The Effects of Poria cocos Polysaccharides on Growth Performance, Immunity, and Cecal Microflora Composition of Weaned Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Poria cocos Polysaccharide Preparation
2.3. Animals, Trial Design, and Management
2.4. Sample Collection
2.4.1. Serum Collection
2.4.2. Lymphocyte Isolation
2.4.3. Tissue Sample Collection
2.4.4. Intestinal Sample Collection
2.5. Determination of Immunoglobulin and Cytokine Contents and the CD4+/CD8+ T Cell Ratio
2.6. Measurement of Cytokine mRNA Levels in the Spleen
2.7. Analysis of the Cecal Microflora
2.8. Statistical Analysis
3. Results
3.1. The Effect of PCP on Growth Performance
3.2. The Effect of PCP on Serum Immunoglobulin and Cytokine Contents
3.3. The Effect of PCP on the CD4+/CD8+ T Cell Ratio
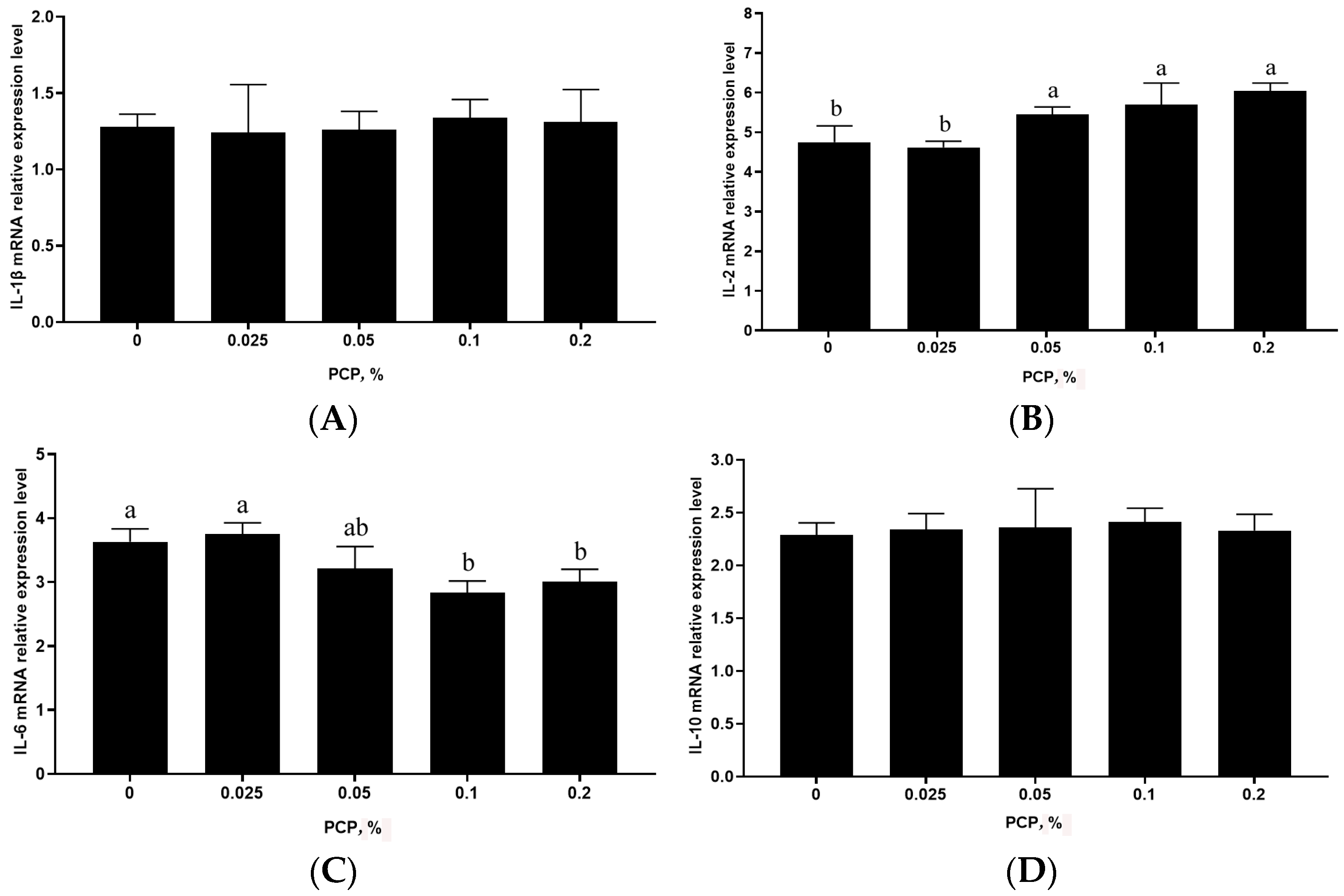
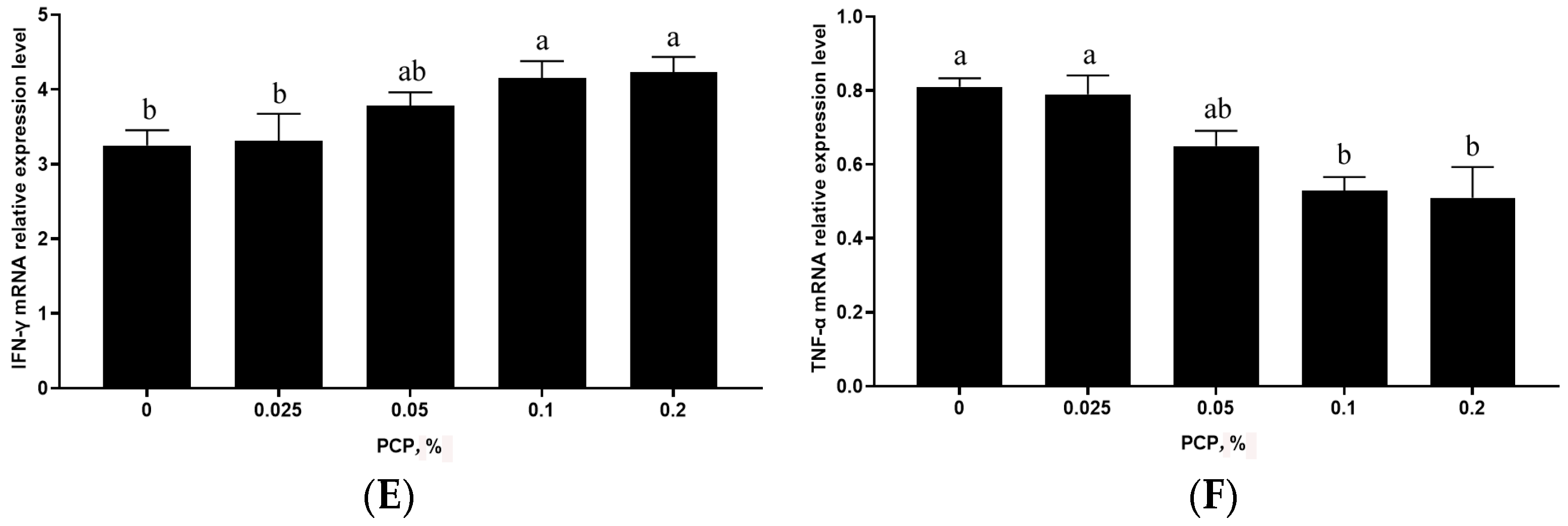
3.4. The Effect of PCP on Cytokine mRNA Levels in the Spleen
3.5. The Effect of PCP on the Cecal Microflora
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechnol. 2013, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Krishnasamy, V.; Otte, J.; Silbergeld, E. Antimicrobial use in Chinese swine and broiler poultry production. Antimicrob. Resis. Infect. Control 2015, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef]
- Marshall, B.M.; Levy, S.B. Food animals and antimicrobials: Impacts on human health. Clin. Microbiol. Rev. 2011, 24, 718–733. [Google Scholar] [CrossRef]
- Yang, H.; Paruch, L.; Chen, X.; van Eerde, A.; Skomedal, H.; Wang, Y.; Liu, D.; Liu, C.J. Antibiotic application and resistance in swine production in China: Current situation and future perspectives. Front. Vet. Sci. 2019, 6, 136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Shu, X.H.; Zhang, X.; Liu, Y.B.; Zhang, Y.J.; Lv, T.; Huang, X.; Song, C.L. Effects of two polysaccharides from traditional Chinese medicines on rat immune function. Front. Vet. Sci. 2021, 8, 703956. [Google Scholar] [CrossRef]
- Zou, T.; Yang, J.; Guo, X.; He, Q.; Wang, Z.; You, J. Dietary seaweed-derived polysaccharides improve growth performance of weaned pigs through maintaining intestinal barrier function and modulating gut microbial populations. J. Anim. Sci. Biotechnol. 2021, 12, 28. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, C.; Xie, H.; Wang, L.; Hu, J. Effect of Gan Cao (Glycyrrhiza uralensis Fisch) polysaccharide on growth performance, immune function, and gut microflora of broiler chickens. Poult. Sci. 2022, 101, 102068. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Z.; He, J.H. Achyranthes bidentata polysaccharide enhances immune response in weaned piglets. Immunopharmacol. Immunotoxicol. 2009, 31, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Maggi, E.; Vultaggio, A. Cellular and humoral immune responses during tuberculosis infection: Useful knowledge in the era of biological agents. J. Rheumatol. Suppl. 2014, 91, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, Z.; Pu, Y.; Bao, Y. Immunomodulatory effects exerted by Poria cocos polysaccharides via TLR4/TRAF6/NF-κB signaling in vitro and in vivo. Biomed. Pharmacother. 2019, 112, 108709. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Huang, G.; Khan, I.; Su, L.; Xia, W.; Law, B.Y.K.; Wong, V.K.W.; Wu, Q.; Wang, J.; Leong, W.K.; et al. Poria cocos polysaccharides exert prebiotic function to attenuate the adverse effects and improve the therapeutic outcome of 5-FU in ApcMin/+ mice. Chin. Med. 2022, 17, 116. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Ma, S.; Qiu, Z.; Huang, S.; Deng, G.; Li, Y.; Xu, S.; Yang, M.; Shi, H.; Wu, C.; et al. Poria cocos polysaccharides rescue pyroptosis-driven gut vascular barrier disruption in order to alleviates non-alcoholic steatohepatitis. J. Ethnopharmacol. 2022, 296, 115457. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, S.; Jiang, Y.; Wu, J.; Chen, L.; Ding, Y.; Zhou, Y.; Deng, L.; Chen, X. Poria cocos polysaccharide ameliorated antibiotic-associated diarrhea in mice via regulating the homeostasis of the gut microbiota and intestinal mucosal barrier. Int. J. Mol. Sci. 2023, 24, 1423. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Li, B.; Yu, B.; Chen, T.; Hu, Q.; Peng, B.; Sheng, W. Poria cocos polysaccharide induced Th1-type immune responses to ovalbumin in mice. PLoS ONE 2021, 16, e0245207. [Google Scholar] [CrossRef] [PubMed]
- Lestari, D.; Murtini, S.; Ulupi, N.; Gunawan, A.; Sumantri, C. Flow cytometric evaluation of CD4+ and CD8+ T-cell in IPB-D2 chickens with different Newcastle disease antibody titers level. Vet. World 2023, 16, 1161–1164. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, J.; Shu, D.; Cheng, X.; Tian, T.; Xiao, K.; Zhang, D.; Yang, J. Effect of plant polysaccharides (Poria cocos and Astragalus polysaccharides) on immune responses and intestinal microbiota of Dabry’s sturgeons. Biosci. Microbiota Food Health 2023, 49, 6772–6782. [Google Scholar] [CrossRef]
