Impact of Bacillus licheniformis-Fermented Products on Growth and Productivity in Heat-Stressed Laying Ducks
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Experimental Design
2.3. Animal Subjects
2.4. Experimental Diets
2.5. Data Collection
2.6. Growth Performance
2.7. Rectal Temperatures and Respiratory Rate
2.8. Intestinal Morphology
2.9. Egg Production and Egg Quality
2.10. Quantitative Polymerase Chain Reaction and Western Blotting
2.11. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Rectal Temperatures and Respiratory Rate
3.3. Intestinal Morphology
3.4. Egg Production and Egg Quality
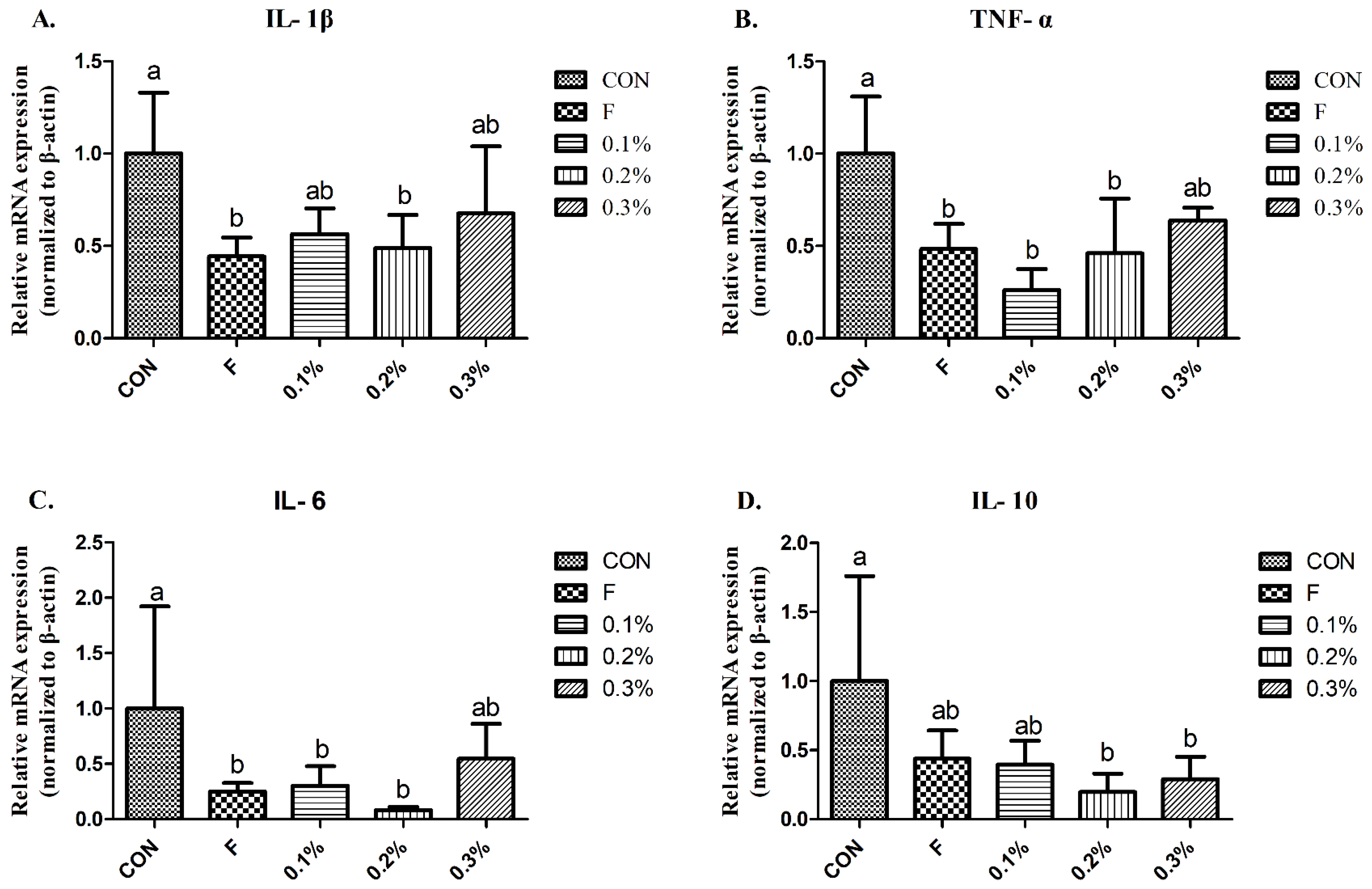
3.5. Pro-Inflammatory Cytokines
3.6. HSP70 and Tight Junction-Associated Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shukla, P.R.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Pörtner, H.O.; Roberts, D.; Zhai, P.; Slade, R.; Connors, S.; Van Diemen, R. IPCC, 2019: Climate Change and Land: An IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems. In Proceedings of the Bonn Climate Change Conference, Bonn, Germany, 17–27 June 2019. [Google Scholar]
- Ahmad, R.; Yu, Y.-H.; Hsiao, F.S.-H.; Su, C.-H.; Liu, H.-C.; Tobin, I.; Zhang, G.; Cheng, Y.-H. Influence of heat stress on poultry growth performance, intestinal inflammation, and immune function and potential mitigation by probiotics. Animals 2022, 12, 2297. [Google Scholar] [CrossRef] [PubMed]
- Bilal, R.M.; Hassan, F.U.; Farag, M.R.; Nasir, T.A.; Ragni, M.; Mahgoub, H.A.; Alagawany, M. Thermal stress and high stocking densities in poultry farms: Potential effects and mitigation strategies. J. Therm. Biol. 2021, 99, 102944. [Google Scholar] [CrossRef] [PubMed]
- Abbas, G. Combating heat stress in laying hens a review. Pak. J. Sci. 2021, 99, 102944. [Google Scholar]
- Roufayel, R.; Kadry, S. Molecular chaperone HSP70 and key regulators of apoptosis-a review. Curr. Mol. Med. 2019, 19, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Alberti, S.; Hyman, A.A. Biomolecular condensates at the nexus of cellular stress, protein aggregation disease and ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 196–213. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.-U.; Nawaz, A.; Rehman, M.S.; Ali, M.A.; Dilshad, S.M.; Yang, C. Prospects of HSP70 as a genetic marker for thermo-tolerance and immuno-modulation in animals under climate change scenario. Anim. Nutr. 2019, 5, 340–350. [Google Scholar] [CrossRef]
