Analysis of Endangered Andalusian Black Cattle (Negra Andaluza) Reveals Genetic Reservoir for Bovine Black Trunk
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Pedigree Database and Software Tool
2.2. Population Summary Statistics
2.3. Inbreeding, Coancestry, and Assortative Mating Degree
2.4. Ancestral Contributions and Probabilities of Gene Origin
2.5. Herd, Municipality, and Province Relationships
3. Results
3.1. Population Summary Statistics
3.2. Inbreeding, Coancestry, and Degree of Non-Random Mating
3.3. Probabilities of Gene Origin and Ancestral Contributions
3.4. Herd, Municipality, and Province Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- González, A.; Herrera, M.; Villafuerte, J.; Peña Blanco, F.; Rodero, E. Caracterización etnológica de la raza bovina negra andaluza. In Proceedings of the IV Jornadas Ibéricas de Razas Autóctonas y Sus Productos Tradicionales: Innovación, Seguridad y Cultura Alimentaria, Seville, Spain, 14–16 November 2007; pp. 379–384. [Google Scholar]
- Spanish Ministry of Agricultrue, Fisheries and Food. Raza Bovina NEGRA ANDALUZA. Available online: https://www.mapa.gob.es/es/ganaderia/temas/zootecnia/razas-ganaderas/razas/catalogo-razas/bovino/negra-andaluza/default.aspx (accessed on 1 February 2024).
- Consejería de Agricultura, Ganadería, Pesca Y Desarrollo Sostenible. Programa De Cría De La Raza Bovina Negra Andaluza; Juanta de Andalucñía: Sevilla, Spain, 2020. [Google Scholar]
- Nogales, S.; Albardonedo, D.; Recio, J.; Delgado, J.; Camacho, M. First results in the study of the morphology present status of the Andalusian Black cattle breed. Arch. Zootec. 2011, 60, 397–399. [Google Scholar] [CrossRef]
- FEAGAS. Negra Andaluza. Available online: https://rfeagas.es/razas/bovino/negra-andaluza/ (accessed on 1 February 2024).
- Rodero Serrano, E. Las razas autóctonas y los sistemas extensivos tradicionales. In Proceedings of the IV Jornadas Ibéricas de Razas Autóctonas y sus Productos Tradicionales: Innovación, Seguridad y Cultura Alimentaria, Seville, Spain, 14–16 November 2007; pp. 329–337. [Google Scholar]
- Carmona Ruiz, M.A. La penetración de las redes de trashumancia castellana en la Sierra Norte de Sevilla. An. Est. Mediev. 1993, 23, 111–118. [Google Scholar] [CrossRef]
- Sanjuán Benito, J.F. La Mesta. 2016, p. 277. Available online: https://juansanjuanbenito.es/jsb/wp-content/uploads/2016/12/LA-MESTA.pdf (accessed on 1 December 2023).
- Silva, J.B. Las vías pecuarias en el término municipal de Cabra. Contraluz Rev. De La Asoc. Cult. Arturo Cerdá Y Rico 2008, 5, 124–136. [Google Scholar]
- Casanovas Arias, D.; León Jurado, J.M.; Bermejo Asensio, L.A.; Navas González, F.J.; Marín Navas, C.; Barba Capote, C.J. Genetic diversity evolution of a sheep breed reintroduced after extinction: Tracing back Christopher Columbus’ first imported sheep. Res. Vet. Sci. 2020, 132, 207–216. [Google Scholar] [CrossRef]
- Navas, F.; Jordana, J.; León, J.; Barba, C.; Delgado, J. A model to infer the demographic structure evolution of endangered donkey populations. Animal 2017, 11, 2129–2138. [Google Scholar] [CrossRef]
- Gutiérrez, J.P.; Marmi, J.; Goyache, F.; Jordana, J. Pedigree information reveals moderate to high levels of inbreeding and a weak population structure in the endangered Catalonian donkey breed. J. Anim. Breed. Genet. 2005, 122, 378–386. [Google Scholar] [CrossRef]
