Schooling Fish from a New, Multimodal Sensory Perspective
Abstract
Simple Summary
Abstract
1. Introduction
1.1. Descriptions and Definitions
1.2. Schooling-like Behavior in Other Animals
2. Schooling Fish
2.1. The Evolution of Schooling
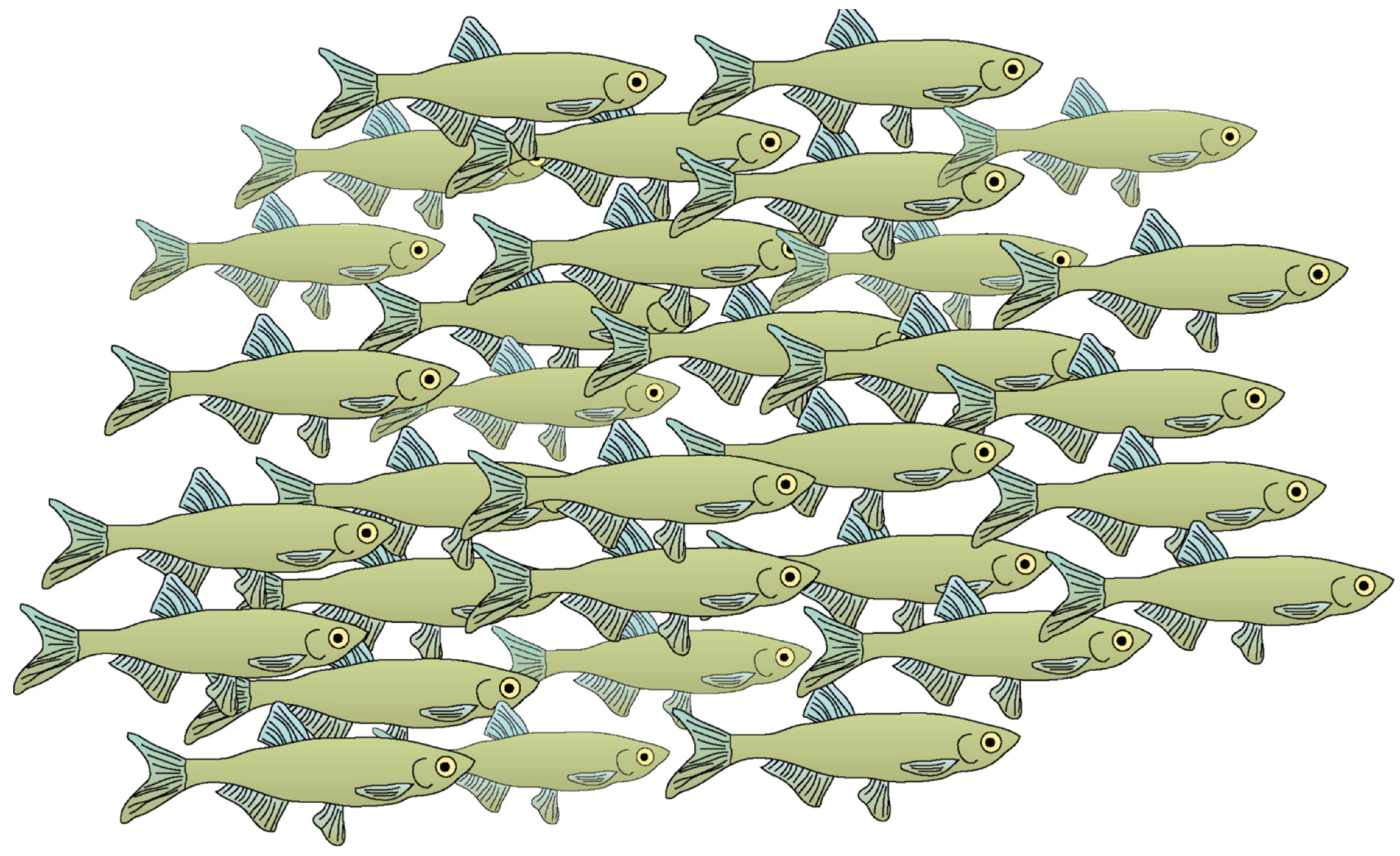
2.2. How Do Fish School?
