The Effect of a Direct Fed Microbial on Liveweight and Milk Production in Dairy Cattle
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location and Herd
2.2. Study Animals
2.3. Statistical Analysis and Data Management
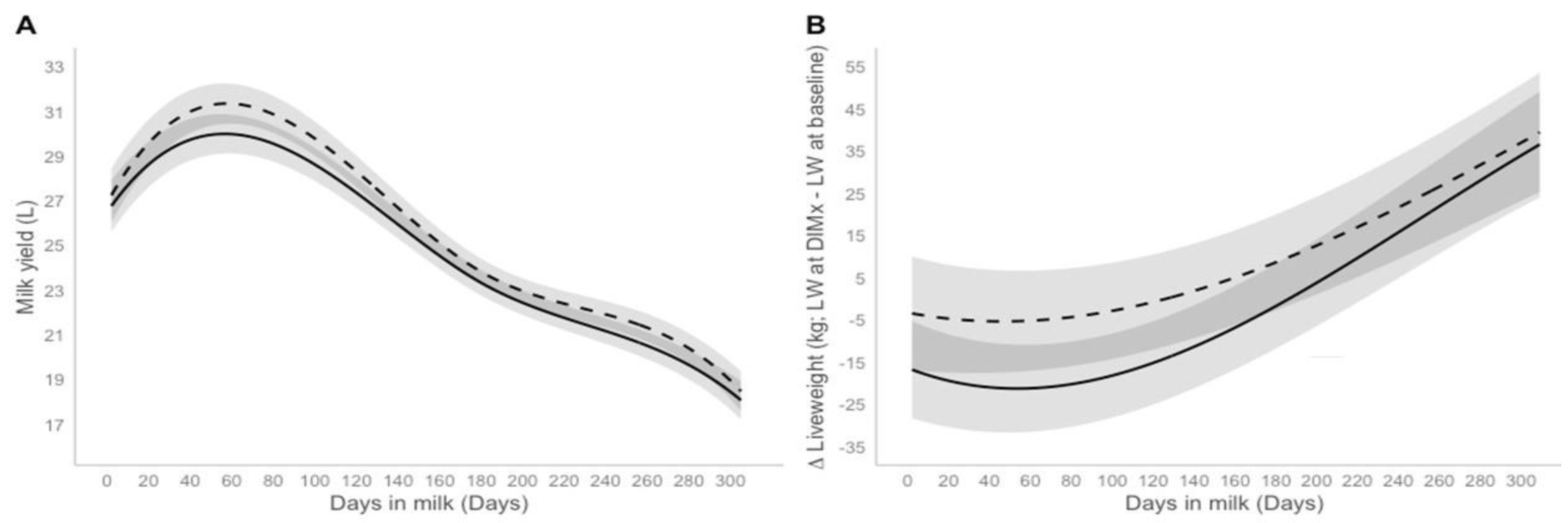
3. Results
3.1. Milk Yield and Liveweight
3.2. Milk Components
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leistikow, K.R.; Beattie, R.E.; Hristova, K.R. Probiotics beyond the farm: Benefits, costs, and considerations of using antibiotic alternatives in livestock. Front. Antibiot. 2022, 11, 1003912. [Google Scholar] [CrossRef]
- Robles-Jimenez, L.E.; Aranda-Aguirre, E.; Castelan-Ortega, O.A.; Shettino-Bermudez, B.S.; Ortiz-Salinas, R.; Miranda, M.; Li, X.; Angeles-Hernandez, J.C.; Vargas-Bello-Pérez, E.; Gonzalez-Ronquillo, M. Worldwide Traceability of Antibiotic Residues from Livestock in Wastewater and Soil: A Systematic Review. Animals 2022, 12, 60. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, R.; Wang, Y.; Gilbert, M.; Van Boeckel, T.P. Global trends in antimicrobial use in food-producing animals: 2020 to 2030. PLOS Glob. Public Health 2023, 3, e0001305. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Shabana, N.; Kuldeep, D.; Karthik, K.; Ruchi, T.; Abdelrahman, M.M.; Alhidary, I.A.; Arshad, Z. Direct-fed microbial: Beneficial applications, modes of action and prospects as a safe tool for enhancing ruminant production and safeguarding health. Int. J. Pharmacol. 2016, 12, 220–231. [Google Scholar] [CrossRef]
- Kalebich, C.C.; Cardoso, F.C. Chapter 9—Effects of Direct-Fed Microbials on Feed Intake, Milk Yield, Milk Composition, Feed Conversion, and Health Condition of Dairy Cows. In Nutrients in Dairy and Their Implications on Health and Disease; Watson, R.R., Collier, R.J., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 111–121. [Google Scholar] [CrossRef]
- Bhogoju, S.; Nahashon, S. Recent Advances in Probiotic Application in Animal Health and Nutrition: A Review. Agriculture 2022, 12, 304. [Google Scholar] [CrossRef]
- Kulkarni, N.A.; Chethan, H.S.; Srivastava, R.; Gabbur, A.B. Role of probiotics in ruminant nutrition as natural modulators of health and productivity of animals in tropical countries: An overview. Trop. Anim. Health Prod. 2022, 54, 110. [Google Scholar] [CrossRef] [PubMed]
- Nocek, J.E.; Kautz, W.P.; Leedle, J.A.Z.; Block, E. Direct-Fed Microbial Supplementation on the Performance of Dairy Cattle During the Transition Period1. J. Dairy Sci. 2003, 86, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Boyd, J.; West, J.W.; Bernard, J.K. Effects of the addition of direct-fed microbials and glycerol to the diet of lactating dairy cows on milk yield and apparent efficiency of yield. J. Dairy Sci. 2011, 94, 4616–4622. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, J.; Rico, D.E.; Perdomo, C.M.; Ronholm, J.; Gervais, R.; Chouinard, P.Y. Effects of direct-fed Bacillus subtilis and Bacillus licheniformis on production performance and milk fatty acid profile in dairy cows. J. Dairy Sci. 2023, 106, 1815–1825. [Google Scholar] [CrossRef] [PubMed]
- Nalla, K.; Manda, N.K.; Dhillon, H.S.; Kanade, S.R.; Rokana, N.; Hess, M.; Puniya, A.K. Impact of Probiotics on Dairy Production Efficiency. Front. Microbiol. 2022, 13, 805963. [Google Scholar] [CrossRef] [PubMed]