- Lindgren, D. Quantitative Genetics Concepts (A4); Swedish University of Agricultural Sciences: Uppsala, Sweden, 2016. [Google Scholar]
- Meuwissen, T.; Luo, Z. Computing inbreeding coefficients in large populations. Genet. Sel. Evol. 1992, 24, 305–313. [Google Scholar] [CrossRef]
- Leroy, G.; Mary-Huard, T.; Verrier, E.; Danvy, S.; Charvolin, E.; Danchin-Burge, C. Methods to estimate effective population size using pedigree data: Examples in dog, sheep, cattle and horse. Genet. Sel. Evol. 2013, 45, 1–10. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Cervantes, I.; Goyache, F. Improving the estimation of realized effective population sizes in farm animals. J. Anim Breed. Genet. 2009, 126, 327–332. [Google Scholar] [CrossRef]
- Cervantes, I.; Goyache, F.; Molina, A.; Valera, M.; Gutiérrez, J. Estimation of effective population size from the rate of coancestry in pedigreed populations. J. Anim Breed. Genet. 2011, 128, 56–63. [Google Scholar] [CrossRef]
- Caballero, A.; Toro, M.A. Interrelations between effective population size and other pedigree tools for the management of conserved populations. Gen. Res. 2000, 75, 331–343. [Google Scholar] [CrossRef]
- Lacy, R.C. Analysis of founder representation in pedigrees: Founder equivalents and founder genome equivalents. Zoo Zoobiol. 1989, 8, 111–123. [Google Scholar] [CrossRef]
- Boichard, D.; Maignel, L.; Verrier, E. The value of using probabilities of gene origin to measure genetic variability in a population. Gent. Sel. Evol. 1997, 29, 5–23. [Google Scholar] [CrossRef]
- Colleau, J.-J.; Sargolzaei, M. A proximal decomposition of inbreeding, coancestry and contributions. Gen. Res. 2008, 90, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Sargolzaei, M.; Iwaisaki, H.; Colleau, J.J. CFC: A tool for monitoring genetic diversity. In Proceedings of the 8th World Congress on Genetics Applied to Livestock Production, Belo Horizonte, Brazil, 13–18 August 2006. [Google Scholar]
- Wright, S. Evolution and the Genetics of Populations: Vol. 2. The Theory of Gene Frequencies; Wright, S., Ed.; University of Chicago Press: Chicago, IL, USA, 1969. [Google Scholar]
- Cervantes, I.; Goyache, F.; Molina, A.; Valera, M.; Gutiérrez, J. Application of individual increase in inbreeding to estimate realized effective sizes from real pedigrees. J. Anim. Breed. Genet. 2008, 125, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Nei, M. Molecular Evolutionary Genetics; Columbia University Press: New York, NY, USA, 1987. [Google Scholar]
- Horcada Ibáñez, A.L.; Polvillo Polo, O. Conceptos básicos sobre la carne. In La Producción de carne en Andalucía; Junta de Andalucía, Consejería de Agricultura y Pesca 201: Sevilla, Spain, 2010. [Google Scholar]
- Carolino, N.; Vitorino, A.; Carolino, I.; Pais, J.; Henriques, N.; Silveira, M.; Vicente, A. Genetic Diversity in the Portuguese Mertolenga Cattle Breed Assessed by Pedigree Analysis. Animals 2020, 10, 1990. [Google Scholar] [CrossRef]