- Ali, I.; Li, C.; Kuang, M.; Shah, A.U.; Shafiq, M.; Ahmad, M.A.; Abdalmegeed, D.; Li, L.; Wang, G. Nrf2 Activation and NF-Kb & caspase/bax signaling inhibition by sodium butyrate alleviates LPS-induced cell injury in bovine mammary epithelial cells. Mol. Immunol. 2022, 148, 54–67. [Google Scholar] [PubMed]
- Hu, C.; Yang, J.; Qi, Z.; Wu, H.; Wang, B.; Zou, F.; Mei, H.; Liu, J.; Wang, W.; Liu, Q. Heat shock proteins: Biological functions, pathological roles, and therapeutic opportunities. MedComm. 2022, 3, e161. [Google Scholar] [CrossRef] [PubMed]
- Zbikowska, K.; Michalczuk, M.; Dolka, B. The use of bacteriophages in the poultry industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Muhammad, J.; Khan, S.; Su, J.Q.; Hesham, A.E.-L.; Ditta, A.; Nawab, J.; Ali, A. Antibiotics in poultry manure and their associated health issues: A systematic review. J. Soil. Sediment. 2020, 20, 486–497. [Google Scholar] [CrossRef]
- Ahmad, R.; Yu, Y.-H.; Hsiao, F.S.-H.; Dybus, A.; Ali, I.; Hsu, H.-C.; Cheng, Y.-H. Probiotics as a Friendly Antibiotic Alternative: Assessment of Their Effects on the Health and Productive Performance of Poultry. Fermentation 2022, 8, 672. [Google Scholar] [CrossRef]
- Li, Y.; Ali, I.; Lei, Z.; Li, Y.; Yang, M.; Yang, C.; Li, L. Effect of a Multistrain Probiotic on Feline Gut Health through the Fecal Microbiota and Its Metabolite SCFAs. Metabolites 2023, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Malyar, R.M.; Naseri, E.; Li, H.; Ali, I.; Farid, R.A.; Liu, D.; Maroof, K.; Nasim, M.; Banuree, S.A.H.; Huang, K. Hepatoprotective effects of selenium-enriched probiotics supplementation on heat-stressed wistar rat through anti-inflammatory and antioxidant effects. Biol. Trace Elem. Res. 2021, 199, 3445–3456. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, N.A.H.; Omer, N.A.; Yi-Ru, W.; Mei-Qian, K.; Ilyas, A.; Abdurahim, Y.; Wang, G.L. Protective effect of betaine against lead-induced testicular toxicity in male mice. Andrologia 2020, 52, e13600. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Li, Y.; Yu, D.; Rajput, I.; Li, W. Influence of dietary inclusion of Bacillus licheniformis on laying performance, egg quality, antioxidant enzyme activities, and intestinal barrier function of laying hens. Poult. Sci. 2013, 92, 2389–2395. [Google Scholar] [CrossRef] [PubMed]