- Oyebade, A.O.; Lee, S.; Sultana, H.; Arriola, K.; Duvalsaint, E.; Nino De Guzman, C.; Fernandez Marenchino, I.; Marroquin Pacheco, L.; Amaro, F.; Ghedin Ghizzi, L.; et al. Effects of direct-fed microbial supplementation on performance and immune parameters of lactating dairy cows. J. Dairy Sci. 2023, 106, 8611–8626. [Google Scholar] [CrossRef] [PubMed]
- Ban, Y.; Guan, L.L. Implication and challenges of direct-fed microbial supplementation to improve ruminant production and health. J. Anim. Sci. Biotechnol. 2021, 12, 109. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.R.O.; Jacobs, J.L.; Chandra, S.; Soust, M.; Russo, V.M.; Douglas, M.L.; Hess, P.S.A. The Effect of Direct-Fed Lactobacillus Species on Milk Production and Methane Emissions of Dairy Cows. Animals 2023, 13, 1018. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Huang, W.; Hou, Q.; Kwok, L.-Y.; Sun, Z.; Ma, H.; Zhao, F.; Lee, Y.-K.; Zhang, H. The effects of probiotics administration on the milk production, milk components and fecal bacteria microbiota of dairy cows. Sci. Bull. 2017, 62, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Oetzel, G.R.; Emery, K.M.; Kautz, W.P.; Nocek, J.E. Direct-Fed Microbial Supplementation and Health and Performance of Pre- and Postpartum Dairy Cattle: A Field Trial. J. Dairy Sci. 2007, 90, 2058–2068. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Wang, J.Q.; Deng, L.F. Effects of Bacillus subtilis natto on milk production, rumen fermentation and ruminal microbiome of dairy cows. Animal 2013, 7, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Raeth-Knight, M.L.; Linn, J.G.; Jung, H.G. Effect of direct-fed microbials on performance, diet digestibility, and rumen characteristics of Holstein dairy cows. J. Dairy Sci. 2007, 90, 1802–1809. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.; Richardson, E.; Teets, C.; Akay, V. Production performance and nutrient digestibility of lactating dairy cows fed low-forage diets with and without the addition of a live-yeast supplement. J. Dairy Sci. 2019, 102, 6174–6179. [Google Scholar] [CrossRef] [PubMed]
- Malekkhahi, M.; Tahmasbi, A.M.; Naserian, A.A.; Danesh-Mesgaran, M.; Kleen, J.L.; AlZahal, O.; Ghaffari, M.H. Effects of supplementation of active dried yeast and malate during sub-acute ruminal acidosis on rumen fermentation, microbial population, selected blood metabolites, and milk production in dairy cows. Anim. Feed. Sci. Technol. 2016, 213, 29–43. [Google Scholar] [CrossRef]
- Luan, S.; Duersteler, M.; Galbraith, E.A.; Cardoso, F.C. Effects of direct-fed Bacillus pumilus 8G-134 on feed intake, milk yield, milk composition, feed conversion, and health condition of pre- and postpartum Holstein cows. J. Dairy Sci. 2015, 98, 6423–6432. [Google Scholar] [CrossRef]
- Hetti Arachchige, A.D.; Fisher, A.D.; Wales, W.J.; Auldist, M.J.; Hannah, M.C.; Jongman, E.C. Space allowance and barriers influence cow competition for mixed rations fed on a feed-pad between bouts of grazing. J. Dairy Sci. 2014, 97, 3578–3588. [Google Scholar] [CrossRef] [PubMed]
- Bates, D. lme4: Linear Mixed-Effects Models Using S4 Classes; R Package Version 0.999375-37; 2010. Available online: http://www.r-project.org (accessed on 1 July 2023).
