Potential Food Inclination of Crab-Eating Macaques in Laboratory Environments: Enhancing Positive Reinforcement Training and Health Optimization
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Study Animals
2.3. Animal Husbandry
2.4. Experimental Procedure
2.5. Analysis of Potential Food Inclination
2.6. Comparison of Weight Gain and Food Item Eaten/Provided Rates
2.7. Comparison of Total Calorie Intake and Food Item Eaten/Provided Rates
2.8. Statistical Analysis
3. Results
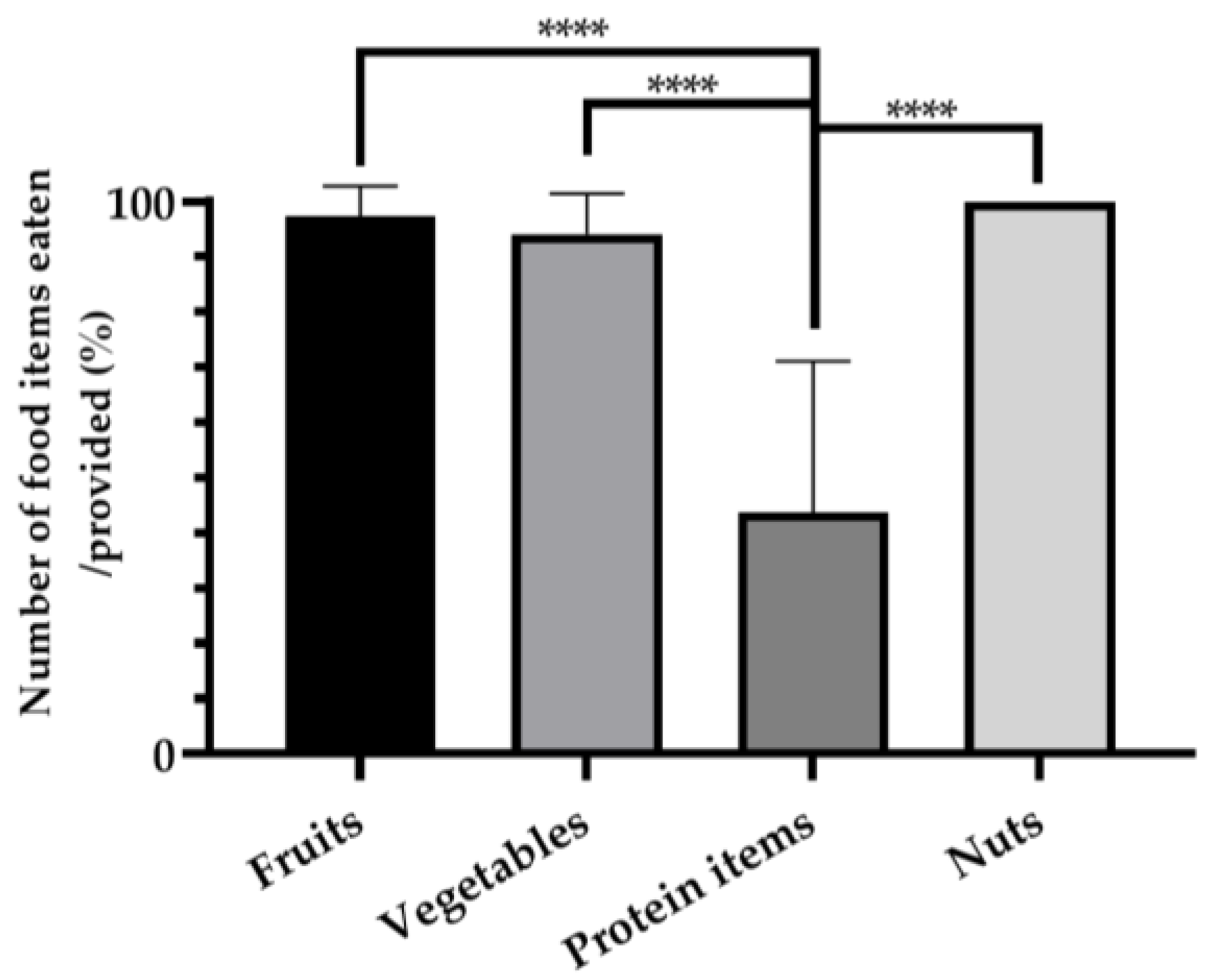
3.1. Consumption Ratios per Food Item Category
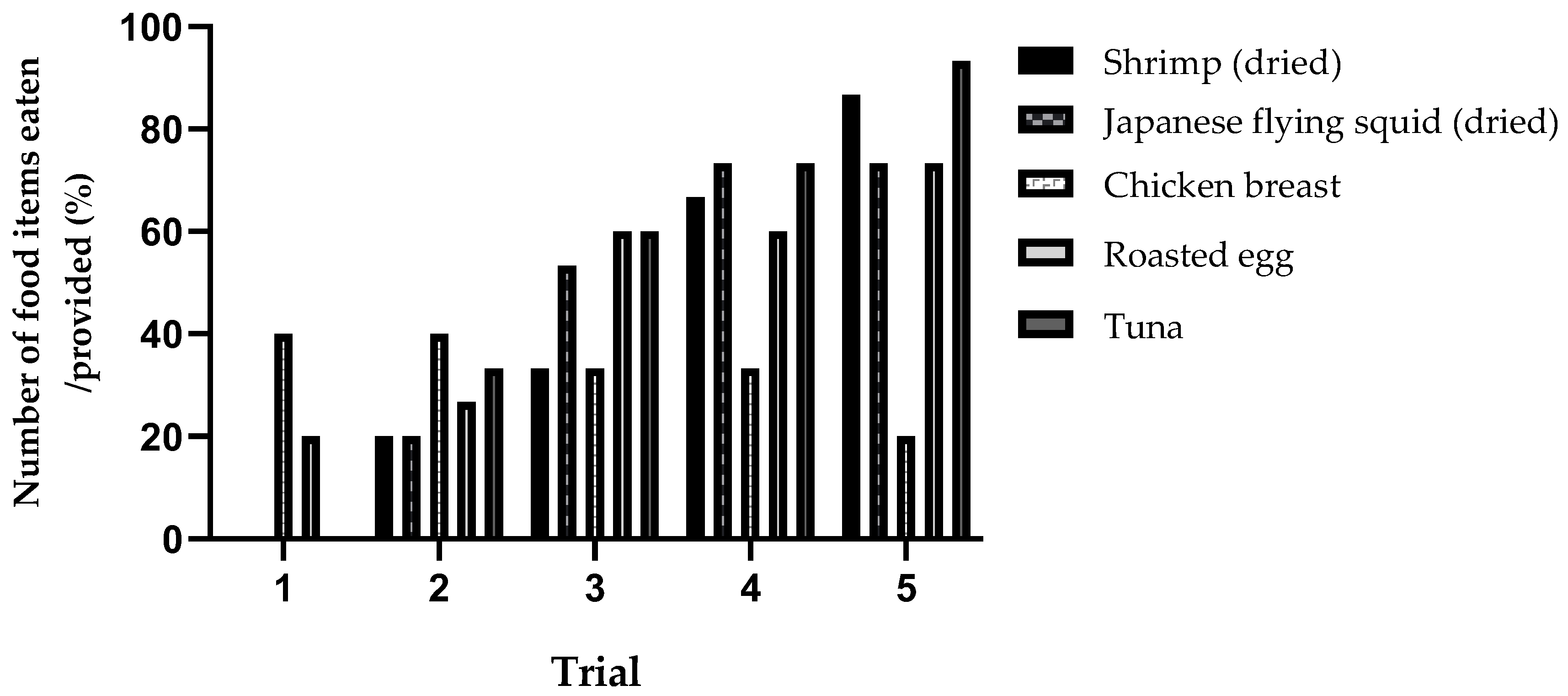
3.2. Variations in Food Consumption across Trials
3.3. Individual Consumption Ratio of Protein Items across Trials
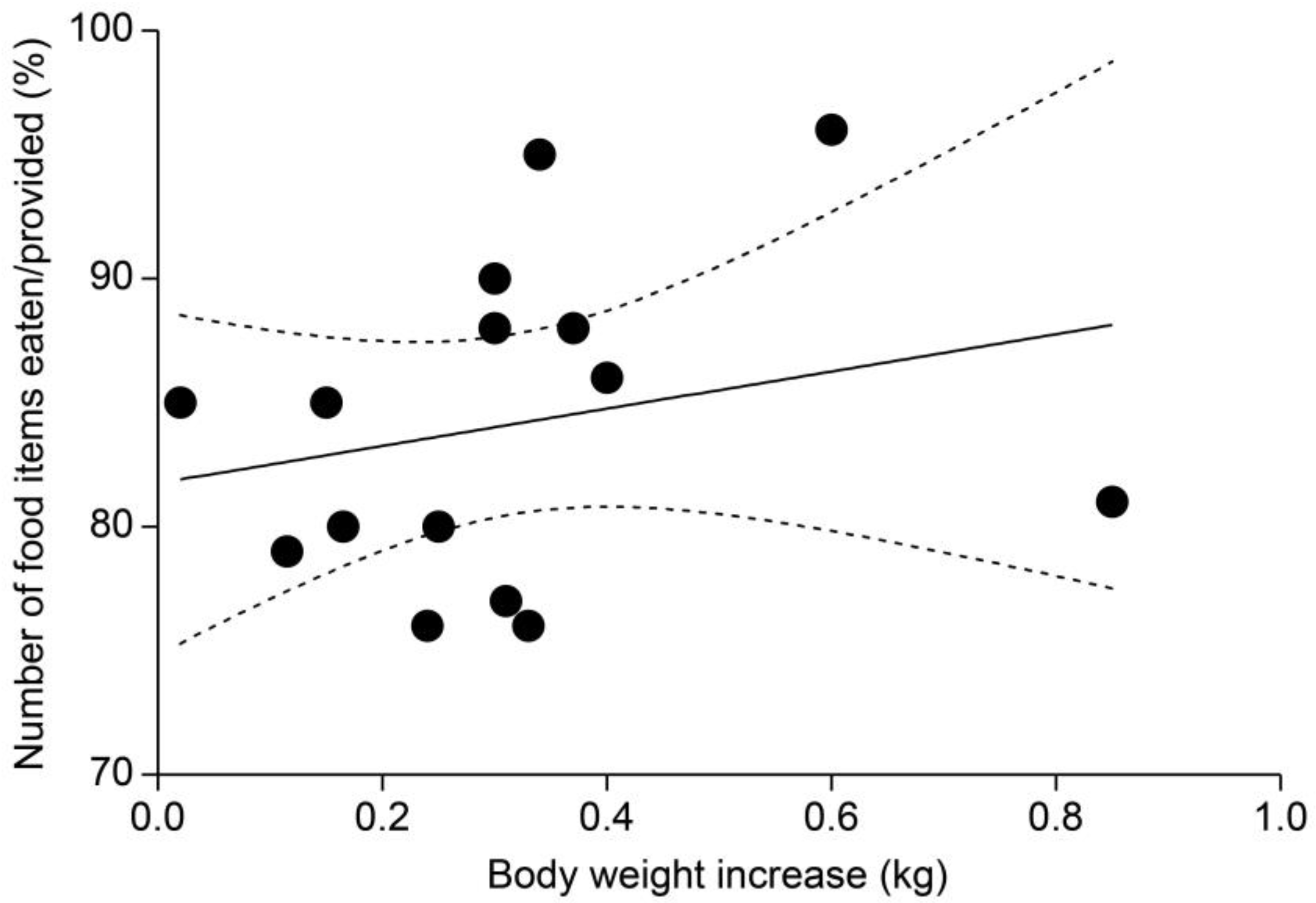
3.4. Comparison of Weight Gain and Food Item Consumption Ratios
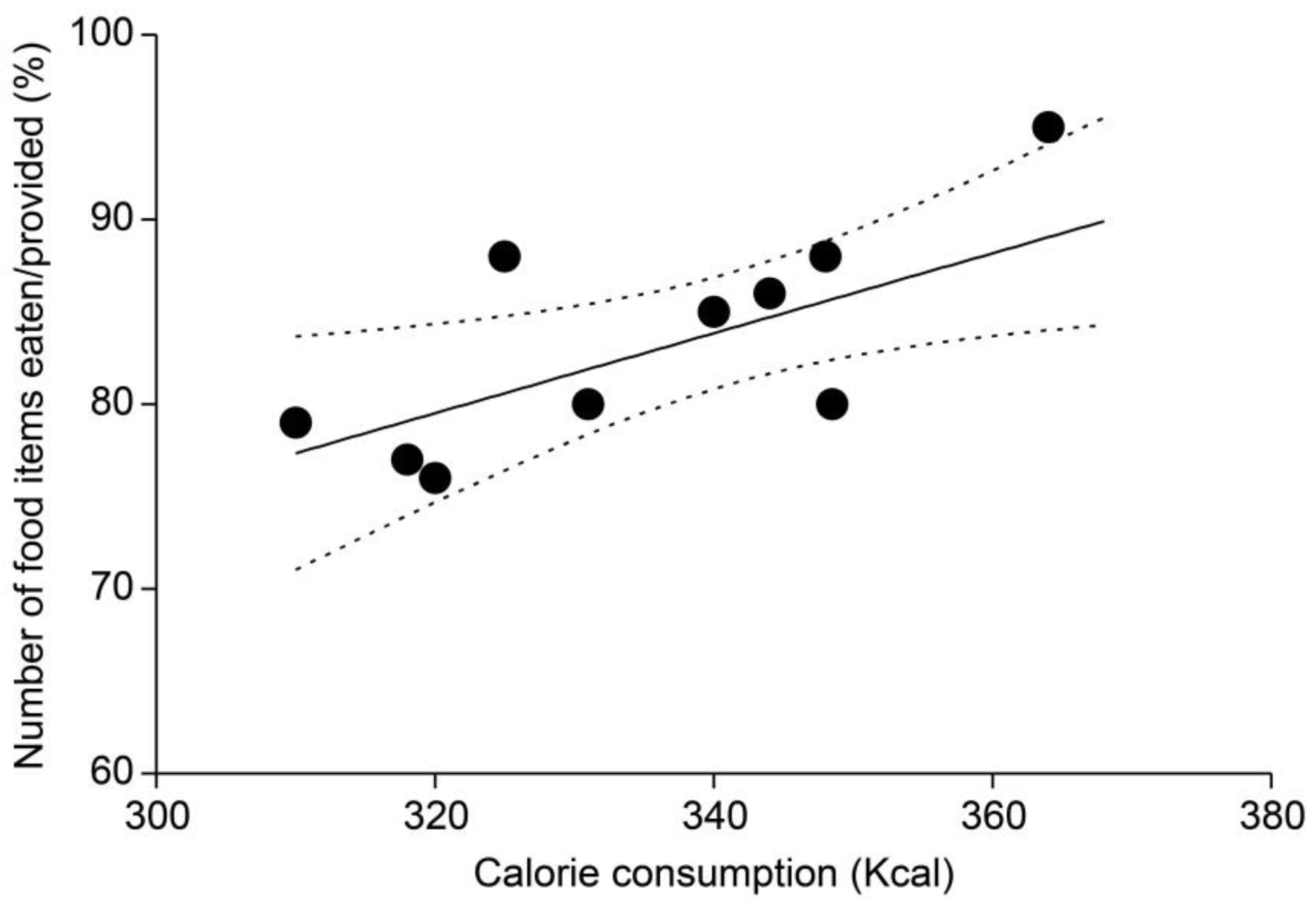
3.5. Comparison of Total Caloric Consumption and Food Item Consumption Ratios
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Groves, C. Primate Taxonomy; Smithsonian Institution Press: Washington, DC, USA, 2001. [Google Scholar]
- Gamalo, L.E.; Ilham, K.; Jones-Engel, L.; Gill, M.; Sweet, R.; Aldrich, B.; Phiapalath, P.; Van Bang, T.; Ahmed, T.; Kite, S. Removal from the wild endangers the once widespread long-tailed macaque. Am. J. Primatol. 2023, 86, e23547. [Google Scholar] [CrossRef] [PubMed]
- Fooden, J. Systematic review of Southeast Asian long-tailed macaques, Macaca fasciculari (Raffles, 1821). Fieldiana Zool. 1995, 64, iv+44. [Google Scholar]
- Zhang, S.-J.; Liu, C.-J.; Shi, M.; Kong, L.; Chen, J.-Y.; Zhou, W.-Z.; Zhu, X.; Yu, P.; Wang, J.; Yang, X. RhesusBase: A knowledgebase for the monkey research community. Nucleic Acids Res. 2013, 41, D892–D905. [Google Scholar] [CrossRef] [PubMed]
- Cyranoski, D. Monkey kingdom. Nature 2016, 532, 300–302. [Google Scholar] [CrossRef]