2.3. Sensory Systems Involved
2.3.1. Vision
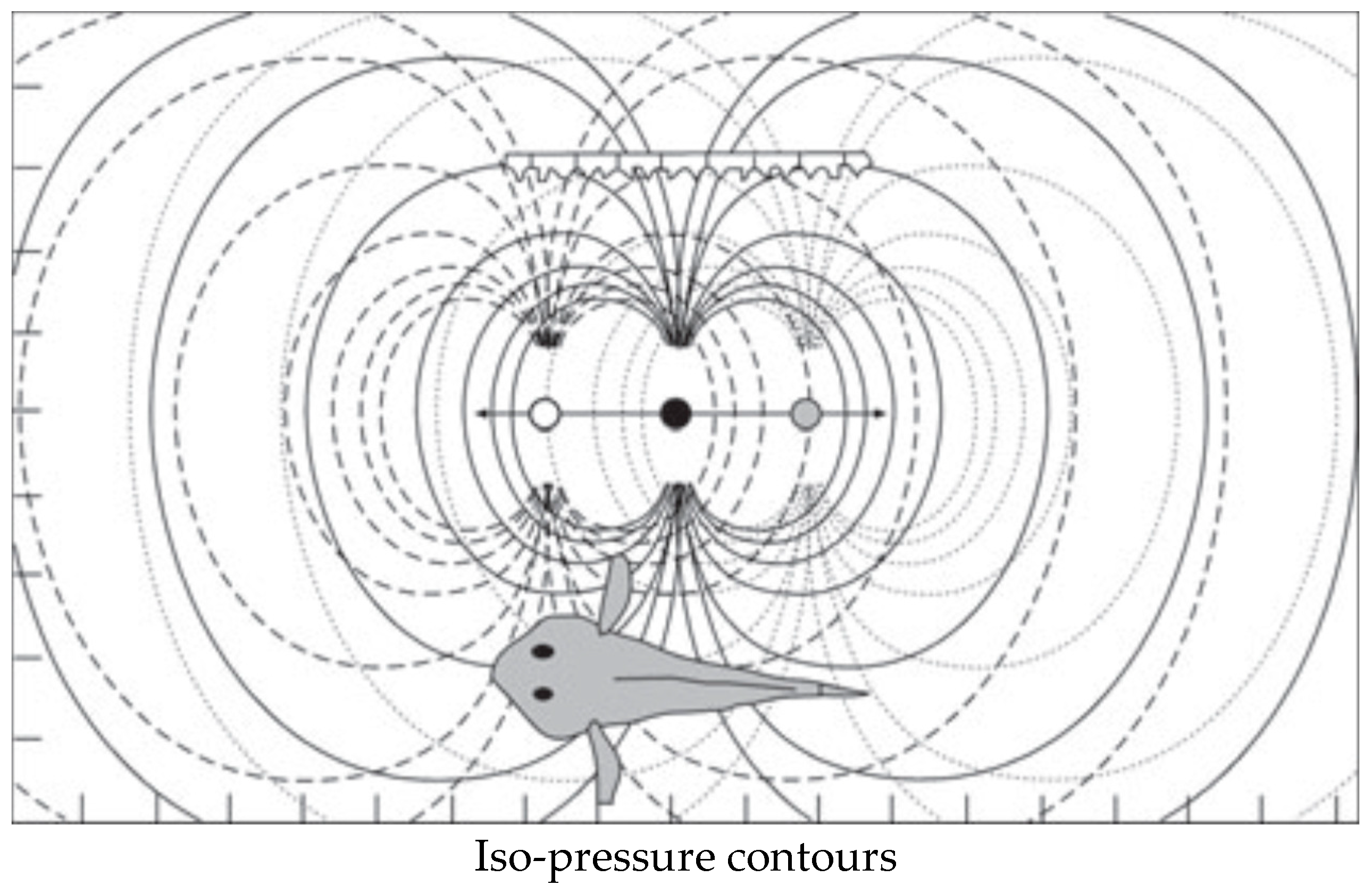
2.3.2. The Octavo-Lateralis System (Lateral Line and Inner Ear)
2.3.3. Fact Sheet
- neuromasts within the lateral line canal;
- the superficial, or free, neuromasts in the epithelium of the head, trunk and caudal fin.
