New Data on the Larval Stages of Leptophallus nigrovenosus (Digenea, Plagiorchiata)
Abstract
:Simple Summary
Abstract
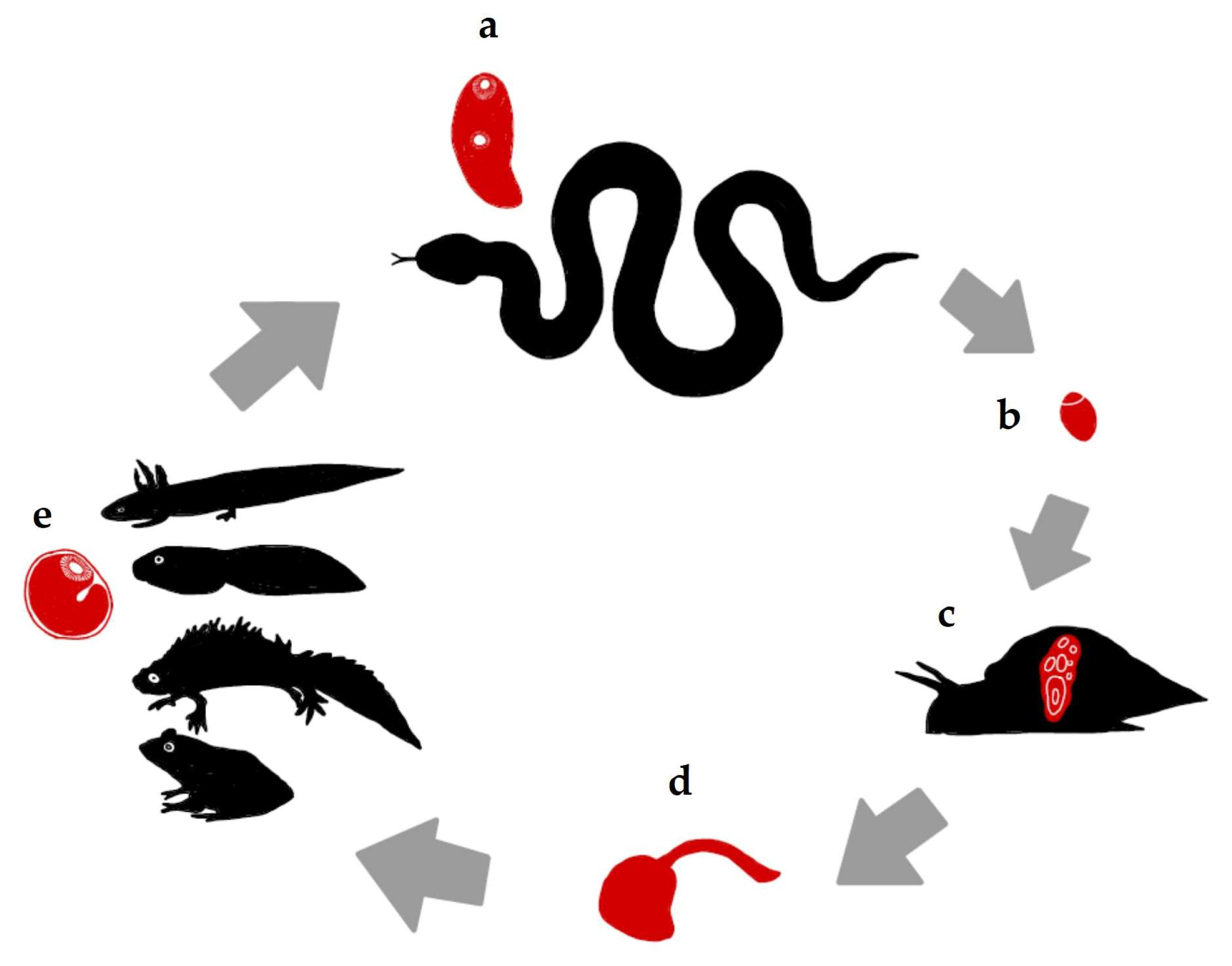
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Cercaria and Metacercaria Isolation
2.2. Cercarial Sensory Receptors
2.3. Histology
3. Results
3.1. Cercariae
3.2. Metacercariae
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dollfus, R.P. Miscelania Helminthological Maroccana XV. Presence Au Maroc de Leptophallus Nigrovenosus (Bellingham) (Trematodes, Distomes). Arch. Inst. Pasteur Maroc. 1954, 6, 612–624. [Google Scholar]
- Zając, B.; Bury, S.; Kuśmierek, N.; Okarma, H. Frequent Infection of Urban Grass Snakes (Natrix Natrix) Oral Cavity with Leptophallus Nigrovenosus Trematode. Parasitol. Res. 2022, 121, 2167–2171. [Google Scholar] [CrossRef] [PubMed]
- Luiselli, L.; Filippi, E.; Capula, M. Geographic Variation in Diet Composition of the Grass Snake (Natrix Natrix) along the Mainland and an Island of Italy: The Effects of Habitat Type and Interference with Potential Competitors. Herpetol. J. 2005, 15, 221–230. [Google Scholar]
- Arabuli, L.; Murvanidze, L.; Faltynkova, A.; Mumladze, L. Checklist of Digeneans (Platyhelminthes, Trematoda, Digenea) of Georgia. Biodivers. Data J. 2024, 12, e110201. [Google Scholar] [CrossRef] [PubMed]
- Edelényi, B. A Haza i Hüllők Néhány Belsőélősködő Férge. Acta Acad. Paedagog. Agriensis 1962, 272, 561–578. [Google Scholar]
- Matskási, I.; Mészáros, F.; Murai, E.; Gubányi, A. Helminthological Investigations of Vertebrates in the Bukk National Park (Monogenea, Digenea, Cestoda, Acanthocephala, Nematoda). In The Fauna of the Bukk National Park; Hungarian Natural History Museum: Budapest, Hungary, 1996; pp. 11–32. [Google Scholar]
- Santoro, M.; Tkach, V.; Mattiucci, S.; Kinsella, M.; Nascetti, G. Renifer Aniarum (Digenea: Reniferidae), an Introduced North American Parasite in Grass Snakes Natrix Natrix in Calabria, Southern Italy. Dis. Aquat. Organ. 2011, 95, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Bertman, M. Helmintofauna Zaskrońca –Natrix Natrix (L.) Okolic Kielc. Rocz. Świętokrzyski Ser. B Nauk. Przyr. 1998, 25, 61–68. [Google Scholar]
- Lewin, J. Parasites of the Water Snake, Natrix Natrix L., in Poland. Acta Parasitol. 1992, 37, 195–199. [Google Scholar]
- Mihalca, A.D.; Gherman, C.; Ghira, I.; Cozma, V. Helminth Parasites of Reptiles (Reptilia) in Romania. Parasitol. Res. 2007, 101, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.A.; Kirillova, N.Y.; Chikhlyaev, I.V. Trematodes of Terrestrial Vertebrates of the Middle Volga Region; Institute of Ecology of Volga River basin of Russian Academy of Sciences: Togliatti, Russia, 2012. [Google Scholar]