- Carolino, N.; Gama, L. Indicators of genetic erosion in an endangered population: The Alentejana cattle breed in Portugal. J. Anim. Sci. 2008, 86, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Fioretti, M.; Negrini, R.; Biffani, S.; Quaglia, A.; Valentini, A.; Nardone, A. Demographic structure and population dynamics of Maremmana cattle local breed after 35 years of traditional selection. Livest. Sci. 2020, 232, 103903. [Google Scholar] [CrossRef]
- Adamczyk, K.; Felenczak, A.; Jamrozy, J.; Szarek, J.; Bulla, J. Conservation of polish red cattle. Slovak J. Anim. Sci. 2008, 41, 72–76. [Google Scholar]
- Szarek, J.; Adamczyk, K.; Felenczak, A. Polish Red Cattle breeding: Past and present. Anim. Gen. Res. 2004, 35, 21–35. [Google Scholar] [CrossRef]
- Jarnecka, O.; Bauer, E.; Jagusiak, W. Pedigree analysis in the Polish Red cattle population. Animal 2021, 15, 100238. [Google Scholar] [CrossRef]
- James, W.H. The categories of evidence relating to the hypothesis that mammalian sex ratios at birth are causally related to the hormone concentrations of both parents around the time of conception. J. Biosoc. Sci. 2011, 43, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Santana, M., Jr.; Pereira, R.; Bignardi, A.; Ayres, D.; Menezes, G.d.O.; Silva, L.; Leroy, G.; Machado, C.; Josahkian, L.; Albuquerque, L. Structure and genetic diversity of Brazilian Zebu cattle breeds assessed by pedigree analysis. Livest. Sci. 2016, 187, 6–15. [Google Scholar] [CrossRef]
- Santana, M., Jr.; Pereira, R.; Bignardi, A.; El Faro, L.; Tonhati, H.; Albuquerque, L. History, structure, and genetic diversity of Brazilian Gir cattle. Livest. Sci. 2014, 163, 26–33. [Google Scholar] [CrossRef]
- Ribeiro, N.; Medeiros, G.; Nascimento, G.; Arandas, J.; Ribeiro, M. Analysis of the population structure of a cattle conservation nucleus Curraleiro Pé Duro. Arq. Bras. Med. Vet. Zootec. 2021, 73, 231–238. [Google Scholar] [CrossRef]
- Fabbri, M.C.; Gonçalves de Rezende, M.P.; Dadousis, C.; Biffani, S.; Negrini, R.; Souza Carneiro, P.L.; Bozzi, R. Population structure and genetic diversity of Italian beef breeds as a tool for planning conservation and selection strategies. Animals 2019, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Addo, S.; Schäler, J.; Hinrichs, D.; Thaller, G. Genetic diversity and ancestral history of the German Angler and the Red-and-White dual-purpose cattle breeds assessed through pedigree analysis. Agric. Sci. 2017, 8, 1033–1047. [Google Scholar] [CrossRef][Green Version]
- Melka, M.; Sargolzaei, M.; Miglior, F.; Schenkel, F. Genetic diversity of Guernsey population using pedigree data and gene-dropping simulations. Animal 2013, 7, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Worede, G.; Forabosco, F.; Zumbach, B.; Palucci, V.; Jorjani, H. Evaluation of genetic variation in the international Brown Swiss population. Animal 2013, 7, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Marquez, G.; Speidel, S.; Enns, R.; Garrick, D. Genetic diversity and population structure of American Red Angus cattle. J. Anim. Sci. 2010, 88, 59–68. [Google Scholar] [CrossRef]
- Ríos-Utrera, Á.; Montaño-Bermúdez, M.; Vega-Murillo, V.E.; Martínez-Velázquez, G.; Baeza-Rodríguez, J.J.; Román-Ponce, S.I. Genetic diversity evolution in the Mexican Charolais cattle population. Anim. Biosci. 2021, 34, 1116. [Google Scholar] [CrossRef]
