Aging 2007, 4, 31–60. [Google Scholar] [CrossRef]
- Fanta, E.; Meyer, A.A.; Grotzner, S.R.; Luvizotto, M.F. Comparative study on feeding strategy and activity patterns of two Antarctic fish: Trematomus newnesi Boulenger, 1902 and Gobionotothen gibberifrons (Lonnberg, 1905) (Pisces, Nototheniidae) under different light conditions. Antarct. Rec. 1994, 38, 13–29. [Google Scholar]
- Collin, S.P.; Collin, H. Retinal and lenticular ultrastructure in the aestivating salamanderfish, Lepidogalaxias salamandroides (Galaxiidae, Teleostei) with special reference to a new type of photoreceptor mosaic. Histol. Histopathol. 1998, 13, 1037–1048. [Google Scholar]
- Zaunreiter, M.; Junger, H.; Hotrschal, K. Retinal structure; physiology and pharmacology: Retinal morphology of cyprinid fishes: A quantitative histological study of ontogenetic changes and interspecific variation. Vis. Res. 1991, 31, 383–394. [Google Scholar] [CrossRef]
- Collin, S.; Collin, H.; Ali, M. Ultrastructure and organisation of the retina and pigment epithelium in the cutlips minnow, Exoglossum maxillingua (Cyprinidae, Teleostei). Histol. Histopathol. 1996, 11, 55–69. [Google Scholar]
- Kunz, Y.W.; Ennis, S. Ultrastructural diurnal changes of the retinal photoreceptors in the embryo of a viviparous teleost (Poecilia reticulata P.). Cell Differ. 1983, 13, 115–123. [Google Scholar] [CrossRef]
- Kunz, Y.W. Cone mosaics in a teleost retina: Changes during light and dark adaptation. Experientia 1980, 36, 1371–1374. [Google Scholar] [CrossRef]
- Van der Meer, H. Constructional morphology of photoreceptor patterns in percomorph fish. Acta Biotheor. 1992, 40, 51–85. [Google Scholar] [CrossRef]
- Blaxter, J.; Jones, M.P. The development of the retina and retinomotor responses in the herring. J. Mar. Biol. Assoc. U. K. 1967, 47, 677–697. [Google Scholar] [CrossRef]
- Eng, L.F. Glial fibrillary acidic protein (GFAP): The major protein of glial intermediate filaments in differentiated astrocytes. J. Neuroimmunol. 1985, 8, 203–214. [Google Scholar] [CrossRef]
- Koke, J.R.; Mosier, A.L.; García, D.M. Intermediate filaments of zebrafish retinal and optic nerve astrocytes and Müller glia: Differential distribution of cytokeratin and GFAP. BMC Res. Notes 2010, 3, 50. [Google Scholar] [CrossRef]
- Stafford, C.; Shehab, S.; Nona, S.; Cronly-Dillon, J. Expression of glial fibrillary acidic protein (GFAP) in goldfish optic nerve following injury. Glia 1990, 3, 33–42. [Google Scholar] [CrossRef] [PubMed]
- De Guevara, R.; Pairault, C.; Pinganaud, G. Expression de la vimentine et de la GFAP et développement de la rétine chez la truite. Comptes Rendus l’Acad. Sci. Série 3 Sci. 1994, 317, 737–741. [Google Scholar]
- Casañas, M.N.; Santos, E.; Yanes, C.; Romero-Alemán, M.M.; Viñoly, R.; Alfayate, M.C.; Monzón-Mayor, M. Development of astroglia heterogeneously expressing Pax2, vimentin and GFAP during the ontogeny of the optic pathway of the lizard (Gallotia galloti): An immunohistochemical and ultrastructural study. Cell Tissue Res. 2011, 345, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, C.; Bachir, A.S.; Idder, T. New record and biology of the Molly Poecilia sphenops (Poeciliidae), discovered in the Northern Sahara of Algeria. J. Ichthyol. 2019, 59, 602–609. [Google Scholar] [CrossRef]
- Cummings, M.E.; Rosenthal, G.G.; Ryan, M.J. A private ultraviolet channel in visual communication. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2003, 270, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Schlupp, I.; Riesch, R.; Tobler, M.; Plath, M.; Parzefall, J.; Schartl, M. A novel, sexually selected trait in poeciliid fishes: Female preference for mustache-like, rostral filaments in male Poecilia sphenops. Behav. Ecol. Sociobiol. 2010, 64, 1849–1855. [Google Scholar] [CrossRef]
- Kodric-Brown, A.; Nicoletto, P.F. Female choice in the guppy (Poecilia reticulata): The interaction between male color and display. Behav. Ecol. Sociobiol. 2001, 50, 346–351. [Google Scholar] [CrossRef]