- Kirillova, N.Y.; Ruchin, A.B.; Kirillov, A.A.; Chikhlyaev, I.V.; Alpeev, A. Overview of Helminths in Land Vertebrates from the Mordovia Nature Reserve, European Russia. Nat. Environ. Pollut. Technol. 2023, 22, 1667–1690. [Google Scholar] [CrossRef]
- Cordero del Campillo, M.; Castañón, L.; Reguera, A. Índice-Catálogo de Zooparasitos Ibéricos; Universidad de León: León, Spain, 1994. [Google Scholar]
- Galeano, M.; Navarro, P.; Lluch, J. Helmintofauna de Algunos Herpetos Del Sistema Ibérico Español. An. Biol. 1996, 21, 23–30. [Google Scholar]
- Marushchak, O.; Syrota, Y.; Dmytrieva, I.; Kuzmin, Y.; Nechai, A.; Lisitsyna, O.; Svitin, R. Helminths Found in Common Species of the Herpetofauna in Ukraine. Biodivers. Data J. 2024, 12, e113770. [Google Scholar] [CrossRef] [PubMed]
- Bakiev, A.; Kirillov, A.; Mebert, K. Diet and Parasitic Helminths of Dice Snakes from the Volga Basin, Russia. Mertensiella 2011, 18, 325–329. [Google Scholar]
- Brumpt, E. Cycle Évolutif Du Trématode Leptophallus Nigrovenosus, Parasite de La Couleuvre à Collier (Tropinodotus Natrix), et Expérimentalement de La Vipère (Vipera Aspis). Ann. Parasitol. Hum. Comparée 1944, 20, 244–262. [Google Scholar] [CrossRef]
- Sharpilo, V.P.; Iskova, N.I. The Fauna of the Ukraine. Trematodes: Plagiorchiata. Fauna Ukr. 1989, 34, 1–278. [Google Scholar]
- Richard, J. La Chétotaxie Des Cercaires. Valeur Systématique et Phylétique. Mém. Mus. Nat. Hist. Nat. Ser. A Zool. 1971, 67, 1–179. [Google Scholar]
- Grabda-Kazubska, B. Current Status of Studies on Amphibian and Reptile Parasites in Poland. Wiad Parazytol. 1963, 9, 325–331. [Google Scholar] [PubMed]
- Bayssade-Dufour, C.; Albaret, J.L.; Grabda-Kazubska, B.; Chabaud, A.G. Nomenclature Proposée Pour La Chétotaxie Des Cercaires de Plagiorchiata. Ann. Parasitol. Hum. Comp. 1989, 64, 426–432. [Google Scholar] [CrossRef]
- Shimalov, V.V.; Shimalov, V.T. Helminth Fauna of Snakes (Reptilia, Serpentes) in Belorussian Polesye. Parasitol. Res. 2000, 86, 340–341. [Google Scholar] [CrossRef] [PubMed]
- Kirillov, A.A.; Kirillova, N.Y. First Finding of Spirurid Larva (Chromadorea, Spirurida) in the Common European Viper Vipera Berus (Linnaeus, 1758) of the Russian Fauna. IOP Conf. Ser. Earth Environ. Sci. 2021, 818, 012017. [Google Scholar] [CrossRef]
- Luiselli, L.; Anibaldi, C.; Capula, M. The Diet of Juvenile Adders, Vipera Berus, in an Alpine Habitat. Amphibia-Reptilia 1995, 16, 404–407. [Google Scholar] [CrossRef]
- Biserkov, V.; Kostadinova, A. Intestinal Helminth Communities in the Green Lizard, Lacerta Viridis, from Bulgaria. J. Helminthol. 1998, 72, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Pekár, S.; Gajski, D.; Mifková, T.; Smolinský, R.; Gojak, T.; Martišová, M. Natural Diet of European Green Lizards, Lacerta Viridis (Squamata: Lacertidae): A Comparison of Macroscopic and Molecular Identification Methods. Herpetologica 2023, 79, 135–143. [Google Scholar] [CrossRef]