- Kito, G.; Nishimura, A.; Susumu, T.; Nagata, R.; Kuge, Y.; Yokota, C.; Minematsu, K. Experimental thromboembolic stroke in cynomolgus monkey. J. Neurosci. Methods 2001, 105, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Buse, E. Development of the immune system in the cynomolgus monkey: The appropriate model in human targeted toxicology? J. Immunotoxicol. 2005, 2, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yang, Z.; Rodrigues, A.D. Cynomolgus monkey as an emerging animal model to study drug transporters: In vitro, in vivo, in vitro-to-in vivo translation. Drug Metab. Dispos. 2022, 50, 299–319. [Google Scholar] [CrossRef]
- Liu, N.; Yue, F.; Tang, W.; Chan, P. An objective measurement of locomotion behavior for hemiparkinsonian cynomolgus monkeys. J. Neurosci. Methods 2009, 183, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Camus, S.M.; Blois-Heulin, C.; Li, Q.; Hausberger, M.; Bezard, E. Behavioural profiles in captive-bred cynomolgus macaques: Towards monkey models of mental disorders? PLoS ONE 2013, 8, e62141. [Google Scholar] [CrossRef]
- Buchanan, K.; Burt de Perera, T.; Carere, C.; Carter, T.; Hailey, A.; Hubrecht, R.; Jennings, D.; Metcalfe, N.; Pitcher, T.; Peron, F. Guidelines for the treatment of animals in behavioural research and teaching. Anim. Behav. 2012, 83, 301–309. [Google Scholar]
- Martin, A.L.; Franklin, A.N.; Perlman, J.E.; Bloomsmith, M.A. Systematic assessment of food item preference and reinforcer effectiveness: Enhancements in training laboratory-housed rhesus macaques. Behav. Process. 2018, 157, 445–452. [Google Scholar] [CrossRef]
- Schapiro, S.J.; Bloomsmith, M.A.; Laule, G.E. Positive reinforcement training as a technique to alter nonhuman primate behavior: Quantitative assessments of effectiveness. J. Appl. Anim. Welf. Sci. 2003, 6, 175–187. [Google Scholar] [CrossRef]
- Prescott, M.J.; Brown, V.J.; Flecknell, P.A.; Gaffan, D.; Garrod, K.; Lemon, R.N.; Parker, A.J.; Ryder, K.; Schultz, W.; Scott, L. Refinement of the use of food and fluid control as motivational tools for macaques used in behavioural neuroscience research: Report of a Working Group of the NC3Rs. J. Neurosci. Methods 2010, 193, 167–188. [Google Scholar] [CrossRef]