- Lu, J.; Huang, Z.; Ye, Y.; Xu, A.; Li, Z. Effects of Poria cocos polysaccharide on growth performance, physiological parameters, and lipid metabolism of spotted sea bass Lateolabrax maculatus. J. Ocean. Limnol. 2023, 11, 8. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, B.; Wang, Z.; Li, M.; Zhao, W. Natural polysaccharides with immunomodulatory activities. Mini. Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, S.; Li, H.; Zhao, C.; Ma, H.; Zhao, X.; Wu, J.; Liu, K.; Shan, J.; Wang, Y. Effect of a polysaccharide from Poria cocos on humoral response in mice immunized by H1N1 influenza and HBsAg vaccines. Int. J. Biol. Macromol. 2016, 91, 248–257. [Google Scholar] [CrossRef]
- Zhang, W.; Cheng, N.; Wang, Y.; Zheng, X.; Zhao, Y.; Wang, H.; Wang, C.; Han, Q.; Gao, Y.; Shan, J.; et al. Adjuvant activity of PCP-II, a polysaccharide from Poria cocos, on a whole killed rabies vaccine. Virus Res. 2019, 270, 197638. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Wang, L.; Chen, W.D.; Duan, Y.T.; Sun, M.J.; Huang, J.J.; Peng, D.Y.; Yu, N.J.; Wang, Y.Y.; Zhang, Y. Poria cocos polysaccharide prevents alcohol-induced hepatic injury and inflammation by repressing oxidative stress and gut leakiness. Front. Nutr. 2022, 9, 963598. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, L.; Feng, X.; Ibrahim, S.A.; Huang, W.; Liu, Y. Immunomodulatory activity of carboxymethyl pachymaran on immunosuppressed mice induced by cyclophosphamide. Molecules 2021, 26, 5733. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Ying, M.; Yu, Q.; Zheng, B.; Wang, H.; Wang, J.; Chen, S.; Nie, S.; Xie, M. Cultured Cordyceps sinensis polysaccharides modulate intestinal mucosal immunity and gut microbiota in cyclophosphamide-treated mice. Carbohydr. Polym. 2020, 235, 115957. [Google Scholar] [CrossRef]
- Liu, J.; Song, J.; Gao, F.; Chen, W.; Zong, Y.; Li, J.; He, Z.; Du, R. Extraction, purification, and structural characterization of polysaccharides from Sanghuangporus vaninii with anti-inflammatory activity. Molecules 2023, 28, 6081. [Google Scholar] [CrossRef]
- Alagbaoso, C.A.; Mizuno, M. Lentinula edodes polysaccharides suppressed pro-inflammatory cytokines expression and colitis in mice. Arq. Gastroenterol. 2022, 59, 288–295. [Google Scholar] [CrossRef]