2.4. Smell
2.5. Electrosense
2.6. Pros and Cons for the Relative Importance of Vision vs. the OLS
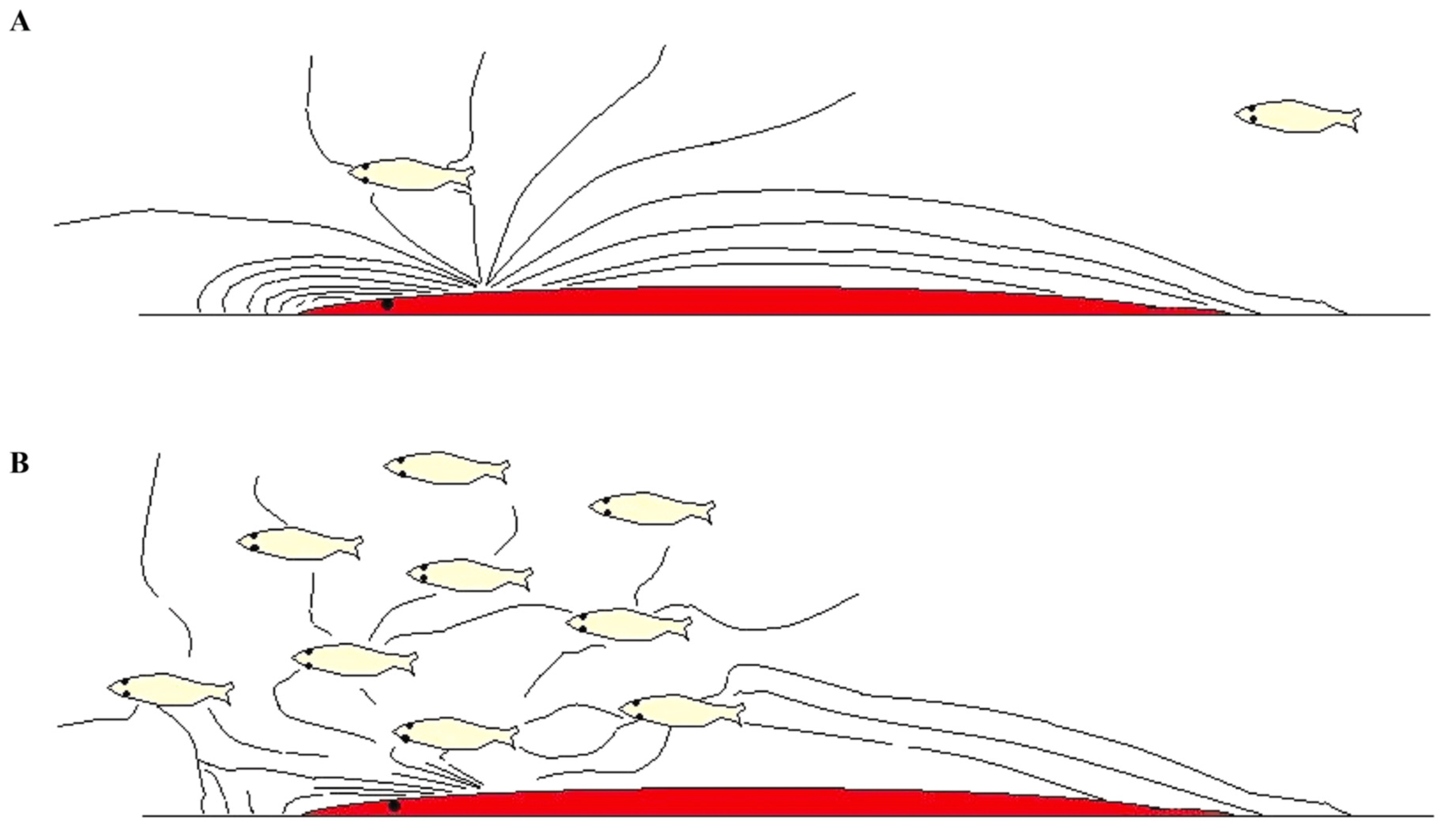
2.7. Synchronized Locomotion May Boost the Perception of the Sounds of the Surroundings
2.8. Can Fish Use Locomotion Sound as Signals within the School?
2.9. Shoal Assortment/Join Leave-or-Stay?
3. Why Do Fish School?
Schooling to Avoid Risk
4. Predator Confusion
4.1. Visual Confusion
4.2. Confusion of the Octavo-Lateralis System
4.3. The Electrosensory System
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Masuda, R.; Tsukamoto, K. School formation and concurrent developmental changes in carangid fish with reference to dietary conditions. Environ. Biol. Fishes 1999, 56, 243–252. [Google Scholar] [CrossRef]
- Dostálková, I.; Spinka, M. When to go with the crowd: Modelling synchronization of all-or-nothing activity transitions in grouped animals. J. Theor. Biol. 2010, 263, 437–448. [Google Scholar] [CrossRef][Green Version]
- Radakov, D.V. Schooling in the Ecology of Fish; John Wiley: New York, NY, USA, 1973. [Google Scholar]
- Kasumyan, A.O.; Pavlov, D.S. On the Problem of the Evolutionary Origin of Schooling Behavior of Fish. J. Ichthyol. 2023, 63, 1374–1389. [Google Scholar] [CrossRef]
- Parrish, J.K.; Edelstein-Keshet, L. Complexity, pattern, and evolutionary trade-offs in animal aggregation. Science 1999, 284, 99–101. [Google Scholar] [CrossRef] [PubMed]
- Couzin, I.D.; Krause, J.; James, R.; Ruxton, G.D.; Franks, N.R. Collective memory and spatial sorting in animal groups. J. Theor. Biol. 2002, 218, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, Y.; Cherel, Y. Synchronous underwater foraging behavior in penguins. Condor 1999, 101, 179–185. [Google Scholar] [CrossRef]
- McCue, L.M.; Cioffi, W.R.; Heithaus, M.R.; Barre, L.; Connor, R.C. Synchrony, leadership, and association in male Indo-pacific bottlenose dolphins (Tursiops aduncus). Ethology 2020, 126, 741–750. [Google Scholar] [CrossRef]
- Becker, A.D.; Masoud, H.; Newbolt, J.W.; Shelley, M.; Ristroph, L. Hydrodynamic schooling of flapping swimmers. Nat. Commun. 2015, 6, 8514. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M. Incidental sounds of locomotion in animal cognition. Anim. Cogn. 2012, 15, 1–13. [Google Scholar] [CrossRef]
- Larsson, M. Tool-use-associated sound in the evolution of language. Anim. Cogn. 2015, 18, 993–1005. [Google Scholar] [CrossRef]
- Larsson, M.; Abbott, B. Is the capacity for vocal learning in vertebrates rooted in fish schooling behavior? Evol. Biol. 2018, 45, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.; Richter, J.; Ravignani, A. Bipedal Steps in the Development of Rhythmic Behavior in Humans. Music. Sci. 2019, 2, 1–14. [Google Scholar] [CrossRef]
- Janik, V.M.; Knörnschild, M. Vocal production learning in mammals revisited. Philos. Trans. R. Soc. B—Biol. Sci. 2021, 376, 20200244. [Google Scholar] [CrossRef] [PubMed]