- Fischer, B.; Wegener, D. Emphasizing the “positive” in positive reinforcement: Using nonbinary rewarding for training monkeys on cognitive tasks. J. Neurophysiol. 2018, 120, 115–128. [Google Scholar] [CrossRef]
- Clay, A.W.; Bloomsmith, M.A.; Marr, M.J.; Maple, T.L. Systematic investigation of the stability of food preferences in captive orangutans: Implications for positive reinforcement training. J. Appl. Anim. Welf. Sci. 2009, 12, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Laule, G.; Whittaker, M. Enhancing nonhuman primate care and welfare through the use of positive reinforcement training. J. Appl. Anim. Welf. Sci. 2007, 10, 31–38. [Google Scholar] [CrossRef]
- Laule, G.E.; Bloomsmith, M.A.; Schapiro, S.J. The use of positive reinforcement training techniques to enhance the care, management, and welfare of primates in the laboratory. In Training Nonhuman Primates Using Positive Reinforcement Techniques; Psychology Press: New York, NY, USA, 2016; pp. 163–173. [Google Scholar]
- Renner, M.J.; Feiner, A.J.; Orr, M.G.; Delaney, B.A. Environmental enrichment for New World primates: Introducing food-irrelevant objects and direct and secondary effects. J. Appl. Anim. Welf. Sci. 2000, 3, 23–32. [Google Scholar] [CrossRef]
- Turner, P.V.; Grantham II, L.E. Short-term effects of an environmental enrichment program for adult cynomolgus monkeys. J. Am. Assoc. Lab. Anim. Sci. 2002, 41, 13–17. [Google Scholar]
- Verspeek, J.; Stevens, J.M. Food preference and nutrient composition in captive bonobos (Pan paniscus). Primates 2020, 61, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Scherbaum, C.; Estrada, A. Selectivity in feeding preferences and ranging patterns in spider monkeys Ateles geoffroyi yucatanensis of northeastern Yucatan peninsula, Mexico. Curr. Zool. 2013, 59, 125–134. [Google Scholar] [CrossRef][Green Version]
- Addessi, E.; Stammati, M.; Sabbatini, G.; Visalberghi, E. How tufted capuchin monkeys (Cebus apella) rank monkey chow in relation to other foods. Anim. Welf. 2005, 14, 215–222. [Google Scholar] [CrossRef]