- Vojtek, J. The Present Situation of the Research into the Stages of Development of Trematodes in Czechoslovakia. Scr. Fac. Sci. Nat. Univ. Purkynianae Brun. 1989, 19, 339–352. [Google Scholar]
- Barsiene, J.; Grabda-Kazubska, B. A Comparative Study on Chromosomes in Plagiorchiid Trematodes. 1. Karyotypes of Opisthioglyphe Ranae (Frolich, 1791), Haplometra Cylindracea (Zeder, 1800) and Leptophallus Nigrovenosus (Bellingham, 1844). Acta Parasitol. Pol. 1988, 33, 249–255. [Google Scholar]
- Cichy, A.; Faltýnková, A.; Żbikowska, E. Cercariae (Trematoda, Digenea) in European Freshwater Snails—A Checklist of Records from over One Hundred Years. Folia Malacol. 2011, 19, 165–189. [Google Scholar] [CrossRef]
- Buttner, A. Première Démonstration Expérimentale d’un Cycle Abrégé Chez Les Trématodes Digénétiques. Cas Du Plagiorchis Brumpti. Ann. Parasitol. Hum. Comparée 1950, 25, 21–26. [Google Scholar] [CrossRef]
- Buttner, A. La Proqenèse Chez Les Trématodes Digénétiques (Suite)—Recherches Personnelles Sur Deux Espèces Progénétiques Déjà Connues: Ratzia Joyeuxi (E. Brumpt, 1922) et Pleurogenes Medians (Olsson, 1876). Ann. Parasitol. Hum. Comparée 1951, 26, 138–189. [Google Scholar] [CrossRef]
- Dobrovolsky, A.A. The Life Cycle of Paralepoderma Cloacicola (Luhe, 1909) Dollfus, 1950 (Trematoda, Plagiorchiidae). Vestn. Leningr. Gos. Univ. Seriya Biol. 1969, 9, 28–38. [Google Scholar]
- Dobrovolsky, A.A. Life-Cycle of Macrodera Longicottis (Abildgaard, 1788) Luhe, 1909 (Trematoda: Ochetosomatoidea). Vestn. Leningr. Gos. Univ. Seriya Biol. 1971, 26, 9–20. [Google Scholar]
- Grabda-Kazubska, B.; Bayssade-Dufour, C. Comparative Chaetotaxy of Five Species of Plagiorchiid Cercariae, Genera Leptophallus, Metaleptophallus, Macrodera and Paralepoderma. Acta Parasitol. 1999, 44, 215–221. [Google Scholar]
- Tkach, V.V.; Grabda-Kazubska, B.; Pawlowski, J.W.; Swiderski, Z. Molecular and Morphological Evidence for Close Phylogenetic Affinities of the Genera Macrodera, Leptophallus, Metaleptophallus and Paralepoderma (Digenea, Plagiorchiata). Acta Parasitol. 1999, 44, 170–179. [Google Scholar]
- Ribas, A.; Roca, S.L.; Roca, V. Helminths from Snakes in Northeast Spain. Boletín la Asoc. Herpetológica Española 2010, 21, 44–46. [Google Scholar]
- Navarro, P.; Lluch, J. Helminth Communities of Two Green Frogs (Rana Perezi and Rana Saharica) from Both Shores of the Alboran Sea. Parasite 2006, 13, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Montori, A.; Llorente, G.A. Tritón Pirenaico—Calotriton Asper. In Enciclopedia Virtual de los Vertebrados Españoles; Salvador, A., Ed.; Museo Nacional de Ciencias Naturales: Madrid, Spain, 2009. [Google Scholar]