- Cavani, L.; Silva, R.M.d.O.; Carreño, L.O.D.; Ono, R.K.; Bertipaglia, T.S.; Farah, M.M.; Millen, D.D.; Fonseca, R.d. Genetic diversity of Brazilian Brahman cattle by pedigree analysis. Pesqui. Agropecu. Bra. 2018, 53, 74–79. [Google Scholar] [CrossRef]
- Bouquet, A.; Venot, E.; Laloë, D.; Forabosco, F.; Fogh, A.; Pabiou, T.; Moore, K.; Eriksson, J.-Å.; Renand, G.; Phocas, F. Genetic structure of the European Charolais and Limousin cattle metapopulations using pedigree analyses. J. Anim Sci. 2011, 89, 1719–1730. [Google Scholar] [CrossRef] [PubMed]
- Brito, F.; Sargolzaei, M.; Braccini Neto, J.; Cobuci, J.; Pimentel, C.; Barcellos, J.; Schenkel, F. In-depth pedigree analysis in a large Brazilian Nellore herd. Genet. Mol. Res. 2013, 12, 5758–5765. [Google Scholar] [CrossRef]
- Cañas-Álvarez, J.; Mouresan, E.; Varona, L.; Díaz, C.; Avilés, C.; Baró, J.; Altarriba, J.; Casellas, J.; Piedrafita, J. Linkage disequilibrium and persistence of phase in five spanish local beef cattle breeds. In Proceedings of the 10th World Congress of Genetics Applied to Livestock Production, Vancouver, BC, Canada, 18–22 August 2014. [Google Scholar]
- Valera Córdoba, M.M.; Vázquez González, I.; Fernández Martín, J. Programa de conservación ex situ. In La conservación de la Diversidad de Razas Autóctonas de Andalucía. Patrimonio Ganadero Andaluz; Junta de Andalucía: Sevilla, Spain, 2007; Volume 3. [Google Scholar]
- Benavente, M.; Santana, R.; Delgado, J. Gestión de la consanguinidad en la raza bovina Palmera. AICA 2017, 10, 78–82. [Google Scholar]
- Marín Navas, C.; Delgado Bermejo, J.V.; McLean, A.K.; León Jurado, J.M.; Rodriguez de la Borbolla y Ruiberriz de Torres, A.; Navas González, F.J. Discriminant canonical analysis of the contribution of Spanish and Arabian purebred horses to the genetic diversity and population structure of Hispano-Arabian horses. Animals 2021, 11, 269. [Google Scholar] [CrossRef]
- Figueredo, J.; Cruz, J.; Sousa, L.; Neto, M.T.; Carneiro, P.; Brito, N.; Pinheiro, R.; Lacerda, K.; Mottin, V. Genetic diversity and population structure estimation of Brazilian Somali sheep from pedigree data. Small Rumin. Res. 2019, 179, 64–69. [Google Scholar] [CrossRef]
- Restoux, G.; Rognon, X.; Vieaud, A.; Guemene, D.; Petitjean, F.; Rouger, R.; Brard-Fudulea, S.; Lubac-Paye, S.; Chiron, G.; Tixier-Boichard, M. Managing genetic diversity in breeding programs of small populations: The case of French local chicken breeds. Genet. Sel. Evol. 2022, 54, 56. [Google Scholar] [CrossRef] [PubMed]
- McHugo, G.P.; Dover, M.J.; MacHugh, D.E. Unlocking the origins and biology of domestic animals using ancient DNA and paleogenomics. BMC Biol. 2019, 17, 98. [Google Scholar] [CrossRef] [PubMed]
- (EU) 2016/1012; Regulation (EU) 2016/1012 of the European Parliament and of the Council of 8 June 2016 on Zootechnical and Genealogical Conditions for the Breeding, Trade in and Entry into the Union of Purebred Breeding Animals, Hybrid Breeding Pigs and the Germinal Products Thereof and Amending Regulation (EU) No 652/2014, Council Directives 89/608/EEC and 90/425/EEC and Repealing Certain Acts in the Area of Animal Breeding (‘Animal Breeding Regulation’). European Parliament and of the Council: Strasbourg, France, 2016.