- Huskisson, S.M.; Jacobson, S.L.; Egelkamp, C.L.; Ross, S.R.; Hopper, L.M. Using a touchscreen paradigm to evaluate food preferences and response to novel photographic stimuli of food in three primate species (Gorilla gorilla gorilla, Pan troglodytes, and Macaca fuscata). Int. J. Primatol. 2020, 41, 5–23. [Google Scholar] [CrossRef]
- Rehrig, A.; DiVincenti, L.; Napolitano, D.; McAdam, D. Assessing Food Preference and Reinforcer Effectiveness in Laboratory-Housed Cynomolgus Macaques (Macaca fascicularis): A Refinement for Positive Reinforcement Training. Am. J. Primatol. 2019, 81. [Google Scholar]
- Visalberghi, E.; Fragaszy, D. The behaviour of capuchin monkeys, Cebus apella, with novel food: The role of social context. Anim. Behav. 1995, 49, 1089–1095. [Google Scholar] [CrossRef]
- Addessi, E.; Chiarotti, F.; Visalberghi, E.; Anzenberger, G. Response to novel food and the role of social influences in common marmosets (Callithrix jacchus) and Goeldi’s monkeys (Callimico goeldii). Am. J. Primatol. 2007, 69, 1210–1222. [Google Scholar] [CrossRef] [PubMed]
- Visalberghi, E.; Addessi, E. Seeing group members eating a familiar food enhances the acceptance of novel foods in capuchin monkeys. Anim. Behav. 2000, 60, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Springer, D.A.; Baker, K.C. Effect of ketamine anesthesia on daily food intake in Macaca mulatta and Cercopithecus aethiops. Am. J. Primatol. 2007, 69, 1080–1092. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.F. Nutritional maintenance of the oral fracture patient. Oral Surg. Oral Med. Oral Pathol. 1965, 19, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Foltin, R.W. The behavioral pharmacology of anorexigenic drugs in nonhuman primates: 30 years of progress. Behav. Pharmacol. 2012, 23, 461–477. [Google Scholar] [CrossRef][Green Version]