- Kasumyan, A.O.; Pavlov, D.S. Evolution of Schooling Behavior in Fish. J. Ichthyol. 2018, 58, 670–678. [Google Scholar] [CrossRef]
- Mizumoto, N.; Miyata, S.; Pratt, S.C. Inferring collective behaviour from a fossilized fish shoal. Proc. R. Soc. B—Biol. Sci. 2019, 286, 20190891. [Google Scholar] [CrossRef] [PubMed]
- Lauder, G.V.; Liem, K.F. The evolution and interrelationships of the actinopterygian fishes. Bull. Mus. Comp. Zool. 1983, 150, 95–197. [Google Scholar]
- Pitcher, T.J. Shoaling and Shoaling Behaviour in Fishes. In Comparative Psychology: A Handbook. Garland; Greenberg, G., Hararway, M.M., Eds.; Garland: New York, NY, USA, 1998; pp. 748–760. [Google Scholar]
- Pitcher, T.J. Fish Schooling: Implications for Pattern in the Oceans and Impacts on Human Fisheries. In Encyclopedia of Ocean Sciences; Steele, J.H., Turekian, K.K., Thorpe, S.A., Eds.; Academic Press: London, UK, 2001; pp. 975–987. [Google Scholar]
- Pitcher, T.J.; Magurran, A.E.; Edwards, J.I. Schooling mackerel and herring choose neighbours of similar size. Mar. Biol. 1985, 86, 319–322. [Google Scholar] [CrossRef]
- Pitcher, T.J.; Parrish, J.K. Functions of shoaling behaviour in teleosts. In The Behaviour of Teleost Fishes, 2nd ed.; Pitcher, T.J., Ed.; Croom Helm: London, UK, 1993; pp. 364–439. [Google Scholar]
- Keenleyside, M.H.A. Some aspects of the schooling behaviour of fish. Behav. Genet. 1955, 8, 183–248. [Google Scholar] [CrossRef]
- Gregson, J.N.S.; De Perera, T.B. Shoaling in eyed and blind morphs of the characin Astyanax fasciatus under light and dark conditions. J. Fish Biol. 2007, 70, 1615–1619. [Google Scholar] [CrossRef]
- Gray, J.A.B.; Denton, E.J. Fast pressure pulses and communication between fish. J. Mar. Biol. Assoc. United Kingd. 1991, 71, 83–106. [Google Scholar] [CrossRef]
- Braun, C.B.; Coombs, S. The overlapping roles of the inner ear and lateral line: The active space of dipole source detection. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Kalmijn, A.J. Hydrodynamic and acoustic field detection. In Sensory Biology of Aquatic Animals; Atema, J., Fay, R.R., Popper, A.N., Tavolga, W.N., Eds.; Springer: New York, NY, USA, 1988; pp. 83–130. [Google Scholar]
- Popper, A.N.; Platt, C. Inner ear and lateral line. In The Physiology of Fishes; CRC Press: Boca Raton, FL, USA, 1993; pp. 99–136. [Google Scholar]
- Popper, A.N.; Fay, R.R. Sound detection and processing by fish: Critical review and major research questions. Brain Behav. Evol. 1993, 41, 14–38. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M. Schooling Fish: A Multisensory Approach. In Reference Module in Earth Systems and Environmental Sciences; Elias, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar] [CrossRef]
- Larsson, M. Why do fish school? Curr. Zool. 2012, 58, 116–128. [Google Scholar] [CrossRef]
- Larsson, M. Possible functions of the octavolateralis system in fish schooling. Fish Fish 2009, 10, 344–355. [Google Scholar] [CrossRef]