- Posner, L.P.; Scott, G.N.; Law, J.M. Repeated exposure of goldfish (Carassius auratus) to tricaine methanesulfonate (MS-222). J. Zoo Wildl. Med. 2013, 44, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.; Gamble, M. Theory and Practice of Histological Techniques; Gamble, M., Ed.; Churchill Livingstone: London, UK, 2002. [Google Scholar]
- Mokhtar, D.M.; Attaai, A.; Zaccone, G.; Alesci, A.; Alonaizan, R.; Hussein, M.T. Morphological Distribution Patterns and Neuroimmune Communication of Ganglia in Molly Fish (Poecilia sphenops, Valenciennes 1846). Fishes 2023, 8, 289. [Google Scholar] [CrossRef]
- Raine, S.-M.L. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques. J. Histochem. Cytochem. 1981, 29, 577–580. [Google Scholar]
- Karnovsky, M. A formaldehyde glutaraldehyde fixative of high osmolality in electron microscopy. J. Cell Biol. 1965, 27, 137–138A. [Google Scholar]
- Reynolds, E.S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208. [Google Scholar] [CrossRef]
- Hajar, M.A.I.; Inada, H.; Hasobe, M.; Arimoto, T. Visual acuity of Pacific Saury Cololabis saira for understanding capture process. Fish. Sci. 2008, 74, 461–468. [Google Scholar] [CrossRef]
- Hunt, D.; Rawlinson, N.; Thomas, G.; Cobcroft, J.M. Investigating photoreceptor densities, potential visual acuity, and cone mosaics of shallow water, temperate fish species. Vis. Res. 2015, 111, 13–21. [Google Scholar] [CrossRef]
- Khatlan Hameed, B. Comparative Study between the Eyeball in the Cat and Hens (Histological Investigation). Assiut Vet. Med. J. 2019, 65, 305–309. [Google Scholar]
- Aly, K.; Imam, H. Comparative Morphological Studies on the Vascular Tunic of the eyeball of Two Species of Fishes: Oreochromis Niloticus and Mugil Cephalus. Assiut Vet. Med. J. 2006, 52, 1–12. [Google Scholar]
- Bowmaker, J.; Kunz, Y. Ultraviolet receptors, tetrachromatic colour vision and retinal mosaics in the brown trout (Salmo trutta): Age-dependent changes. Vis. Res. 1987, 27, 2101–2108. [Google Scholar] [CrossRef]
- Kunz, Y.; Wildenburg, G.; Goodrich, L.; Callaghan, E. The fate of ultraviolet receptors in the retina of the Atlantic salmon (Salmo salar). Vis. Res. 1994, 34, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Hibbard, E. Grid patterns in the retinal organization of the cichlid fish Astronotus ocellatus. Exp. Eye Res. 1971, 12, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Mednick, A.S.; Berk, M.F.; Springer, A.D. Asymmetric distribution of cells in the inner nuclear and cone mosaic layers of the goldfish retina. Neurosci. Lett. 1988, 94, 241–246. [Google Scholar] [CrossRef]
- Cameron, D.A.; Pugh Jr, E.N. Double cones as a basis for a new type of polarization vision in vertebrates. Nature 1991, 353, 161–164. [Google Scholar] [CrossRef]
- Hart, N.S.; Lisney, T.J.; Marshall, N.J.; Collin, S.P. Multiple cone visual pigments and the potential for trichromatic colour vision in two species of elasmobranch. J. Exp. Biol. 2004, 207, 4587–4594. [Google Scholar] [CrossRef]
- Sillman, A.; O’Leary, C.; Tarantino, C.; Loew, E. The photoreceptors and visual pigments of two species of Acipenseriformes, the shovelnose sturgeon (Scaphirhynchus platorynchus) and the paddlefish (Polyodon spathula). J. Comp. Physiol. A 1999, 184, 37–47. [Google Scholar] [CrossRef]
- Bailes, H.J.; Robinson, S.R.; Trezise, A.E.; Collin, S.P. Morphology, characterization, and distribution of retinal photoreceptors in the Australian lungfish Neoceratodus forsteri (Krefft, 1870). J. Comp. Neurol. 2006, 494, 381–397. [Google Scholar] [CrossRef]
- Parry, J.W.; Carleton, K.L.; Spady, T.; Carboo, A.; Hunt, D.M.; Bowmaker, J.K. Mix and match color vision: Tuning spectral sensitivity by differential opsin gene expression in Lake Malawi cichlids. Curr. Biol. 2005, 15, 1734–1739. [Google Scholar] [CrossRef]
- Collin, S. Evolution of the visual system in fishes. In Encyclopedia of Neuroscience; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1459–1466. [Google Scholar]