- Al-Khalaifah, H. Benefits of probiotics and prebiotics for antibiotic-reduced poultry. Poult. Sci. 2018, 97, 3807–3815. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhou, B.; Xi, Y.; Huan, H.; Li, M.; Yu, J.; Zhu, H.; Dai, Z.; Ying, S.; Zhou, W. Fermented feed regulates growth performance and the cecal microbiota community in geese. Poult. Sci. 2019, 98, 4673–4684. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yan, H.; Lv, L.; Xu, Q.; Yin, C.; Zhang, K.; Wang, P.; Hu, J. Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas. J. Anim. Sci. 2012, 25, 682. [Google Scholar] [CrossRef]
- Gong, L.; Wang, B.; Mei, X.; Xu, H.; Qin, Y.; Li, W.; Zhou, Y. Effects of three probiotic Bacillus on growth performance, digestive enzyme activities, antioxidative capacity, serum immunity, and biochemical parameters in broilers. Anim. Sci. J. 2018, 89, 1561–1571. [Google Scholar] [CrossRef]
- Li, L.; Han, X.; Sun, C.; An, K.; Gao, W.; Xia, Z. Effect of Dietary Bacillus licheniformis supplementation on growth performance and microbiota diversity of Pekin ducks. Front. Vet. Sci. 2022, 9, 832141. [Google Scholar] [CrossRef]
- Deng, W.; Dong, X.; Tong, J.; Zhang, Q. The probiotic Bacillus licheniformis ameliorates heat stress-induced impairment of egg production, gut morphology, and intestinal mucosal immunity in laying hens. Poult. Sci. 2012, 91, 575–582. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020, 99, 1432–1443. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Horng, Y.B.; Dybus, A.; Yu, Y.H. Bacillus licheniformis-fermented products improve growth performance and intestinal gut morphology in broilers under Clostridium perfringens challenge. J. Poult. Sci. 2021, 58, 30–39. [Google Scholar] [CrossRef]
- Ma, X.; Lin, Y.; Zhang, H.; Chen, W.; Wang, S.; Ruan, D.; Jiang, Z. Heat stress impairs the nutritional metabolism and reduces the productivity of egg-laying ducks. Anim. Reprod. Sci. 2014, 145, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Yu, Y.H. Differential effects of Bacillus subtilis–and Bacillus licheniformis–fermented products on growth performance, intestinal morphology, intestinal antioxidant and barrier function gene expression, cecal microbiota community, and microbial carbohydrate-active enzyme composition in broilers. Poult. Sci. 2023, 102, 102670. [Google Scholar] [PubMed]