- Lian, Y.Z.; Chang, C.C.; Chen, Y.S.; Tinkov, A.A.; Skalny, A.V.; Chao, J.C. Lycium barbarum polysaccharides and capsaicin modulate inflammatory cytokines and colonic microbiota in colitis rats induced by dextran sulfate sodium. J. Clin. Biochem. Nutr. 2022, 71, 229–237. [Google Scholar] [CrossRef]
- Dong, C. Cytokine regulation and function in T cells. Annu. Rev. Immunol. 2021, 39, 51–76. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Hou, L.; Sun, L.; Gao, J.; Gao, K.; Yang, X.; Jiang, Z.; Wang, L. Intestinal morphology and immune profiles are altered in piglets by early-weaning. Int. Immunopharmacol. 2022, 105, 108520. [Google Scholar] [CrossRef]
- Shan, Y.; Jiang, B.; Yu, J.; Wang, J.; Wang, X.; Li, H.; Wang, C.; Chen, J.; Sun, J. Protective effect of Schisandra chinensis polysaccharides against the immunological liver injury in mice based on Nrf2/ARE and TLR4/NF-κB signaling pathway. J. Med. Food 2019, 22, 885–895. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, W.; Zhang, S.; Meng, G.; Qi, C.; Fan, W.; Wang, Y.; Liu, J. The immune adjuvant response of polysaccharides from Atractylodis macrocephalae Koidz in chickens vaccinated against Newcastle disease (ND). Carbohydr. Polym. 2016, 141, 190–196. [Google Scholar] [CrossRef]
- Zhen, R.; Feng, J.; He, D.; Chen, Y.; Chen, T.; Cai, W.; Xiong, Y.; Qiu, Y.; Jiang, Z.; Wang, L.; et al. Effects of niacin on resistance to enterotoxigenic Escherichia coli infection in weaned piglets. Front. Nutr. 2022, 9, 865311. [Google Scholar] [CrossRef]
- Li, L.; Yin, F.; Wang, X.; Yang, C.; Yu, H.; Lepp, D.; Wang, Q.; Lessard, M.; Lo Verso, L.; Mondor, M.; et al. Microencapsulation protected Lactobacillus viability and its activity in modulating the intestinal microbiota in newly weaned piglets. J. Anim. Sci. 2023, 101, skad193. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.R.; Jian, C.; Uddin, M.K.; Huhtinen, M.; Salonen, A.; Peltoniemi, O.; Venhoranta, H.; Oliviero, C. Impact of intestinal microbiota on growth performance of suckling and weaned piglets. Microbiol. Spectr. 2023, 11, e0374422. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and utility of the antioxidant potential of probiotic Lactobacilli and Bifidobacteria as representatives of the human gut microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef]
- Novik, G.; Savich, V. Beneficial microbiota. Probiotics and pharmaceutical products in functional nutrition and medicine. Microbes Infect. 2020, 22, 8–18. [Google Scholar] [CrossRef]