- Mogdans, J. Sensory ecology of the fish lateral-line system: Morphological and physiological adaptations for the perception of hydrodynamic stimuli. J. Fish Biol. 2019, 95, 53–72. [Google Scholar] [CrossRef] [PubMed]
- Satou, M.; Shiraishi, A.; Matsushima, T.; Okumoto, N. Vibrational communication during spawning behavior in the himé salmon (landlocked red salmon, Oncorhynchus nerka). J. Comp. Physiol. A—Sens. Neural Behav. Physiol. 1991, 168, 417–428. [Google Scholar] [CrossRef]
- Satou, M.; Takeuchi, H.A.; Tanabe, M.; Kitamura, S.; Okumoto, N.; Iwata, M.; Nishii, J. Behavioral and electrophysiological evidences that the lateral line is involved in the inter-sexual vibrational communication of the himé salmon (landlocked red salmon, Oncorhynchus nerka). J. Comp. Physiol. A—Neuroethol. Sens. Neural Behav. Physiol. 1994, 174, 539–549. [Google Scholar] [CrossRef]
- Gardiner, J.M.; Atema, J.; Hueter, R.E.; Motta, P.J. Multisensory Integration and Behavioral Plasticity in Sharks from Different Ecological Niches. PLoS ONE 2014, 9, e93036. [Google Scholar] [CrossRef] [PubMed]
- Von Uexküll, J. A Foray into the Worlds of Animals and Humans: With a Theory of Meaning; University of Minnesota Press: Minneapolis, MN, USA, 2010. [Google Scholar]
- Atema, J. Aquatic odor dispersal fields: Opportunities and limits of detection, communication and navigation. In Chemical Ecology in Aquatic Systems; Bronmark, C., La, H., Eds.; Oxford University Press: Oxford, UK, 2012; pp. 1–18. [Google Scholar]
- Baker, C.V.H.; O’Neill, P.; McCole, R.B. Lateral line, otic and epibranchial placodes: Developmental and evolutionary links? J. Exp. Zool. B Mol. Dev. Evol. 2008, 310B, 370–383. [Google Scholar] [CrossRef]
- Cernuda-Cernuda, R.; Garcia-Fernandez, J.M. Structural diversity of the ordinary and specialized lateral line organs. Microsc. Res. Tech. 1996, 34, 302–312. [Google Scholar] [CrossRef]
- Curcic-Blake, B.; van Netten, S.M. Source location encoding in the fish lateral line canal. J. Exp. Biol. 2006, 209, 1548–1559. [Google Scholar] [CrossRef] [PubMed]
- Bullock, T.H. Electric Fish Electric Organ Discharges, and electroreception. In Evolution of Nervous Systems; Kaas, J.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 417–422. [Google Scholar]
- Moller, P. Electric signals and schooling behavior in a weakly electric fish, Marcusenius cyprinoides L. (Mormyriformes). Science 1976, 193, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Novitskaya, L.I. Predshestvenniki Ryb, Beschelyustnye-Nachalo Puti k Cheloveku (Agnatha Species as the Ancestors of Fishes and New Way to a Man); GEOS: Moscow, Russia, 2015. [Google Scholar]
- Northcutt, R.G.; Gans, C. The genesis of neural crest and epidermal placodes: A reinterpretation of vertebrate origins. Q. Rev. Biol. 1983, 58, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Partridge, B.L.; Pitcher, T.J. The sensory basis of fish schools: Relative roles of lateral line and vision. J. Comp. Physiol. 1980, 135, 315–325. [Google Scholar] [CrossRef]