- Wang, W.; Yan, F.; Hu, J.; Amen, O.; Cheng, H. Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. Anim. Sci. J. 2018, 96, 1654–1666. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Yu, Y.H. Bacillus licheniformis–fermented products and enramycin differentially modulate microbiota and antibiotic resistome in the cecal digesta of broilers. Poult. Sci. 2022, 101, 102010. [Google Scholar] [CrossRef]
- Balakrishnan, K.N.; Ramiah, S.K.; Zulkifli, I. Heat shock protein response to stress in poultry: A review. Animals 2023, 13, 317. [Google Scholar] [CrossRef]
- Pawar, S.S.; Kurade, N.P.; Bhendarkar, M.P.; Bhosale, S.V.; Nirmale, A.V.; Kochewad, S.A. Modulation of heat shock protein 70 (HSP70) gene expression ex vivo in response to heat stress in chicken. Anim. Biotechnol. 2023, 34, 5168–5172. [Google Scholar] [CrossRef]
- Kojima, K.; Musch, M.W.; Ren, H.; Boone, D.L.; Hendrickson, B.A.; Ma, A.; Chang, E.B. Enteric flora and lymphocyte-derived cytokines determine expression of heat shock proteins in mouse colonic epithelial cells. Gastroenterology 2003, 124, 1395–1407. [Google Scholar] [CrossRef]
- Yang, P.C.; He, S.H.; Zheng, P.Y. Investigation into the signal transduction pathway via which heat stress impairs intestinal epithelial barrier function. J. Gastroenterol. Hepatol. 2007, 22, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Du, W.; Lei, K.; Wang, B.; Wang, Y.; Zhou, Y.; Li, W. Effects of dietary Bacillus licheniformis on gut physical barrier, immunity, and reproductive hormones of laying hens. Probiotics Antimicrob. 2017, 9, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Musa, B.B.; Duan, Y.; Khawar, H.; Sun, Q.; Ren, Z.; Elsiddig Mohamed, M.A.; Abbasi, I.H.R.; Yang, X. Bacillus subtilis B21 and Bacillus licheniformis B26 improve intestinal health and performance of broiler chickens with Clostridium perfringens-induced necrotic enteritis. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1039–1049. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Chen, J.; Xu, Z.; Wang, S.; Xia, X.; Liu, D.; Wang, S.; Xie, C.; Wu, J. Preventive effects of Bacillus licheniformis on heat stroke in rats by sustaining intestinal barrier function and modulating gut microbiota. Front. Microbiol. 2021, 12, 630841. [Google Scholar] [CrossRef] [PubMed]