- Bates, D.M.; Sarkar, D. lme4: Linear Mixed-Effects Models Using S4 Classes; R-Package Version 09975-12; 2007. Available online: http://cran.r-project.org/src/contrib/Descriptions/lme4.html (accessed on 1 July 2023).
- Monteiro, H.F.; Lelis, A.L.J.; Fan, P.; Calvo Agustinho, B.; Lobo, R.R.; Arce-Cordero, J.A.; Dai, X.; Jeong, K.C.; Faciola, A.P. Effects of lactic acid-producing bacteria as direct-fed microbials on the ruminal microbiome. J. Dairy Sci. 2022, 105, 2242–2255. [Google Scholar] [CrossRef] [PubMed]
- Ferraretto, L.F.; Shaver, R.D. Effect of direct-fed microbial supplementation on lactation performance and total-tract starch digestibility by midlactation dairy cows. Prof. Anim. Sci. 2015, 31, 63–67. [Google Scholar] [CrossRef]
- Lawrence, M.; Polukis, S.; Barnard, A.M.; Miller, M.A.; Kung, L.; Gressley, T.F. Evaluating the effects of Lactobacillus animalis and Propionibacterium freudenreichii on performance and rumen and fecal measures in lactating dairy cows. J. Dairy Sci. 2021, 104, 4119–4133. [Google Scholar] [CrossRef] [PubMed]
- Asil, A.K.; Mohammadabadi, T.; Chaji, M.; Direkvandi, E. Evaluating the effects of direct-fed microbial supplementation on the performance, milk quality and fatty acid of mid-lactating dairy cows. Vet. Med. Sci. 2023, 9, 2212–2220. [Google Scholar] [CrossRef] [PubMed]
- Walters, A.H.; Pryor, A.W.; Bailey, T.L.; Pearson, R.E.; Gwazdauskas, F.C. Milk yield, energy balance, hormone, follicular and oocyte measures in early and mid-lactation Holstein cows. Theriogenology 2002, 57, 949–961. [Google Scholar] [CrossRef] [PubMed]
- Coppock, C.E. Energy Nutrition and Metabolism of the Lactating Dairy Cow1. J. Dairy Sci. 1985, 68, 3403–3410. [Google Scholar] [CrossRef]
- Goetz, B.M.; Lefler, J.; Abeyta, M.A.; Horst, E.A.; Mayorga, E.J.; Al-Qaisi, M.; Rodriguez-Jimenez, S.; Martino, C.; Izzo, A.; La, R.; et al. Effects of dietary microbial feed supplement on production efficacy in lactating dairy cows. JDS Commun. 2021, 2, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, K.; Lefler, J.; Embree, M.; VandeHaar, M.J. The effect of supplementing native rumen microbes on milk production of dairy cows. JDS Commun. 2023, 4, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Piantoni, P.; VandeHaar, M.J. Symposium review: The impact of absorbed nutrients on energy partitioning throughout lactation. J. Dairy Sci. 2023, 106, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Carpinelli, N.A.; Halfen, J.; Trevisi, E.; Chapman, J.D.; Sharman, E.D.; Anderson, J.L.; Osorio, J.S. Effects of peripartal yeast culture supplementation on lactation performance, blood biomarkers, rumen fermentation, and rumen bacteria species in dairy cows. J. Dairy Sci. 2021, 104, 10727–10743. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.R.; Allen, D.T.; Perry, E.B.; Bruner, J.C.; Gates, K.W.; Rehberger, T.G.; Mertz, K.; Jones, D.; Spicer, L.J. Effects of feeding propionibacteria to dairy cows on milk yield, milk components, and reproduction. J. Dairy Sci. 2006, 89, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Qiao, G.H.; Shan, A.S.; Ma, N.; Ma, Q.Q.; Sun, Z.W. Effect of supplemental Bacillus cultures on rumen fermentation and milk yield in Chinese Holstein cows. J. Anim. Physiol. Anim. Nutr. 2010, 94, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Peiter, M.; Caixeta, L.; Endres, M. Association between change in body weight during early lactation and milk production in automatic milking system herds. JDS Commun. 2023, 4, 369–372. [Google Scholar] [CrossRef] [PubMed]
- So, S.; Wanapat, M.; Cherdthong, A. Effect of sugarcane bagasse as industrial by-products treated with Lactobacillus casei TH14, cellulase and molasses on feed utilization, ruminal ecology and milk production of mid-lactating Holstein Friesian cows. J. Sci. Food Agric. 2021, 101, 4481–4489. [Google Scholar] [CrossRef] [PubMed]
- Wondatir Workie, Z.; Gibson, J.P.; van der Werf, J.H.J. Analysis of culling reasons and age at culling in Australian dairy cattle. Anim. Prod. Sci. 2021, 61, 680–689. [Google Scholar] [CrossRef]
- Bureau of Meteorology. Climate Data Online. Available online: http://www.bom.gov.au/climate/data/ (accessed on 3 October 2023).
| Ingredient | Mean | Min | Max |
|---|---|---|---|
| Grain (barley, wheat, sorghum) | 19.3 | 15.8 | 27.7 |
| Protein Meal (canola meal, soybean meal) | 7.7 | 0 | 17.4 |
| Byproducts (flour bread, carrots, sweet corn waste, chickpea millrun) | 14.3 | 3.1 | 35.3 |
| Lucerne Hay | 5.8 | 3.3 | 9.9 |
| Silage (corn, barley, oats, soybean) | 34.7 | 14.8 | 49.2 |
| Pasture (kikuyu, ryegrass) | 15.3 | 0 | 43 |
| Bypass Fat | 0.6 | 0 | 1.9 |
| Minerals (macro minerals, trace minerals premix, urea, mycotoxin binder) | 2.4 | 1.2 | 3.5 |
| Diet Composition | |||
| Crude Protein (CP, %) | 16.9 | 13.8 | 22.8 |
| Neutral Detergent Fiber (NDF, %) | 35.9 | 31.2 | 40 |
| Acid Detergent Fiber (ADF, %) | 23.2 | 19.3 | 26.2 |
| Non-Fibrous Carbohydrate (NFC, %) | 34.3 | 28.1 | 40.2 |
| Fat (%) | 4.4 | 3.6 | 5.6 |
| MJ ME/Kg DM | 9.5 | 8.6 | 10.8 |
| Starch (%) | 23 | 20.1 | 26.5 |
| Control | DFM | |||||
|---|---|---|---|---|---|---|
| Mean (SD) | Median (Q1–Q3) | Min–Max | Mean (SD) | Median (Q1–Q3) | Min–Max | |
| Baseline (before start of study period) | ||||||
| Milk yield (L) | 33 (7) | 33 (27–38) | 19, 50 | 32 (8) | 31 (26–37) | 11, 49 |
| Days in milk (days) | 131 (53) | 145 (87–172) | 6, 226 | 135 (54) | 146 (88–174) | 5, 423 |
| Parity | 2 (1) | 2 (1–3) | 1, 3 | 2 (1) | 2 (1–3) | 1, 3 |
| Fat yield (kg) | 3 (1) | 3 (3–4) | 2, 10 | 3 (1) | 3 (3–4) | 2, 7 |
| Protein yield (kg) | 3 (0) | 3 (3–3) | 3, 4 | 3 (0) | 3 (3–3) | 3, 5 |
| Somatic cell counts (×1000 cells/mL) | 70 (73) | 39 (20–93) | 8, 356 | 98 (92) | 61 (31–139) | 9, 354 |
| Liveweight (kg) | 582 (57) | 584 (534–612) | 465, 707 | 577 (62) | 577 (524–622) | 437, 733 |
| Second production year (2022/2023) | ||||||
| Milk yield (L) | 26 (6) | 25 (21–29) | 10, 50 | 25 (6) | 24 (21–29) | 8, 55 |
| Days in milk (days) | 193 (98) | 207 (114–266) | 2, 493 | 208 (94) | 222 (142–276) | 2, 526 |