| Ingredients | Content, % | Nutrient Levels 2 | Content, % |
|---|---|---|---|
| Corn | 59.4 | Digestible energy (MJ/kg) | 13.62 |
| Soybean meal | 19.7 | Crude protein (%) | 19.58 |
| Expanded soybean | 8.0 | Crude fat (%) | 3.90 |
| Whey | 3.6 | Calcium (%) | 0.91 |
| Soya oil | 2.0 | Available phosphorus (%) | 0.62 |
| Fish meal | 3.0 | Lysine (%) | 1.48 |
| NaCl | 0.3 | ||
| Premix 1 | 4.0 |
| Gene | ID | Sequence (5′–3′) | Annealing, °C | Extension, S | Product Length, bp |
|---|---|---|---|---|---|
| IL-1β | NM_214055 | GCTGGATGCTCCCATTTCTCA GAAAGCCCAATTCAGGGACCC | 60 | 40 | 217 |
| IL-2 | JN851821 | AACTGTAAATCCAGCAGCAAT ACAATGGGTAAGATGCAGCTC | 60 | 40 | 131 |
| IL-6 | AF518322 | TTTGCCGAGGATGTACTTAA ATGAACTCCCTCTCCACAAGC | 60 | 40 | 190 |
| IL-10 | HQ236499 | ACGCCCATCTGGTCCTTCGTT ATGCCCAGCTCAGCACTGCTC | 60 | 40 | 176 |
| TNF-α | JF831365 | CGGGCTTATCTGAGGTTTGAG GACACCATGAGCACTGAGAGC | 60 | 40 | 268 |
| IFN-γ | NM_213948 | AAAAGAGGTCCACCATTAGG CAGAAGCTAACTCTCTCCGAA | 60 | 40 | 179 |
| β-actin | U07786 | CGTGGTGGTGAAGCTGTAGCC ATGTTTGAGACCTTCAACACGC | 60 | 40 | 243 |
| PCP Level, % | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | 0 | 0.025 | 0.05 | 0.1 | 0.2 | SEM | Groups | Linear | Quadratic |
| IBW, kg | 8.51 | 8.58 | 8.46 | 8.48 | 8.52 | 0.126 | 0.983 | 0.842 | 0.968 |
| FBW, kg | 24.34 b | 24.81 b | 25.72 ab | 26.31 a | 26.13 a | 0.641 | 0.031 | 0.066 | 0.311 |
| ADG, g/d | 376.90 c | 386.43 c | 410.95 b | 424.52 a | 419.29 a | 13.332 | 0.001 | 0.021 | 0.239 |
| ADFI, g/d | 723.07 | 732.84 | 722.44 | 731.19 | 734.52 | 35.403 | 0.683 | 0.304 | 0.601 |
| Gain/feed ratio | 0.52 b | 0.53 b | 0.57 a | 0.58 a | 0.57a | 0.002 | 0.001 | 0.031 | 0.467 |
| PCP Level, % | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | 0 | 0.025 | 0.05 | 0.1 | 0.2 | SEM | Groups | Linear | Quadratic |
| IgG (g/L) | 3.45 c | 4.66 b | 5.16 a | 5.48 a | 5.61 a | 0.130 | 0.001 | 0.009 | 0.878 |
| IgA (g/L) | 0.20 b | 0.27 ab | 0.33 a | 0.34 a | 0.33 a | 0.051 | 0.028 | 0.298 | 0.456 |
| IgM (g/L) | 0.67 | 0.64 | 0.67 | 0.70 | 0.65 | 0.011 | 0.423 | 0.654 | 0.751 |
| IL-1β (ng/L) | 2.33 | 2.26 | 2.37 | 2.41 | 2.34 | 0.156 | 0.541 | 0.342 | 0.611 |
| IL-2 (ng/L) | 36.51 c | 40.46 b | 42.64 a | 44.60 a | 44.63 a | 1.317 | 0.005 | 0.011 | 0.722 |
| IL-6 (ng/L) | 16.45 a | 15.61 ab | 14.29 b | 14.33 b | 14.38 b | 0.803 | 0.030 | 0.053 | 0.675 |