- Combes, C.; Bayssade-Dufour, C.; Cassone, J. Sur l’imprégnation et Le Montage Des Cercaires Pour l’étude Chétotaxique. Ann. Parasitol. Hum. Comparée 1976, 51, 399–400. [Google Scholar] [CrossRef]
- Pereira, A.S.A.; Cavalcanti, N.L.; Nascimento, G.A.F.; Nascimento-Silva, J.L.G.; Padilha, R.J.R.; Viegas, L.F.W.; Alves, L.C.; Lima-Filho, J.L.; Chaves, M.E.C. Morphological and Morphometric Study of Cercariae and Adult Worms of Schistosoma Mansoni (SLM Strain) Isolated from Infected Mice. Parasitol. Res. 2013, 112, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Denisova, S.A.; Shchenkov, S.V. New Data on the Nervous System of Cercaria Parvicaudata Stunkard & Shaw, 1931 (Trematoda: Renicolidae): Revisiting Old Hypotheses. J. Helminthol. 2020, 94, e52. [Google Scholar] [CrossRef]
- Yoneva, A.; van Beest, G.S.; Born-Torrijos, A. Search, Find, and Penetrate: Ultrastructural Data of Furcocercariae of Cardiocephaloides Longicollis (Digenea, Strigeidae) Explain Their Transmission and Infection Strategy into Fish Hosts. Parasitol. Res. 2022, 121, 877–889. [Google Scholar] [CrossRef]
- Bogéa, T.; Caira, J.N. Ultrastructure and Chaetotaxy of Sensory Receptors in the Cercaria of a Species of Allopodocotyle Pritchard, 1966 (Digenea: Opecoelidae). Mem. Inst. Oswaldo Cruz 2001, 96, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Czubaj, A.; Niewiadomska, K. Ultrastructure of Sensory Endings in Diplostomum Pseudospathaceum Niewiadomska, 1984 Cercariae (Digenea, Diplostomidae). Int. J. Parasitol. 1996, 26, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Faltýnková, A.; Sures, B.; Kostadinova, A. Biodiversity of Trematodes in Their Intermediate Mollusc and Fish Hosts in the Freshwater Ecosystems of Europe. Syst. Parasitol. 2016, 93, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Densmore, C.L.; Green, D.E. Diseases of Amphibians. ILAR J. 2007, 48, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Bevier, C.R.; Gelder, A.M.G. The Effect of Parasite Infection on Phonotactic Response in the Mink Frog, Lithobates Septentrionalis. J. Herpetol. 2018, 52, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Calhoun, D.M.; Leslie, K.L.; Riepe, T.B.; Achatz, T.J.; McDevitt-Galles, T.; Tkach, V.V.; Johnson, P.T.J. Patterns of Clinostomum Marginatum Infection in Fishes and Amphibians: Integration of Field, Genetic, and Experimental Approaches. J. Helminthol. 2019, 94, e44. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Fernández-Beaskoetxea, S. Quince Años de Seguimiento de Las Poblaciones de Anfibios Del Macizo de Peñalara (Parque Nacional de La Sierra de Guadarrama, Madrid). Boletín la Asoc. Herpetológica Española 2014, 25, 30–37. [Google Scholar]