- Fang, W.; Bonaffini, S.; Zou, J.; Wang, X.; Zhang, C.; Tsujimura, T.; Kawamura, S.; Wei, X. Characterization of transgenic zebrafish lines that express GFP in the retina, pineal gland, olfactory bulb, hatching gland, and optic tectum. Gene Expr. Patterns 2013, 13, 150–159. [Google Scholar] [CrossRef]
- Cheng, C.L.; Flamarique, I.N. Chromatic organization of cone photoreceptors in the retina of rainbow trout: Single cones irreversibly switch from UV (SWS1) to blue (SWS2) light sensitive opsin during natural development. J. Exp. Biol. 2007, 210, 4123–4135. [Google Scholar] [CrossRef]
- Reckel, F.; Melzer, R.R. Regional variations in the outer retina of Atherinomorpha (Beloniformes, Atheriniformes, Cyprinodontiformes: Teleostei): Photoreceptors, cone patterns, and cone densities. J. Morphol. 2003, 257, 270–288. [Google Scholar] [CrossRef]
- Lyall, A. Cone arrangements in teleost retinae. J. Cell Sci. 1957, 3, 189–201. [Google Scholar] [CrossRef]
- Cheng, C.L.; Flamarique, I.N.; Hárosi, F.I.; Rickers-Haunerland, J.; Haunerland, N.H. Photoreceptor layer of salmonid fishes: Transformation and loss of single cones in juvenile fish. J. Comp. Neurol. 2006, 495, 213–235. [Google Scholar] [CrossRef] [PubMed]
- Beaudet, L.; Flamarique, I.N.; Hawryshyn, C.W. Cone photoreceptor topography in the retina of sexually mature Pacific salmonid fishes. J. Comp. Neurol. 1997, 383, 49–59. [Google Scholar] [CrossRef]
- Douglas, R.H.; Collin, S.P.; Corrigan, J. The eyes of suckermouth armoured catfish (Loricariidae, subfamily Hypostomus): Pupil response, lenticular longitudinal spherical aberration and retinal topography. J. Exp. Biol. 2002, 205, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- Francke, M.; Kreysing, M.; Mack, A.; Engelmann, J.; Karl, A.; Makarov, F.; Guck, J.; Kolle, M.; Wolburg, H.; Pusch, R. Grouped retinae and tapetal cups in some Teleostian fish: Occurrence, structure, and function. Prog. Retin. Eye Res. 2014, 38, 43–69. [Google Scholar] [CrossRef] [PubMed]
- Jerlov, N.G. Marine Optics; Elsevier: Amsterdam, The Netherlands, 1976. [Google Scholar]
- Wagner, H.-J.; Fröhlich, E.; Negishi, K.; Collin, S. The eyes of deep-sea fish II. Functional morphology of the retina. Prog. Retin. Eye Res. 1998, 17, 637–685. [Google Scholar] [CrossRef] [PubMed]
- McCormack, C.A.; Hayden, T.J.; Kunz, Y.W. Ontogenesis of diurnal rhythms of cAMP concentration, outer segment disc shedding and retinomotor movements in the eye of the brown trout, Salmo trutta. Brain Behav. Evol. 1989, 34, 65–72. [Google Scholar] [CrossRef]
- Kunz, Y.; Wise, C. Ultrastructure of the “oil-droplet” in the retinal twin-cone of Lebistes reticulatus (Peters). Preliminary results. (with 5 figures). Rev. Suisse Zool. Ann. Soc. Zool. Suisse Mus. D’Hist. Nat. Geneve 1973, 80, 694–698. [Google Scholar]
- Appudurai, A.M.; Hart, N.S.; Zurr, I.; Collin, S.P. Morphology, characterization and distribution of retinal photoreceptors in the south american (Lepidosiren paradoxa) and spotted african (Protopterus dolloi) lungfishes. Front. Ecol. Evol. 2016, 4, 78. [Google Scholar] [CrossRef]
- Ahlbert, I.B. Organization of the cone cells in the retinae of salmon (Salmo salar) and trout (Salmo trutta trutta) in relation to their feeding habits. Acta Zool. 1976, 57, 13–35. [Google Scholar] [CrossRef]
- Kunz, Y.W.; Wise, C. Structural differences of cone ‘oil-droplets’ in the light and dark adapted retina of Poecilia reticulata P. Experientia 1978, 34, 246–249. [Google Scholar] [CrossRef]
- Rossetto, E.; Dolder, H.; Sazima, I. Double cone mosaic pattern in the retina of larval and adult piranha, Serrasalmus spilopleura. Experientia 1992, 48, 597–599. [Google Scholar] [CrossRef]