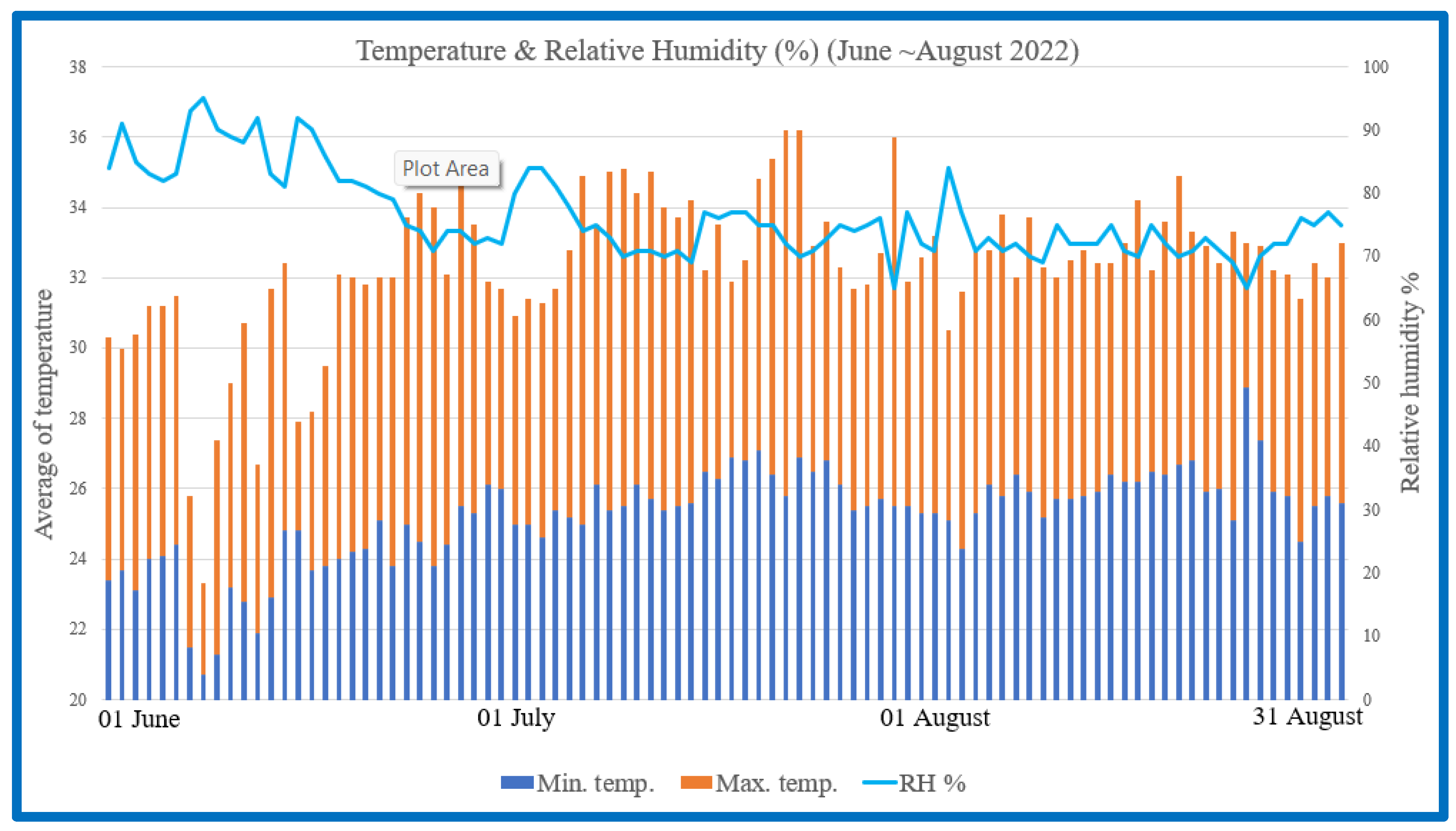

| Ingredients | Starter Diet | Grower Diet | Layer Diet |
|---|---|---|---|
| Yellow corn | 55.54 | 51.94 | 49.93 |
| Soybean meal (44%) | 25.30 | 10.00 | 27.00 |
| Wheat flour middling | 10.30 | 20.00 | - |
| Wheat bran | - | 10.00 | 6.50 |
| Fish meal | 2.00 | - | 3.30 |
| Yeast powder | 3.00 | 2.00 | 2.00 |
| Rice hull powder | - | 2.40 | - |
| Soybean oil | 1.10 | - | 2.50 |
| Limestone | 1.10 | 1.60 | 6.60 |
| Di-calcium phosphate | 1.10 | 1.50 | 1.50 |
| Salt iodized | 0.30 | 0.30 | 0.40 |
| Choline chloride (50%) | 0.08 | 0.08 | 0.08 |
| Lysine-HCl | - | - | 0.01 |
| DL-methionine | 0.05 | 0.05 | 0.05 |
| Vitamin premix a | 0.03 | 0.03 | 0.03 |
| Mineral premix b | 0.10 | 0.10 | 0.10 |
| Calculated Value | |||
| ME (kcal/kg) | 2900 | 2660 | 2700 |
| Crude protein (%) | 19.5 | 13.5 | 20.0 |
| Calcium (%) | 0.81 | 0.94 | 3.05 |
| Available phosphorus (%) | 0.36 | 0.44 | 0.44 |
| Methionine (%) | 0.38 | 0.27 | 0.39 |
| Lysine (%) | 1.05 | 0.60 | 1.11 |
| Gene | Accession No. | Primer Sequence (5′-3′) | Product Size (bp) | |
|---|---|---|---|---|
| β-actin | NM_001310421.1 | Forward: | TGATATTGCTGCGCTCGTTGT | 193 |
| Reverse: | CAGGGTCAGGATACCTCTTTTGC | |||
| HSP70 | GI281323015 | Forward: | CCCCCAGATCGAGGTTACTTT | 200 |
| Reverse: | CTCCCACCCGATCTCTGTTG | |||
| IL-1β | DQ393268 | Forward: | TCGACATCAACCAGAAGTGC | 185 |
| Reverse: | GAGCTTGTAGCCCTTGATGC | |||
| TNF-α | XM_005027491.3 | Forward: | ACCCCGTTACAGTTCAGACG | 140 |
| Reverse: | TAGCCATGTCAATGCTCCTG | |||
| IL-6 | XM_027450925 | Forward: | TTCGACGAGGAGAAATGCTT | 150 |
| Reverse: | CCTTATCGTCGTTGCCAGAT | |||
| IL-10 | NM_001310368.1 | Forward: | GGGAGAGGAAACTGAGAGATGT | 112 |
| Reverse: | TCCTTTCCTCTTAGTCCAGCTC | |||
| IFN-γ | AJ012254 | Forward: | GCTGATGGCAATCCTGTTTT | 247 |
| Reverse: | GGATTTTCAAGCCAGTCAGC | |||
| IL-2 | AF294323 | Forward: | GCCAAGAGCTGACCAACTTC | 137 |
| Reverse: | ATCGCCCACACTAAGAGCAT | |||
| IL-4 | XM_038186630.1 | Forward: | GGCAATGAGGTAAGACGGGA | 232 |
| Reverse: | AGCGTTTTGTGCCCATGGAT | |||
| IL-5 | XM_046927093.1 | Forward: | CACATCAGGACCATGAGGACC | 239 |
| Reverse: | CCGAATCTCCTCATCTCGGG | |||
| Claudin-1 | XM 013108556.1 | Forward: | TCATGGTATGGCAACAGAGTGG | 179 |