- Manenti, R.; De Bernardi, F.; Ficetola, G.F. Pastures vs Forests: Do Traditional Pastoral Activities Negatively Affect Biodiversity? The Case of Amphibians Communities. North-West. J. Zool. 2013, 9, 284–292. [Google Scholar]
- Araújo, M.B.; Calmaestra, R.G.; Picatoste, J.R.; Teira, A.G. Proyecciones de Las Áreas de Distribución Potencial de La Fauna de Vertebrados de La España Peninsular Por Efecto Del Cambio Climático. In Impactos, Vulnerabilidad y Adaptación al Cambio Climático de la Biodiversidad Española; Araújo, M.B., Guilhaumon, F., Rodrigues Neto, D., Pozo Ortego, I., Gómez Calmaestra, R., Eds.; Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente: Madrid, Spain, 2011. [Google Scholar]
- Morelli, F.; Benedetti, Y.; Szkudlarek, M.; Abou Zeid, F.; Delgado, J.D.; Kaczmarski, M. Potential Hotspots of Amphibian Roadkill Risk in Spain. J. Environ. Manag. 2023, 342, 118346. [Google Scholar] [CrossRef] [PubMed]
- Thumsová, B.; Bosch, J.; Martinez-Silvestre, A. Incidence of Emerging Pathogens in Legal and Illegal Amphibian Trade in Spain. Herpetol. Notes 2021, 14, 777–784. [Google Scholar]
- Thumsová, B.; Price, S.J.; González-Cascón, V.; Vörös, J.; Martínez-Silvestre, A.; Rosa, G.M.; Machordom, A.; Bosch, J. Climate Warming Triggers the Emergence of Native Viruses in Iberian Amphibians. iScience 2022, 25, 105541. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Martínez-Solano, I. Chytrid Fungus Infection Related to Unusual Mortalities of Salamandra Salamandra and Bufo Bufo in the Peñalara Natural Park, Spain. Oryx 2006, 40, 84–89. [Google Scholar] [CrossRef]
- Bosch, J.; Martel, A.; Sopniewski, J.; Thumsová, B.; Ayres, C.; Scheele, B.C.; Velo-Antón, G.; Pasmans, F. Batrachochytrium Salamandrivorans Threat to the Iberian Urodele Hotspot. J. Fungi 2021, 7, 644. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Vila-Escale, M.; Fernández-Giberteau, D.; Martinez-Silvestre, A.; Canessa, S.; Van Praet, S.; Pannon, P.; Chiers, K.; Ferran, A.; Kelly, M.; et al. Integral Chain Management of Wildlife Diseases. Conserv. Lett. 2020, 13, e12707. [Google Scholar] [CrossRef]
- Ribas, A.; Amat, F.; Veciana, M. Helmints Paràsits de Salamandra Salamandra Al Parc Natural Del Montseny. Monogr. Montseny 2010, 8, 367–371. [Google Scholar]
- Lluch, J.; Navarro, P.; Pérez-Soler, P. Haematoloechus Carbonelli Sp.n. (Haematoloechidae: Plagiorchiata) Un Nouveau Trématode Parasite d’Amphibiens de La Péninsule Ibérique. Révue Suisse Zool. 1991, 98, 255–260. [Google Scholar] [CrossRef]
- Navarro, P.; Guerrero, F.; Perez-Mellado, V.; Lluch, J. Description of a New Species: Dorylaimus Parasiticus, a Parasite of Amphibians in the Iberian Peninsula (Nematoda: Dorylaimida). J. Zool. 1995, 237, 169–177. [Google Scholar] [CrossRef]