- Hart, N.S.; Partridge, J.; Cuthill, I. Visual pigments, oil droplets and cone photoreceptor distribution in the European starling (Sturnus vulgaris). J. Exp. Biol. 1998, 201, 1433–1446. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, R.; Collin, S.P.; Michiels, N.K. Anatomical analysis of the retinal specializations to a crypto-benthic, micro-predatory lifestyle in the Mediterranean triplefin blenny Tripterygion delaisi. Front. Neuroanat. 2017, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Sulzer, D.; Holtzman, E. Alcian blue and neutral red staining of retinal synaptic layers. J. Histochem. Cytochem. 1986, 34, 1513–1515. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Alesci, A.; Pergolizzi, S.; Zaccone, G. Light and electron microscopic observations on retinal neurons of red-tail shark (Epalzeorhynchos bicolor HM Smith, 1931). Microsc. Res. Tech. 2024, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Roberts, P.A.; Yoshimatsu, T.; Lagnado, L.; Baden, T. Amacrine cells differentially balance zebrafish color circuits in the central and peripheral retina. Cell Rep. 2023, 42, 112055. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.N.; Tsujimura, T.; Kawamura, S.; Dowling, J.E. Bipolar cell–photoreceptor connectivity in the zebrafish (Danio rerio) retina. J. Comp. Neurol. 2012, 520, 3786–3802. [Google Scholar] [CrossRef] [PubMed]
- Fritzsch, B.; Collin, S.P. Dendritic distribution of two populations of ganglion cells and the retinopetal fibers in the retina of the silver lamprey (Ichthyomyzon unicuspis). Vis. Neurosci. 1990, 4, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia–neuron interactions in the mammalian retina. Prog. Retin. Eye Res. 2016, 51, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Ajtai, B.M.; Kálmán, M. Glial fibrillary acidic protein expression but no glial demarcation follows the lesion in the molecular layer of cerebellum. Brain Res. 1998, 802, 285–288. [Google Scholar] [CrossRef]
- Omri, S.; Omri, B.; Savoldelli, M.; Jonet, L.; Thillaye-Goldenberg, B.; Thuret, G.; Gain, P.; Jeanny, J.-C.; Crisanti, P.; Behar-Cohen, F. The outer limiting membrane (OLM) revisited: Clinical implications. Clin. Ophthalmol. 2010, 4, 183–195. [Google Scholar]
- Derbalah, A.; El-Gendy, S.A.; Alsafy, M.A.; Elghoul, M. Micro-morphology of the retina of the light-adapted African catfish (Clarias gariepinus). Microsc. Res. Tech. 2023, 86, 208–215. [Google Scholar] [CrossRef]
- Garcia-Pradas, L.; Gleiser, C.; Wizenmann, A.; Wolburg, H.; Mack, A.F. Glial cells in the fish retinal nerve fiber layer form tight junctions, separating and surrounding axons. Front. Mol. Neurosci. 2018, 11, 367. [Google Scholar] [CrossRef]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A. Late-stage neuronal progenitors in the retina are radial Müller glia that function as retinal stem cells. J. Neurosci. 2007, 27, 7028–7040. [Google Scholar] [CrossRef] [PubMed]
- Raymond, P.A.; Barthel, L.K.; Bernardos, R.L.; Perkowski, J.J. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev. Biol. 2006, 6, 36. [Google Scholar] [CrossRef] [PubMed]




| Dorsal | Ventral | Temporal | Nasal | |
|---|---|---|---|---|
| Cones | ||||
| Mean | 7143 c | 8786 a | 7370 bc | 6733 cd |
| Std. Error | ±144.9 | ±169.3 | ±92.68 | ±90.21 |
| Rods | ||||
| Mean | 6112 a | 5832 ab | 5679 abc | 4932 d |
| Std. Error | ±99 | ±96 | ±107 | ±126 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtar, D.M.; Albano, M.; Alonaizan, R.; Attaai, A. Retinal Structure of Poecilia sphenops: Photoreceptor Mosaics, Synaptic Ribbon Patterns, and Glial Cell Expressions. Animals 2024, 14, 939. https://doi.org/10.3390/ani14060939
Mokhtar DM, Albano M, Alonaizan R, Attaai A. Retinal Structure of Poecilia sphenops: Photoreceptor Mosaics, Synaptic Ribbon Patterns, and Glial Cell Expressions. Animals. 2024; 14(6):939. https://doi.org/10.3390/ani14060939
Chicago/Turabian StyleMokhtar, Doaa M., Marco Albano, Rasha Alonaizan, and Abdelraheim Attaai. 2024. "Retinal Structure of Poecilia sphenops: Photoreceptor Mosaics, Synaptic Ribbon Patterns, and Glial Cell Expressions" Animals 14, no. 6: 939. https://doi.org/10.3390/ani14060939
APA StyleMokhtar, D. M., Albano, M., Alonaizan, R., & Attaai, A. (2024). Retinal Structure of Poecilia sphenops: Photoreceptor Mosaics, Synaptic Ribbon Patterns, and Glial Cell Expressions. Animals, 14(6), 939. https://doi.org/10.3390/ani14060939