- Hogenbirk, R.N.; van der Plas, W.Y.; Hentzen, J.E.; van Wijk, L.; Wijma, A.G.; Buis, C.I.; Viddeleer, A.R.; de Bock, G.H.; van der Schans, C.P.; van Dam, G.M. Postoperative muscle loss, protein intake, physical activity and outcome associations. Br. J. Surg. 2023, 110, 183–192. [Google Scholar] [CrossRef]
- Thomson, R.L.; Buckley, J.D. Protein hydrolysates and tissue repair. Nutr. Res. Rev. 2011, 24, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.G. Laboratory Animal Medicine; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Sandhyamani, S.; Vijayakumari, A.; Nair, M.B. Bonnet monkey model for pancreatic changes in induced malnutrition. Pancreas 1999, 18, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.-S.; Lee, D.-H.; Kang, P.; Jeong, K.-J.; Lee, S.; Kim, K.; Lee, Y.; Huh, J.-W.; Kim, Y.-H.; Park, S.-J. Reference values of hematological and biochemical parameters in young-adult cynomolgus monkey (Macaca fascicularis) and rhesus monkey (Macaca mulatta) anesthetized with ketamine hydrochloride. Lab. Anim. Res. 2019, 35, 7. [Google Scholar] [CrossRef] [PubMed]
- Vonk, J.; Truax, J.; McGuire, M.C. A food for all seasons: Stability of food preferences in gorillas across testing methods and seasons. Animals 2022, 12, 685. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Guide for the Care and Use of Laboratory Animals; US Department of Health and Human Services; Public Health Service; National Institutes of Health: Washington, DC, USA, 2010.
- Food and Nutrition Ingredients Database Ministry of Food and Drug Safey. Available online: https://various.foodsafetykorea.go.kr/nutrient/ (accessed on 24 January 2024).
- Wilson, M.E.; Fisher, J.; Fischer, A.; Lee, V.; Harris, R.B.; Bartness, T.J. Quantifying food intake in socially housed monkeys: Social status effects on caloric consumption. Physiol. Behav. 2008, 94, 586–594. [Google Scholar] [CrossRef]
- Cameron, K.E.; Bizo, L.A.; Starkey, N.J. Food preferences of the brushtail possum (Trichosurus vulpecula). Int. J. Comp. Psychol. 2013, 26, 324–336. [Google Scholar] [CrossRef]