- Blaxter, J.H.S.; Gray, J.A.B.; Denton, E.J. Sound and startle response in herring shoals. J. Mar. Biol. Assoc. United Kingd. 1981, 61, 851–869. [Google Scholar] [CrossRef]
- Blaxter, J.H.S. Structure and development of the lateral line Biol. Rev. Camb. Philos. Soc. 1987, 62, 471–514. [Google Scholar] [CrossRef]
- Pavlov, D.S.; Kasumyan, A.O. Patterns and mechanisms of schooling behavior in fish: A review. J. Ichthyol. 2000, 40 (Suppl. 2), S163–S231. [Google Scholar]
- Wysocki, L.E.; Ladich, F. Effects of noise exposure on click detection and the temporal resolution ability of the goldfish auditory system. Hear. Res. 2005, 201, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Norberg, Å. The flappet lark Mirafra rufocinnamomea doubles its wingbeat rate to 24 Hz in wing-clap display flight: A sexually selected feat. J. Exp. Biol. 1991, 159, 515–523. [Google Scholar] [CrossRef]
- Coleman, S.W. Mourning dove (Zenaida macroura) wing-whistles may contain threat-related information for con- and hetero-specifics. Naturwissenschaften 2008, 95, 981–986. [Google Scholar] [CrossRef]
- Hingee, M.; Magrath, R.D. Flights of fear: A mechanical wing whistle sounds the alarm in a flocking bird. Proc. R. Soc. Lond. B Biol. Sci. 2009, 276, 4173–4179. [Google Scholar] [CrossRef]
- Payne, R.B. Wingflap dialect in flapplet lark Mirafra rufocinnamomea. Ibis 1973, 115, 270–274. [Google Scholar] [CrossRef]
- Coleman, P.D. Failure to localize the source distance of an unfamiliar sound. J. Acoust. Soc. Am. 1962, 34, 345–346. [Google Scholar] [CrossRef]
- Krause, J.; Hoare, D.J.; Croft, D.; Lawrence, J.; Ward, A.; Ruxton, G.D.; Godin, J.G.J.; James, R. Fish shoal composition: Mechanisms and constraints. Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- Krause, J.; Butlin, R.K.; Peuhkuri, N.; Pritchard, V.L. The social organization of fish shoals: A test of the predictive power of laboratory experiments for the field. Biol. Rev. Camb. Philos. Soc. 2000, 75, 477–501. [Google Scholar] [CrossRef]
- Hanke, W.; Brucker, C.; Bleckmann, H. The ageing of the low-frequency water disturbances caused by swimming goldfish and its possible relevance to prey detection. J. Exp. Biol. 2000, 203, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Hanke, W.; Bleckmann, H. The hydrodynamic trails of Lepomis gibbosus (Centrarchidae), Colomesus psittacus (Tetraodontidae) and Thysochromis ansorgii (Cichlidae) investigated with scanning particle image velocimetry. J. Exp. Biol. 2004, 207, 1585–1596. [Google Scholar] [CrossRef]
- Pitcher, T.J.; Partridge, B.L.; Wardle, C.S. Blind fish can school. Science 1976, 194, 963–965. [Google Scholar] [CrossRef] [PubMed]
- Hager, M.C.; Helfman, G.S. Safety in numbers: Shoal size choice by minnows under predatory threat. Behav. Ecol. Sociobiol. 1991, 29, 271–276. [Google Scholar] [CrossRef]