- González-Cano, R.; González-Martínez, A.; Muñoz-Mejías, M.E.; Valera, P.; Rodero, E. Analyses of Genetic Diversity in the Endangered “Berrenda” Spanish Cattle Breeds Using Pedigree Data. Animals 2022, 12, 249. [Google Scholar] [CrossRef]
- Bernardes, P.; Grossi, D.; Savegnago, R.; Buzanskas, M.; Ramos, S.; Romanzini, E.; Guidolin, D.; Bezerra, L.A.F.; Lôbo, R.B.; Munari, D. Population structure of Tabapuã beef cattle using pedigree analysis. Livest. Sci. 2016, 187, 96–101. [Google Scholar] [CrossRef]
- Pezzini, T.; Mariante, A.S.; Martins, E.; Paiva, S.; Seixas, L.; Costa, J.B., Jr.; Rolo, J.; McManus, C. Population structure of Brazilian Crioula lageana cattle (Bos taurus) breed. Rev. Colomb. Cienc. Pecu. 2018, 31, 93–102. [Google Scholar] [CrossRef]
- Rosendo Ponce, A.; Jiménez, A.L.P.; Martínez, F.R.; Hernández, G.T.; Valverde, R.R.; Pérez, C.M.B. Genetic variability of Tropical Milking Criollo cattle of Mexico estimated from genealogy information. Rev. Colomb. Cienc. Pecu. 2018, 31, 196–203. [Google Scholar] [CrossRef]
- Maignel, L.; Boichard, D.; Verrier, E. Genetic variability of French dairy breeds estimated from pedigree information. Interbull Bull. 1996, 14, 49–56. [Google Scholar]
- Sölkner, J.; Filipcic, L.; Hampshire, N. Genetic variability of populations and similarity of subpopulations in Austrian cattle breeds determined by analysis of pedigrees. Anim. Sci. 1998, 67, 249–256. [Google Scholar] [CrossRef]
- Chakraborty, R. Analysis of genetic structure of populations: Meaning, methods, and implications. In Human Population Genetics: A Centennial Tribute to JBS Haldane; Majumder, P.P., Ed.; Plenum Press: New York, NY, USA, 1993; pp. 189–206. [Google Scholar]
- GebreMariam, S.; Amare, S.; Baker, D.; Solomon, A.; Davies, R. Study of the Ethiopian Live Cattle and Beef Value Chain; ILRI Editorial and Publishing Services: Addis Ababa, Ethiopia, 2013. [Google Scholar]
- Ithagu, S.M. Management of Genetic Diversity in Sahiwal Cattle Breed in Kenya; Egerton University: Njoro, Kenya, 2018. [Google Scholar]
- Nomura, T.; Honda, T.; Mukai, F. Inbreeding and effective population size of Japanese Black cattle. J. Anim. Sci. 2001, 79, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Rovelli, G.; Luigi-Sierra, M.G.; Guan, D.; Sbarra, F.; Quaglia, A.; Sarti, F.M.; Amills, M.; Lasagna, E. Evolution of inbreeding: A gaze into five Italian beef cattle breeds history. PeerJ 2021, 9, e12049. [Google Scholar] [CrossRef]
- González, A.R.M.; Navas González, F.J.; Crudeli, G.Á.; Delgado Bermejo, J.V.; Camacho Vallejo, M.E.; Quirino, C.R. Process of introduction of australian braford cattle to south america: Configuration of population structure and genetic diversity evolution. Animals 2022, 12, 275. [Google Scholar] [CrossRef] [PubMed]