- Buchanan-Smith, H.M. Environmental enrichment for primates in laboratories. Adv. Sci. Res. 2011, 5, 41–56. [Google Scholar] [CrossRef]
- Lutz, C.K.; Novak, M.A. Environmental enrichment for nonhuman primates: Theory and application. ILAR J. 2005, 46, 178–191. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-H.; Lee, L.-L. Food habits of Formosan rock macaques (Macaca cyclopis) in Jentse, northeastern Taiwan, assessed by fecal analysis and behavioral observation. Int. J. Primatol. 2001, 22, 359–377. [Google Scholar] [CrossRef]
- Schülke, O.; Pesek, D.; Whitman, B.J.; Ostner, J. Ecology of Assamese macaques (Macaca assamensis) at Phu Khieo Wildlife Sanctuary, Thailand. J. Wildl. Thail. 2011, 18, 1–15. [Google Scholar]
- Wiafe, E.D. Nutrient contents of three commonly consumed fruits of lowe’s monkey (Cercopithecus campbelii lowei). SpringerPlus 2015, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Rowe, N.; Myers, M. All the World’s Primates; Pogonias Press Charlestown: Boston, MA, USA, 2016; Volume 777. [Google Scholar]
- Kitamura, S.; Yumoto, T.; Poonswad, P.; Chuailua, P.; Plongmai, K.; Maruhashi, T.; Noma, N. Interactions between fleshy fruits and frugivores in a tropical seasonal forest in Thailand. Oecologia 2002, 133, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Nila, S.; Suryobroto, B.; Widayati, K.A. Dietary variation of long tailed macaques (Macaca fascicularis) in Telaga Warna, Bogor, West Java. HAYATI J. Biosci. 2014, 21, 8–14. [Google Scholar] [CrossRef]
- Felton, A.M.; Felton, A.; Raubenheimer, D.; Simpson, S.J.; Foley, W.J.; Wood, J.T.; Wallis, I.R.; Lindenmayer, D.B. Protein content of diets dictates the daily energy intake of a free-ranging primate. Behav. Ecol. 2009, 20, 685–690. [Google Scholar] [CrossRef]
- Kassim, N.; Hambali, K.; Amir, A. Nutritional composition of fruits selected by long-tailed macaques (Macaca fascicularis) in Kuala Selangor, Malaysia. Trop. Life Sci. Res. 2017, 28, 91. [Google Scholar] [CrossRef]