- Engeszer, R.E.; Ryan, M.J.; Parichy, D.M. Learned social preference in zebrafish. Curr. Biol. 2004, 14, 881–884. [Google Scholar] [CrossRef]
- Kimbell, H.S.; Morrell, L.J. Turbidity weakens selection for assortment in body size in groups. Behav. Ecol. 2016, 27, 545–552. [Google Scholar] [CrossRef]
- Croft, D.P.; Arrowsmith, B.J.; Bielby, J.; Skinner, K.; White, E.; Couzin, I.D.; Magurran, A.E.; Ramnarine, I.; Krause, J. Mechanisms underlying shoal composition in the Trinidadian guppy, Poecilia reticulata. Oikos 2003, 100, 429–438. [Google Scholar] [CrossRef]
- Krause, J.; Ward, A.J.W.; Jackson, A.L.; Ruxton, G.D.; James, R.; Currie, S. The influence of differential swimming speeds on composition of multi-species fish shoals. J. Fish Biol. 2005, 67, 866–872. [Google Scholar] [CrossRef]
- Turesson, H.; Bronmark, C. Predator-prey encounter rates in freshwater piscivores: Effects of prey density and water transparency. Oecologia 2007, 153, 281–290. [Google Scholar] [CrossRef]
- Partridge, B.L.; Pitcher, T.J. Evidence against a hydrodynamic function for fish schools. Nature 1979, 279, 418–419. [Google Scholar] [CrossRef]
- Domenici, P.; Lefrancois, C.; Shingles, A. Hypoxia and the antipredator behaviours of fishes. Philos. Trans. R. Soc. London. Ser. B Biol. Sci. 2007, 362, 2105–2121. [Google Scholar] [CrossRef]
- Marras, S.; Killen, S.S.; Lindström, J.; McKenzie, D.J.; Steffensen, J.F.; Domenici, P. Fish swimming in schools save energy regardless of their spatial position. Behav. Ecol. Sociobiol. 2015, 69, 219–226. [Google Scholar] [CrossRef]
- Ioannou, C.C.; Guttal, V.; Couzin, I.D. Predatory fish select for coordinated collective motion in virtual prey. Science 2012, 337, 1212–1215. [Google Scholar] [CrossRef]
- Couzin, I.D.; Krause, J. Self-organization and collective behavior in vertebrates. In Advances in the Study of Behavior; Academic Press Inc.: San Diego, CA, USA, 2003; Volume 32, pp. 1–75. [Google Scholar]
- Krause, J. Differential fitness returns in relation to spatial position in groups. Biol. Rev. Camb. Philos. Soc. 1994, 69, 187–206. [Google Scholar] [CrossRef]
- Svendsen, J.C.; Skov, J.; Bildsoe, M.; Steffensen, J.F. Intra-school positional preference and reduced tail beat frequency in trailing positions in schooling roach under experimental conditions. J. Fish Biol. 2003, 62, 834–846. [Google Scholar] [CrossRef]
- Springer, S. Some observation on the behavior of schools of fishes in the Gulf of Mexico and adjacent waters. Ecology 1957, 38, 166–171. [Google Scholar] [CrossRef]
- Breder, C.M. Studies on social groupings in fishes. Bull. Am. Mus. Nat. Hist. 1959, 117, 393–482. [Google Scholar]
- Aivaz, A.N.; Manica, A.; Neuhaus, P.; Ruckstuhl, K.E. Picky predators and odd prey: Colour and size matter in predator choice and zebrafish’s vulnerability—A refinement of the oddity effect. Ethol. Ecol. Evol. 2020, 32, 135–147. [Google Scholar] [CrossRef]