| Reverse: | CGGGTGGGTGGATAGAAG | |||
| Occludin | XM 013109403.1 | Forward: | CAGGATGTGGCAGAGGAATACAA | 160 |
| Reverse: | CCTTGTCGTAGTCGCTCACCAT |
| BLFP | |||||||
|---|---|---|---|---|---|---|---|
| Items | Control | Flavomycin | 0.1% | 0.2% | 0.3% | SEM | p-Value |
| Body weight (g) | |||||||
| 3 wk | 272.09 | 279.00 | 323.64 | 285.40 | 303.08 | 10.95 | 0.58 |
| 6 wk | 838.51 ab | 741.3 b | 882.26 ab | 822.99 ab | 891.31 a | 17.07 | 0.05 |
| Feed intake (g) | |||||||
| 3 wk | 884.08 | 948.70 | 942.50 | 923.02 | 907.90 | 18.96 | 0.89 |
| 6 wk | 3617.50 | 3167.00 | 3277.00 | 3093.00 | 3259.00 | 83.92 | 0.38 |
| 1~6 wk | 4501.58 | 4115.70 | 4219.50 | 4016.02 | 4166.90 | 88.35 | 0.59 |
| Feed conversion ratio (G/F) | |||||||
| 3 wk | 4.35 | 4.21 | 3.26 | 3.59 | 3.51 | 0.22 | 0.47 |
| 6 wk | 6.41 | 7.07 | 6.05 | 5.95 | 5.60 | 0.16 | 0.06 |
| 1~6 wk | 5.63 ab | 5.84 a | 4.99 ab | 5.13 ab | 4.82 b | 0.12 | 0.03 |
| Parameters | Control | Flavomycin | 0.1% | 0.2% | 0.3% | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | Male | Female | SEM | Diet | Gender | Diet × Gender | |
| Rectal temperature | 42.29 | 42.03 | 42.47 | 42.34 | 42.26 | 42.27 | 42.18 | 42.48 | 42.34 | 42.22 | 0.03 | 0.01 | 0.54 | 0.09 |
| Breath count | 22.19 | 28.75 | 24.97 | 32.89 | 25.06 | 29.94 | 25.31 | 28.22 | 25.22 | 34.86 | 0.54 | 0.3363 | <0.0001 | 0.5898 |
| BLPF | ||||||||
|---|---|---|---|---|---|---|---|---|
| Items | Control | Flavomycin | 0.1% | 0.2% | 0.3% | SEM | p-Value | |
| Duodenum | ||||||||
| Villus height (μm) | 811.89 | 812.02 | 701.59 | 776.62 | 709.78 | 29.39 | 0.63 | |
| Crypt depth (μm) | 21.99 | 21.28 | 28.11 | 24.01 | 31.62 | 1.43 | 0.11 | |
| Villus: Crypt | 39.84 | 39.09 | 28.80 | 34.66 | 25.87 | 1.94 | 0.08 | |
| Jejunum | ||||||||
| Villus height (μm) | 695.69 b | 752.44 b | 825.19 b | 995.68 a | 804.33 b | 23.32 | <0.01 | |
| Crypt depth (μm) | 33.86 | 24.88 | 29.31 | 29.11 | 27.59 | 1.29 | 0.28 | |
| Villus: Crypt | 23.08 | 31.74 | 29.39 | 37.74 | 31.74 | 1.64 | 0.07 | |
| Ileum | ||||||||
| Villus height (μm) | 627.1 bc | 596.78 c | 763.57 a | 731.68 ab | 753.69 ab | 21.42 | 0.03 | |
| Crypt depth (μm) | 42.74 | 40.59 | 48.43 | 43.29 | 37.20 | 1.98 | 0.50 | |
| Villus: Crypt | 16.33 | 14.88 | 19.00 | 17.57 | 24.28 | 1.40 | 0.25 | |
| BLFP | |||||||