- McLennan, M.R.; Spagnoletti, N.; Hockings, K.J. The implications of primate behavioral flexibility for sustainable human–primate coexistence in anthropogenic habitats. Int. J. Primatol. 2017, 38, 105–121. [Google Scholar] [CrossRef]
- Hansen, M.; Ang, A.; Trinh, T.; Sy, E.; Paramasivam, S.; Ahmed, T.; Dimalibot, J.; Jones-Engel, L.; Ruppert, N.; Griffioen, C. Macaca fascicularis (amended version of 2022 assessment). IUCN Red List Threat. Species 2022. [Google Scholar]
- Fooden, J. Comparative review of fascicularis-group species of macaques (Primates: Macaca). Fieldiana Zool. 2006, 2006, 1–43. [Google Scholar]
- Hilborn, R.; Smith, D.R. Is the long-tailed macaque at risk of extinction? Am. J. Primatol. 2023, 86, e23590. [Google Scholar] [CrossRef]
- Moore, J.H.; Gibson, L.; Amir, Z.; Chanthorn, W.; Ahmad, A.H.; Jansen, P.A.; Mendes, C.P.; Onuma, M.; Peres, C.A.; Luskin, M.S. The rise of hyperabundant native generalists threatens both humans and nature. Biol. Rev. 2023, 98, 1829–1844. [Google Scholar] [CrossRef]
- Gumert, M.D. The common monkey of Southeast Asia: Longtailed macaque populations, ethnophoresy, and their occurrence in human environments. In Ecology and Management of Long-Tailed Macaques and their Interface with Humans; Cambridge University Press: Cambridge, UK, 2011; pp. 3–44. [Google Scholar]
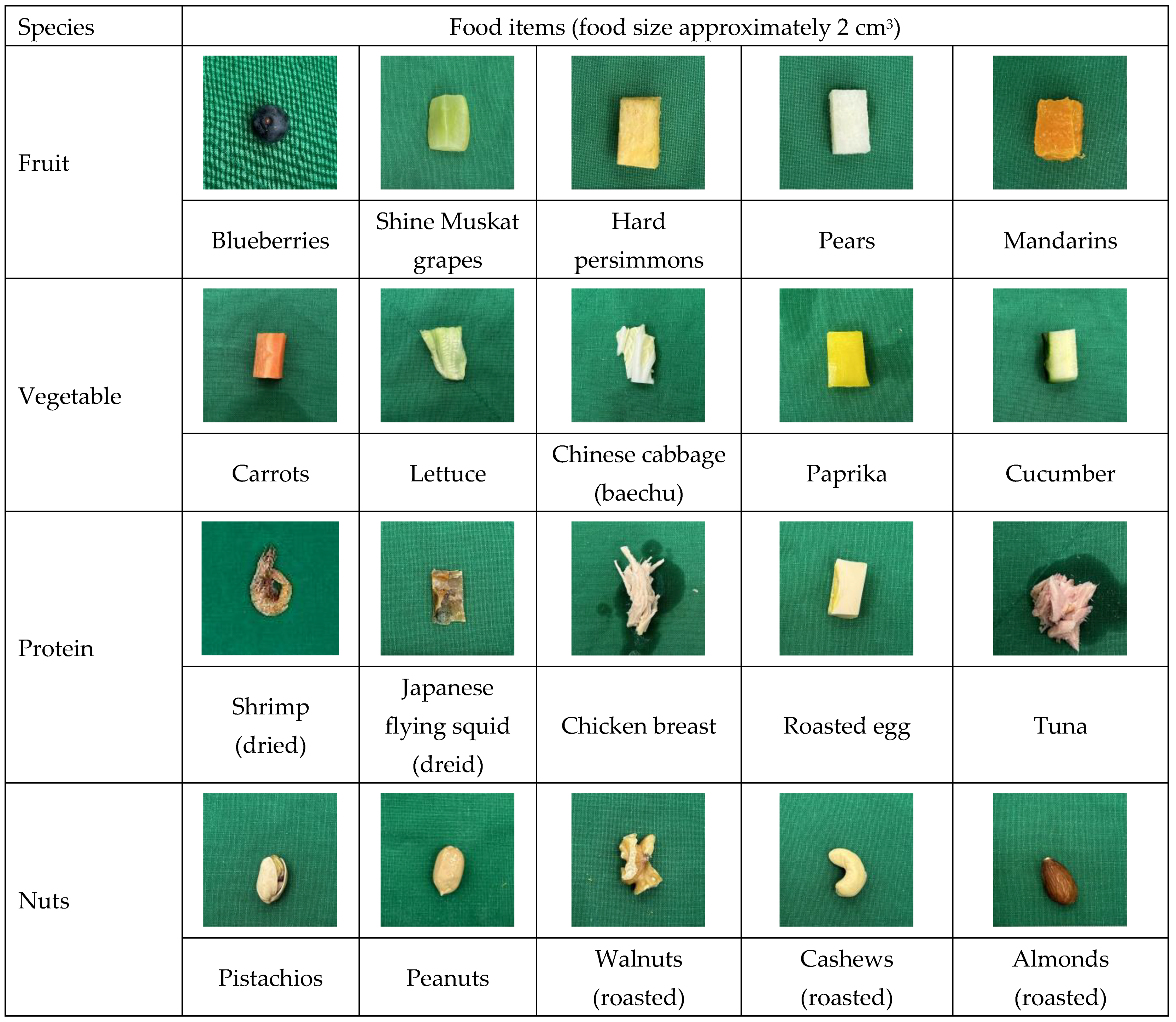

| Energy (Kcal) | Protein (g) | Lipids (g) | Carbohydrates (g) | Total Sugars (g) | Cholesterol (g) | Total Fatty Acids (g) | ||
|---|---|---|---|---|---|---|---|---|
| Fruits | Blueberries | 48 | 0.55 | 0.09 | 12.57 | 9.96 | 0.00 | 0.08 |
| Shine Muskat grapes | 66 | 0.38 | 0.10 | 17.66 | 15.29 | 0.00 | 0.05 | |
| Hard persimmons | 51 | 0.41 | 0.04 | 13.66 | 10.52 | 0.00 | 0.04 | |
| Pears | 45 | 0.29 | 0.04 | 12.24 | 8.30 | 0.00 | 0.04 | |
| Mandarins | 39 | 0.53 | 0.10 | 10.04 | 7.99 | 0.00 | 0.06 | |
| Vegetables | Carrots | 31 | 1.02 | 0.13 | 7.03 | 6.23 | 0.00 | 0.12 |
| Lettuce | 15 | 1.06 | 0.10 | 3.35 | 2.17 | 0.00 | 0.09 | |
| Chinese cabbage (baechu) | 15 | 1.25 | 0.04 | 3.20 | 1.75 | 0.00 | 0.04 | |