- Landeau, L.; Terborgh, J. Oddity and the confusion effect in predation. Anim. Behav. 1986, 34, 1372–1380. [Google Scholar] [CrossRef]
- Theodorakis, C.W. Size segregation and the effects of oddity on predation risk in minnow schools. Anim. Behav. 1989, 38, 496–502. [Google Scholar] [CrossRef]
- Penry-Williams, I.L.; Ioannou, C.C.; Taylor, M.I. The oddity effect drives prey choice but not necessarily attack time. Ethology 2018, 124, 496–503. [Google Scholar] [CrossRef]
- Tosh, C.R.; Jackson, A.L.; Ruxton, G.D. The confusion effect in predatory neural networks. Am. Nat. 2006, 167, E52–E65. [Google Scholar] [CrossRef]
- Ruckstuhl, K.E. Sexual segregation in vertebrates: Proximate and ultimate causes. Integr. Comp. Biol. 2007, 47, 245–257. [Google Scholar] [CrossRef]
- Tang, Z.H.; Huang, Q.; Wu, H.; Kuang, L.; Fu, S.J. The behavioral response of prey fish to predators: The role of predator size. PeerJ 2017, 5, e3222. [Google Scholar] [CrossRef]
- Montgomery, J.C. Lateral line detection of planktonic prey. In The Mechanosensory Lateral Line; Coombs, S., Görner, P., Münz, H., Eds.; Springer: New York, NY, USA, 1989; pp. 561–574. [Google Scholar]
- Palmer, L.M.; Deffenbaugh, M.; Mensinger, A.F. Sensitivity of the anterior lateral line to natural stimuli in the oyster toadfish, Opsanus tau (Linnaeus). J. Exp. Biol. 2005, 208, 3441–3450. [Google Scholar] [CrossRef]
- New, J.G.; Fewkes, L.A.; Khan, A.N. Strike feeding behavior in the muskellunge, Esox masquinongy: Contributions of the lateral line and visual sensory systems. J. Exp. Biol. 2001, 204, 1207–1221. [Google Scholar] [CrossRef] [PubMed]
- Crampton, W.G.R. Electroreception, electrogenesis and electric signal evolution. J. Fish Biol. 2019, 95, 92–134. [Google Scholar] [CrossRef] [PubMed]
- von der Emde, G.; Schwarz, S.; Gomez, L.; Budelli, R.; Grant, K. Electric fish measure distance in the dark. Nature 1998, 395, 890–894. [Google Scholar] [CrossRef] [PubMed]
- Babineau, D.; Lewis, J.E.; Longtin, A. Spatial acuity and prey detection in weakly electric fish. PLoS Comput. Biol. 2007, 3, e38. [Google Scholar] [CrossRef] [PubMed]
- Mogdans, J.; Bleckmann, H. Responses of the goldfish trunk lateral line to moving objects. J. Comp. Physiol. A—Sens. Neural Behav. Physiol. 1998, 182, 659–676. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larsson, M. Schooling Fish from a New, Multimodal Sensory Perspective. Animals 2024, 14, 1984. https://doi.org/10.3390/ani14131984
Larsson M. Schooling Fish from a New, Multimodal Sensory Perspective. Animals. 2024; 14(13):1984. https://doi.org/10.3390/ani14131984
Chicago/Turabian StyleLarsson, Matz. 2024. "Schooling Fish from a New, Multimodal Sensory Perspective" Animals 14, no. 13: 1984. https://doi.org/10.3390/ani14131984
APA StyleLarsson, M. (2024). Schooling Fish from a New, Multimodal Sensory Perspective. Animals, 14(13), 1984. https://doi.org/10.3390/ani14131984