- Zhivotovsky, L.A. Relationships between Wright’s FST and F IS statistics in a context of Wahlund effect. J. Hered. 2015, 106, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Scraggs, E.; Zanella, R.; Wojtowicz, A.; Taylor, J.; Gaskins, C.; Reeves, J.; de Avila, J.; Neibergs, H. Estimation of inbreeding and effective population size of full-blood wagyu cattle registered with the American Wagyu Cattle Association. J. Anim. Breed. Genet. 2014, 131, 3–10. [Google Scholar] [CrossRef]
- Centeno, G.F.; Manso, F.M. La Red Nacional de Vías Pecuarias. Ambient. La Rev. Del Minist. De Medio Ambiente 2017, 120, 4–13. [Google Scholar]
- Ruiz Roldán, Á.J. Análisis y Representación 3D del Proyecto de Mejora y Reforma de la Zona Verde Cañada Real Soriana (Villarrubia de Córdoba). Master’s Thesis, University of Córdoba, Córdoba, Spain, 2022. [Google Scholar]
- Ruiz Ruiz, E. La Cañada Soriana Occidental; Junta de Castilla y León; Consejería de Agricultura y Ganadería: Valladolid, Spain, 1991; pp. 183–201. [Google Scholar]
- Martín-Burriel, I.; Rodellar, C.; Cañón, J.; Cortés, O.; Dunner, S.; Landi, V.; Martínez-Martínez, A.; Gama, L.; Ginja, C.; Penedo, M. Genetic diversity, structure, and breed relationships in Iberian cattle. J. Anim. Sci. 2011, 89, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Alcobendas, M.; Castanet, J. Bone growth plasticity among populations of Salamandra salamandra: Interactions between internal and external factors. Herpetologica 2000, 56, 14–26. [Google Scholar]
- Mendigorri, A.M. Fronteras y territorios: La gestión de las áreas protegidas en cuestión. Cuad. Geográficos 2018, 57, 61–86. [Google Scholar] [CrossRef]
- Cañas-Álvarez, J.; González-Rodríguez, A.; Munilla, S.; Varona, L.; Díaz, C.; Baro, J.; Altarriba, J.; Molina, A.; Piedrafita, J. Genetic diversity and divergence among Spanish beef cattle breeds assessed by a bovine high-density SNP chip. J. Anim. Sci. 2015, 93, 5164–5174. [Google Scholar] [CrossRef] [PubMed]






| Population Sets | Historical | Current | |
|---|---|---|---|
| Parameters | |||
| Total number of herds | 28 | 28 | |
| Total number of provinces | 5 | 5 | |
| Total number of municipalities | 23 | 22 | |
| Average number of animals per herd/average herd size | 305.54 | 88.28 | |
| Total bull percentage (%) | 42.00% | 23.99% | |
| Mean number of calves per bull (n) | 2.05 | 3.87 | |
| Maximum calf number per mated bull (n) (animals with unknown sire excluded) | 379 | 282 | |
| Mean number of calves per mated bull (n) (animals with unknown sire excluded) | 80.18 | 91.76 | |
| Average age of bull in reproduction (years) | 9.51 | 7.87 | |
| Total cow percentage (%) | 58.00 | 76.01 | |
| Mean number of calves per cow (n) | 1.53 | 1.64 | |
| Maximum calf number per mated cow (n) (animals with unknown dam excluded) | 14 | 13 | |
| Mean number of calves per mated cow (n) (animals with unknown dam excluded) | 3.10 | 1.07 | |