|---|---|---|---|---|---|---|---|
| Control | Flavomycin | 0.1% | 0.2% | 0.3% | SEM | p-Value | |
| Feed intake (g) | 161.1 a | 139.1 b | 171.0 a | 164.4 a | 167.1 a | 2.94 | <0.0001 |
| Body weight (g) | 1263.3 ab | 1200.9 b | 1244.9 ab | 1197.6 b | 1340.7 a | 16.08 | 0.043 |
| Production rate (%) | 88.9 | 90.2 | 92 | 89 | 89 | 0.01 | 0.7291 |
| Egg Weight (g) | 64.3 a | 60.2 ab | 61.9 ab | 62.5 a | 60.1 b | 0.54 | 0.0301 |
| Feed conversion ratio | 2.5 c | 2.3 d | 2.8 a | 2.6 bc | 2.8 ab | 0.02 | 0.0027 |
| Egg width (mm) | 42.9 | 42.7 | 43 | 43.3 | 40.6 | 0.58 | 0.2929 |
| Egg height (mm) | 59.1 | 57.7 | 58.9 | 59.1 | 57.9 | 0.26 | 0.1602 |
| Shape index | 72.64 | 74 | 73.2 | 73.3 | 70.6 | 1.01 | 0.6872 |
| Break strength (N) | 48.69 | 52.3 | 47.1 | 50.9 | 54.6 | 1.20 | 0.1791 |
| Albumin height | 8.2 | 7 | 8.2 | 6.9 | 7.8 | 0.23 | 0.0646 |
| Haugh | 88 | 82.5 | 89.1 | 80.7 | 85.6 | 1.60 | 0.1959 |
| Yolk color | 12.1 b | 12.1 b | 12 b | 11.8 b | 12.4 a | 0.06 | 0.0217 |
| Eggshell thickness (mm) | |||||||
| Top | 0.412 | 0.372 | 0.368 | 0.369 | 0.386 | 0.0043 | 0.1293 |
| Middle | 0.400 | 0.408 | 0.387 | 0.416 | 0.400 | 0.0027 | 0.3629 |
| Bottom | 0.382 | 0.374 | 0.357 | 0.38 | 0.405 | 0.0038 | 0.0964 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, R.; Yu, Y.-H.; Hsiao, F.S.-H.; Liu, H.-W.; Su, C.-H.; Cheng, Y.-H. Impact of Bacillus licheniformis-Fermented Products on Growth and Productivity in Heat-Stressed Laying Ducks. Animals 2024, 14, 1164. https://doi.org/10.3390/ani14081164
Ahmad R, Yu Y-H, Hsiao FS-H, Liu H-W, Su C-H, Cheng Y-H. Impact of Bacillus licheniformis-Fermented Products on Growth and Productivity in Heat-Stressed Laying Ducks. Animals. 2024; 14(8):1164. https://doi.org/10.3390/ani14081164
Chicago/Turabian StyleAhmad, Rafiq, Yu-Hsiang Yu, Felix Shih-Hsiang Hsiao, Hsiu-Wei Liu, Chin-Hui Su, and Yeong-Hsiang Cheng. 2024. "Impact of Bacillus licheniformis-Fermented Products on Growth and Productivity in Heat-Stressed Laying Ducks" Animals 14, no. 8: 1164. https://doi.org/10.3390/ani14081164
APA StyleAhmad, R., Yu, Y.-H., Hsiao, F. S.-H., Liu, H.-W., Su, C.-H., & Cheng, Y.-H. (2024). Impact of Bacillus licheniformis-Fermented Products on Growth and Productivity in Heat-Stressed Laying Ducks. Animals, 14(8), 1164. https://doi.org/10.3390/ani14081164