| IL-10 (ng/L) | 11.23 | 11.47 | 12.01 | 11.56 | 11.29 | 0.396 | 0.134 | 0.546 | 0.203 |
| IFN-γ (ng/L) | 24.34 c | 24.52 c | 26.35 b | 28.01 a | 28.16 a | 1.014 | 0.015 | 0.047 | 0.410 |
| TNF-α (ng/L) | 0.63 a | 0.51 ab | 0.47 ab | 0.43 b | 0.41 b | 0.023 | 0.016 | 0.030 | 0.313 |
| PCP Level, % | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | 0 | 0.025 | 0.05 | 0.1 | 0.2 | SEM | Groups | Linear | Quadratic |
| CD3+ | 65.44 | 67.30 | 65.41 | 66.15 | 66.07 | 4.243 | 0.268 | 0.381 | 0.524 |
| CD4+ | 41.19 b | 41.72 b | 43.56 ab | 45.20 a | 45.34 a | 2.327 | 0.021 | 0.046 | 0.365 |
| CD8+ | 33.45 | 33.51 | 34.05 | 34.23 | 34.11 | 2.134 | 0.163 | 0.113 | 0.201 |
| CD4+/CD8+ | 1.23 b | 1.25 b | 1.28 ab | 1.32 a | 1.33 a | 0.057 | 0.036 | 0.025 | 0.337 |
| PCP Level, % | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Item | 0 | 0.025 | 0.05 | 0.1 | 0.2 | SEM | Groups | Linear | Quadratic |
| Lactobacilli | 6.58 b | 6.85 ab | 7.20 a | 7.41 a | 7.36 a | 0.151 | 0.014 | 0.109 | 0.351 |
| Bifidobacteria | 7.32 c | 7.39 c | 7.88 b | 8.34 a | 8.31 a | 0.175 | 0.021 | 0.074 | 0.233 |
| Escherichia coli | 5.24 a | 5.01 a | 4.62 b | 4.45 b | 4.38 b | 0.086 | 0.011 | 0.020 | 0.425 |
| Salmonella | 4.81 a | 4.56 b | 4.33 b | 3.50 c | 3.44 c | 0.062 | 0.003 | 0.012 | 0.316 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Wang, H.; Meng, S.; Zhang, C.; Guo, L.; Miao, Z. The Effects of Poria cocos Polysaccharides on Growth Performance, Immunity, and Cecal Microflora Composition of Weaned Piglets. Animals 2024, 14, 1121. https://doi.org/10.3390/ani14071121
Zhang J, Wang H, Meng S, Zhang C, Guo L, Miao Z. The Effects of Poria cocos Polysaccharides on Growth Performance, Immunity, and Cecal Microflora Composition of Weaned Piglets. Animals. 2024; 14(7):1121. https://doi.org/10.3390/ani14071121
Chicago/Turabian StyleZhang, Jinzhou, Heming Wang, Shuaitao Meng, Chuankuan Zhang, Liping Guo, and Zhiguo Miao. 2024. "The Effects of Poria cocos Polysaccharides on Growth Performance, Immunity, and Cecal Microflora Composition of Weaned Piglets" Animals 14, no. 7: 1121. https://doi.org/10.3390/ani14071121
APA StyleZhang, J., Wang, H., Meng, S., Zhang, C., Guo, L., & Miao, Z. (2024). The Effects of Poria cocos Polysaccharides on Growth Performance, Immunity, and Cecal Microflora Composition of Weaned Piglets. Animals, 14(7), 1121. https://doi.org/10.3390/ani14071121