| Fat yield (kg) | 1 (0) | 1 (1–1) | 0, 2 | 1 (0) | 1 (1–1) | 0, 1 |
| Protein yield (kg) | 195 (451) | 65 (28–163) | 4, 4927 | 246 (561) | 94 (42–207) | 7, 4704 |
| Somatic cell counts (×1000 cells/mL) | 588 (62) | 584 (546–624) | 398, 842 | 591 (67) | 583 (542–636) | 427, 797 |
| Liveweight change (kg) | 6 (43) | 3 (−23–32) | −144, 160 | 14 (43) | 12 (−15–41) | −166, 167 |
| Explanatory Variable | Coefficient (SE) | 95% Confidence Interval | p-Value * |
|---|---|---|---|
| Intercept | 23.06 (1.05) | 19.38; 24.16 | <0.001 |
| Days in milk (DIM) fitted as fourth-order cubic spline ** | |||
| Experimental groups | 0.91 | ||
| Control | Reference | ||
| DFM | 0.09 (0.83) | −1.54; 1.72 | |
| Milk yield at First Production year | 0.23 (0.03) | 0.19; 0.31 | <0.001 |
| Cow parity | <0.001 | ||
| 1st and 2nd (category 1) | Reference | ||
| >2nd (category 2) | 1.77 (0.16) | 1.54; 2.20 | |
| Production year | <0.001 | ||
| First (2021/2022) | Reference | ||
| Second (2022/2023) | −3.59 (0.14) | −3.87; −3.30 | |
| DIM polynomial terms × Experimental group | 0.07 | ||
| Experimental Group × Production year | 0.05 | ||
| Control × 2021/2022 | Reference | ||
| DFM × 2022/2023 | 0.39 (0.19) | 0.10; 0.89 |
| Explanatory Variable | Coefficient (SE) | 95% Confidence Interval | p-Value * |
|---|---|---|---|
| Intercept | −57.73 (6.24) | −69.91; −45.52 | <0.001 |
| Days in milk (DIM) fitted as fourth-order cubic spline ** | <0.001 | ||
| Experimental groups | 0.05 | ||
| Control | |||
| DFM | 19.40 (9.70) | 0.44; 38.30 | 0.05 |
| Liveweight at First Production year | 0.22 (0.03) | 0.16; 0.28 | <0.001 |
| Cow parity | <0.001 | ||
| 1st and 2nd (Category 1) | Reference | ||
| >2nd (Category 2) | −49.43 (3.71) | −56.72; −41.91 | |
| Production year | <0.001 | ||
| First (2021/2022) | |||
| Second (2022/2023) | 40.9 (1.58) | 37.68; 44.00 | |
| DIM polynomial terms × Experimental group | 0.06 | ||
| DFM × Production year | 0.01 | ||
| Control × 2021 | Reference | ||
| DFM × 2022/2023 | −6.06 (2.27) | −10.49; −1.61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramirez-Garzon, O.; Al-Alawneh, J.I.; Barber, D.; Liu, H.; Soust, M. The Effect of a Direct Fed Microbial on Liveweight and Milk Production in Dairy Cattle. Animals 2024, 14, 1092. https://doi.org/10.3390/ani14071092
Ramirez-Garzon O, Al-Alawneh JI, Barber D, Liu H, Soust M. The Effect of a Direct Fed Microbial on Liveweight and Milk Production in Dairy Cattle. Animals. 2024; 14(7):1092. https://doi.org/10.3390/ani14071092
Chicago/Turabian StyleRamirez-Garzon, Orlando, John I. Al-Alawneh, David Barber, Huanle Liu, and Martin Soust. 2024. "The Effect of a Direct Fed Microbial on Liveweight and Milk Production in Dairy Cattle" Animals 14, no. 7: 1092. https://doi.org/10.3390/ani14071092
APA StyleRamirez-Garzon, O., Al-Alawneh, J. I., Barber, D., Liu, H., & Soust, M. (2024). The Effect of a Direct Fed Microbial on Liveweight and Milk Production in Dairy Cattle. Animals, 14(7), 1092. https://doi.org/10.3390/ani14071092