| Paprika | 24 | 0.77 | 0.20 | 5.95 | 2.79 | 0.00 | 0.12 | |
| Cucumber | 14 | 1.22 | 0.02 | 0.05 | 138.00 | 0.00 | 0.02 | |
| Proteinitems | Shrimp (dried) | 319 | 28.50 | 2.00 | 46.00 | 0.00 | 0.00 | 0.00 |
| Japanese flying squid (dried) | 352 | 67.80 | 6.90 | 52.00 | 0.00 | 0.00 | 0.00 | |
| Chicken breast | 127 | 28.00 | 0.93 | 0.00 | 0.00 | 74.54 | 0.89 | |
| Roasted egg | 142 | 14.20 | 6.90 | 5.70 | 0.00 | 250.4 | 2.4 | |
| Tuna | 216 | 19.00 | 15.00 | 0.00 | 0.00 | 45 | 0.00 | |
| Nuts | Pistachios | 584 | 25.99 | 48.89 | 20.82 | 8.15 | 0.00 | 46.76 |
| Peanuts | 567 | 28.50 | 46.24 | 19.91 | 5.18 | 0.00 | 44.23 | |
| Walnuts (roasted) | 713 | 14.60 | 68.80 | 11.70 | 2.50 | 0.00 | 67.41 | |
| Cashews (roasted) | 581 | 16.84 | 47.77 | 30.16 | 5.01 | 0.00 | 42.95 | |
| Almonds (roasted) | 594 | 23.45 | 51.29 | 20.49 | 3.91 | 0.00 | 49.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.W.; Lee, Y.B.; Hong, Y.S.; Jung, H.; Lee, G.-H. Potential Food Inclination of Crab-Eating Macaques in Laboratory Environments: Enhancing Positive Reinforcement Training and Health Optimization. Animals 2024, 14, 1123. https://doi.org/10.3390/ani14071123
Kim JW, Lee YB, Hong YS, Jung H, Lee G-H. Potential Food Inclination of Crab-Eating Macaques in Laboratory Environments: Enhancing Positive Reinforcement Training and Health Optimization. Animals. 2024; 14(7):1123. https://doi.org/10.3390/ani14071123
Chicago/Turabian StyleKim, Ji Woon, Yoon Beom Lee, Yeon Su Hong, Hoesu Jung, and Gwang-Hoon Lee. 2024. "Potential Food Inclination of Crab-Eating Macaques in Laboratory Environments: Enhancing Positive Reinforcement Training and Health Optimization" Animals 14, no. 7: 1123. https://doi.org/10.3390/ani14071123
APA StyleKim, J. W., Lee, Y. B., Hong, Y. S., Jung, H., & Lee, G.-H. (2024). Potential Food Inclination of Crab-Eating Macaques in Laboratory Environments: Enhancing Positive Reinforcement Training and Health Optimization. Animals, 14(7), 1123. https://doi.org/10.3390/ani14071123