| Average age of cows in reproduction (years) | 11.24 | 8.59 | |
| Total cow/bull ratio | 1.38/1 | 3.17/1 | |
| Mated cow/bull ratio | 26.10/1 | 36.71/1 | |
| Progeny from bulls selected for breeding (%) | 59.89 | 4.75 | |
| Progeny from cows selected for breeding (%) | 59.39 | 26.67 | |
| Male selection intensity or portion of male calves born retained for breeding (%) | 11.37 | 0.58 | |
| Female selection intensity or portion of female calves born retained for breeding (%) | 40.57 | 32.32 | |
| Population Set | Historical | Current | |
|---|---|---|---|
| Parameter | |||
| Population size | 8555 | 2472 | |
| Maximum number of traced generations (n) | 7 | 7 | |
| Pedigree completeness level at 1st generation (Known parents) | 0.8763 | 0.9846 | |
| Pedigree completeness level at 2nd generation (Known grandparents) | 0.5538 | 0.7221 | |
| Pedigree completeness level at 3rd generation (Known great-grandparents) | 0.2331 | 0.3260 | |
| Pedigree completeness level at 4th generation (Known great-great-grandparents) | 0.0661 | 0.0871 | |
| Pedigree completeness level at 5th generation (Known great-great-great grandparents) | 0.0098 | 0.0145 | |
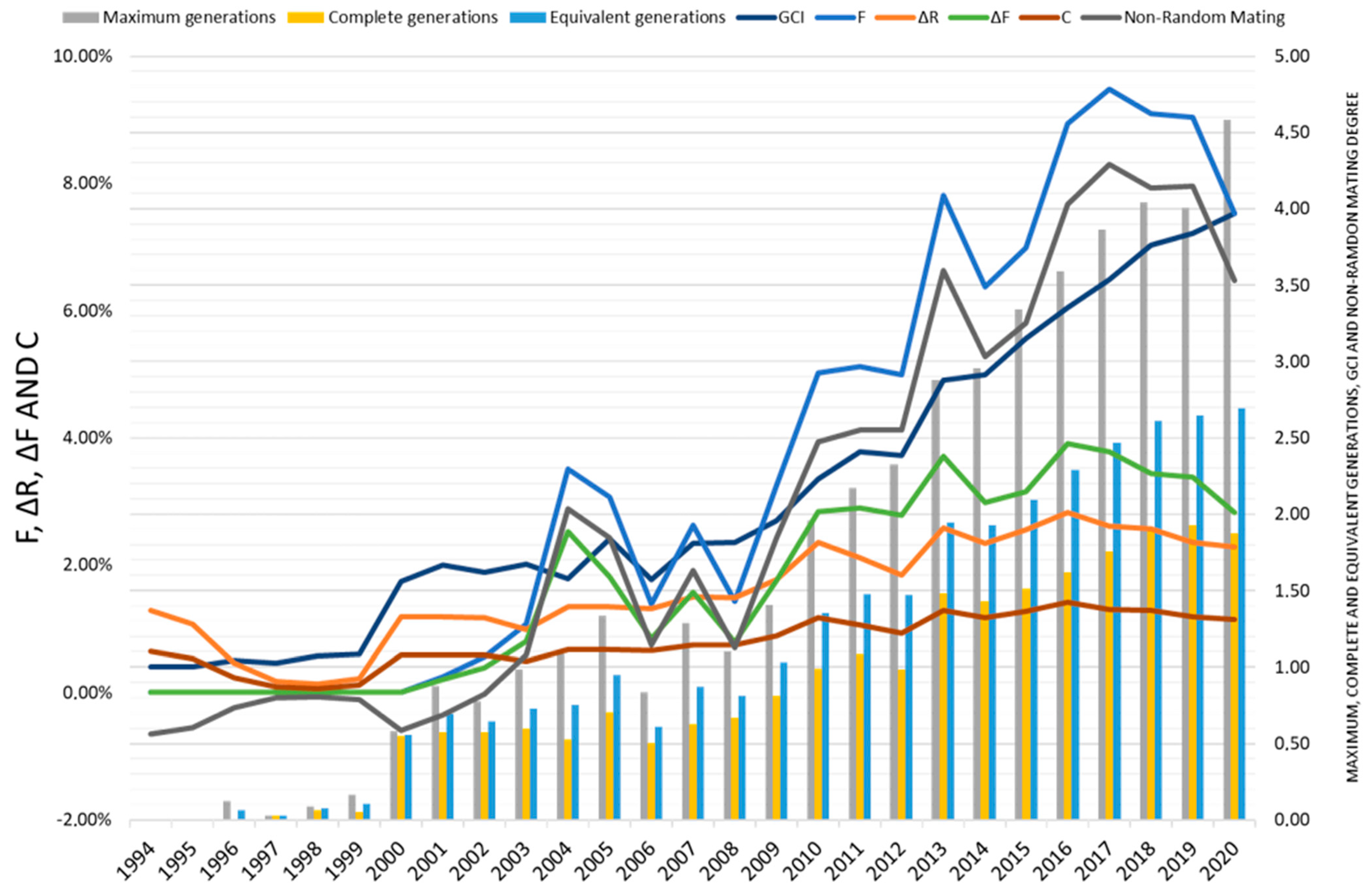
| Number of maximum generations (mean ± SD) | 2.65 ± 1.57 | 3.30 ± 1.32 | |
| Number of complete generations (mean ± SD) | 1.27 ± 0.74 | 1.55 ± 0.63 | |
| Number of equivalent generations (mean ± SD) | 1.74 ± 0.94 | 2.14 ± 0.73 | |
| Populational Sets | Historical (n = 8555) | Current (n = 2472) | |
|---|---|---|---|
| Parameter | |||
| Inbreeding coefficient (F, %) | 5.94 | 7.23 | |
| Average individual increase in inbreeding (ΔF, %) | 2.71 | 3.17 | |
| Maximum coefficient of inbreeding (%) | 43.75 | 43.75 | |
| Inbred animals (%) | 37.80 | 48.82 | |
| Highly inbred animals (%) | 0.193 | 0.185 | |
| Average coancestry coefficient (C, %) | 2.90 | 3.60 | |
| Average relatedness coefficient (ΔR, %) | 2.10 | 2.40 | |
| Non-random mating rate (α) | 0.049 | 0.061 | |
| Genetic Conservation Index (GCI) | 2.75 | 3.19 | |
| Parameter | Reference Population (Both Parents Known) (n = 2396) |
|---|---|
| Historical population | 8555 |
| Current population | 2472 |
| Base population (one or more unknown parents) | 1204 |
| Actual base population (one unknown parent = half founder) | 1058 |
| Number of founders contributing to the reference population (n) | 985 |
| Number of ancestors contributing to the reference population (n) | 981 |
| Effective number of non-founders (Nef) | 96.30 |
| Number of founder equivalents (fe) | 91.14 |
| Effective number of ancestors (fa) | 42 |
| Founder genome equivalents (fg) | 46.83 |
| fa/fe ratio | 0.46 |
| fg/fe ratio | 0.51 |
| Ancestors explaining 25% of the gene pool (n) | 5 |
| Ancestors explaining 50% of the gene pool (n) | 17 |
| Ancestors explaining 75% of the gene pool (n) | 93 |
| Average individual increase in inbreeding (ΔF, %) | 0.21 |
| Average relatedness (R, %) | 0.27 |
| Parameter | Reference Population (Both Parents Known) (n = 2396) |
|---|---|
| Genetic diversity (GD, %) | 98.93 |
| Genetic diversity loss (GDL, %) | 1.07 |
| Genetic diversity in the reference population considered to compute the genetic diversity loss due to the unequal contribution of founders (DG*, %) | 99.45 |
| GDL due to bottlenecks and genetic drift since founders (GL, %) | 1.07 |
| GDL due to unequal founder contributions (%) | 0.55 |
| Parameters | Herd/Breeder | Municipality | Province |
|---|---|---|---|
| Subpopulations a | 28 | 23 | 5 |
| FIS (Inbreeding coefficient relative to the subpopulation) | 0.036 | 0.021 | 0.039 |
| FST (Correlation between random gametes drawn from the subpopulation relative to the total population) | 0.085 | 0.069 | 0.011 |
| FIT (Inbreeding coefficient relative to the total population) | 0.052 | 0.049 | 0.049 |
| Mean inbreeding within subpopulations | 0.095 | 0.078 | 0.022 |
| Mean number of animals per subpopulation | 305.54 | 371.96 | 1711 |
| Number of Nei genetic distances | 378 | 253 | 10 |
| Average Nei genetic distance | 0.084 | 0.068 | 0.011 |
| Mean coancestry within subpopulations | 0.095 | 0.079 | 0.022 |
| Self-coancestry | 0.531 | 0.530 | 0.530 |
| Mean coancestry in the metapopulation | 0.012 | 0.011 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cartuche Macas, L.F.; Camacho Vallejo, M.E.; González Ariza, A.; León Jurado, J.M.; Delgado Bermejo, J.V.; Marín Navas, C.; Navas González, F.J. Analysis of Endangered Andalusian Black Cattle (Negra Andaluza) Reveals Genetic Reservoir for Bovine Black Trunk. Animals 2024, 14, 1131. https://doi.org/10.3390/ani14071131
Cartuche Macas LF, Camacho Vallejo ME, González Ariza A, León Jurado JM, Delgado Bermejo JV, Marín Navas C, Navas González FJ. Analysis of Endangered Andalusian Black Cattle (Negra Andaluza) Reveals Genetic Reservoir for Bovine Black Trunk. Animals. 2024; 14(7):1131. https://doi.org/10.3390/ani14071131
Chicago/Turabian StyleCartuche Macas, Luis Favian, María Esperanza Camacho Vallejo, Antonio González Ariza, José Manuel León Jurado, Juan Vicente Delgado Bermejo, Carmen Marín Navas, and Francisco Javier Navas González. 2024. "Analysis of Endangered Andalusian Black Cattle (Negra Andaluza) Reveals Genetic Reservoir for Bovine Black Trunk" Animals 14, no. 7: 1131. https://doi.org/10.3390/ani14071131
APA StyleCartuche Macas, L. F., Camacho Vallejo, M. E., González Ariza, A., León Jurado, J. M., Delgado Bermejo, J. V., Marín Navas, C., & Navas González, F. J. (2024). Analysis of Endangered Andalusian Black Cattle (Negra Andaluza) Reveals Genetic Reservoir for Bovine Black Trunk. Animals, 14(7), 1131. https://doi.org/10.3390/ani14071131