- Manenti, R.; Mercurio, S.; Melotto, A.; Barzaghi, B.; Pennati, R.; Scarì, G.U.; Ficetola, G.F.; Epis, S.; Tecilla, M. A New Disease Caused by an Unidentified Etiological Agent Affects European Salamanders. Animals 2022, 12, 696. [Google Scholar] [CrossRef] [PubMed]
- Kelehear, C.; Saltonstall, K.; Torchin, M.E. Negative Effects of Parasitic Lung Nematodes on the Fitness of a Neotropical Toad (Rhinella Horribilis). Parasitology 2019, 146, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Thumsová, B.; López-Rojo, N.; Pérez, J.; Alonso, A.; Fisher, M.C.; Boyero, L. Microplastics Increase Susceptibility of Amphibian Larvae to the Chytrid Fungus Batrachochytrium Dendrobatidis. Sci. Rep. 2021, 11, 22438. [Google Scholar] [CrossRef] [PubMed]
- Rohr, J.R.; Schotthoefer, A.M.; Raffel, T.R.; Carrick, H.J.; Halstead, N.; Hoverman, J.T.; Johnson, C.M.; Johnson, L.B.; Lieske, C.; Piwoni, M.D.; et al. Agrochemicals Increase Trematode Infections in a Declining Amphibian Species. Nature 2008, 455, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Buss, N.; Hua, J. Host Exposure to a Common Pollutant Can Influence Diversity-Disease Relationships. J. Anim. Ecol. 2023, 92, 2151–2162. [Google Scholar] [CrossRef]
- Shields, J.D. Pathology and Mortality of the Lung Fluke Haematoloechus Longiplexus (Trematoda) in Rana Catesbeiana. J. Parasitol. 1987, 73, 1005. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Sutherland, D.R. Amphibian Deformities and Ribeiroia Infection: An Emerging Helminthiasis. Trends Parasitol. 2003, 19, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Holland, M.P. Echinostome-Induced Mortality Varies across Amphibian Species in the Field. J. Parasitol. 2010, 96, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Svinin, A.O.; Chikhlyaev, I.V.; Bashinskiy, I.W.; Osipov, V.V.; Neymark, L.A.; Ivanov, A.Y.; Stoyko, T.G.; Chernigova, P.I.; Ibrogimova, P.K.; Litvinchuk, S.N.; et al. Diversity of Trematodes from the Amphibian Anomaly P Hotspot: Role of Planorbid Snails. PLoS ONE 2023, 18, e0281740. [Google Scholar] [CrossRef] [PubMed]
- Doniol-Valcroze, P.; Mazepa, G.; Grimal, F.; Sourrouille, P.; Perrin, N.; Litvinchuk, S.N.; Crochet, P.A. Discovery of a Pelophylax Saharicus (Anura, Ranidae) Population in Southern France: A New Potentially Invasive Species of Water Frogs in Europe. Amphibia-Reptilia 2021, 42, 427–442. [Google Scholar] [CrossRef]
- Koprivnikar, J.; Marcogliese, D.J.; Rohr, J.R.; Orlofske, S.A.; Raffel, T.R.; Johnson, P.T.J. Macroparasite Infections of Amphibians: What Can They Tell Us? Ecohealth 2012, 9, 342–360. [Google Scholar] [CrossRef] [PubMed]
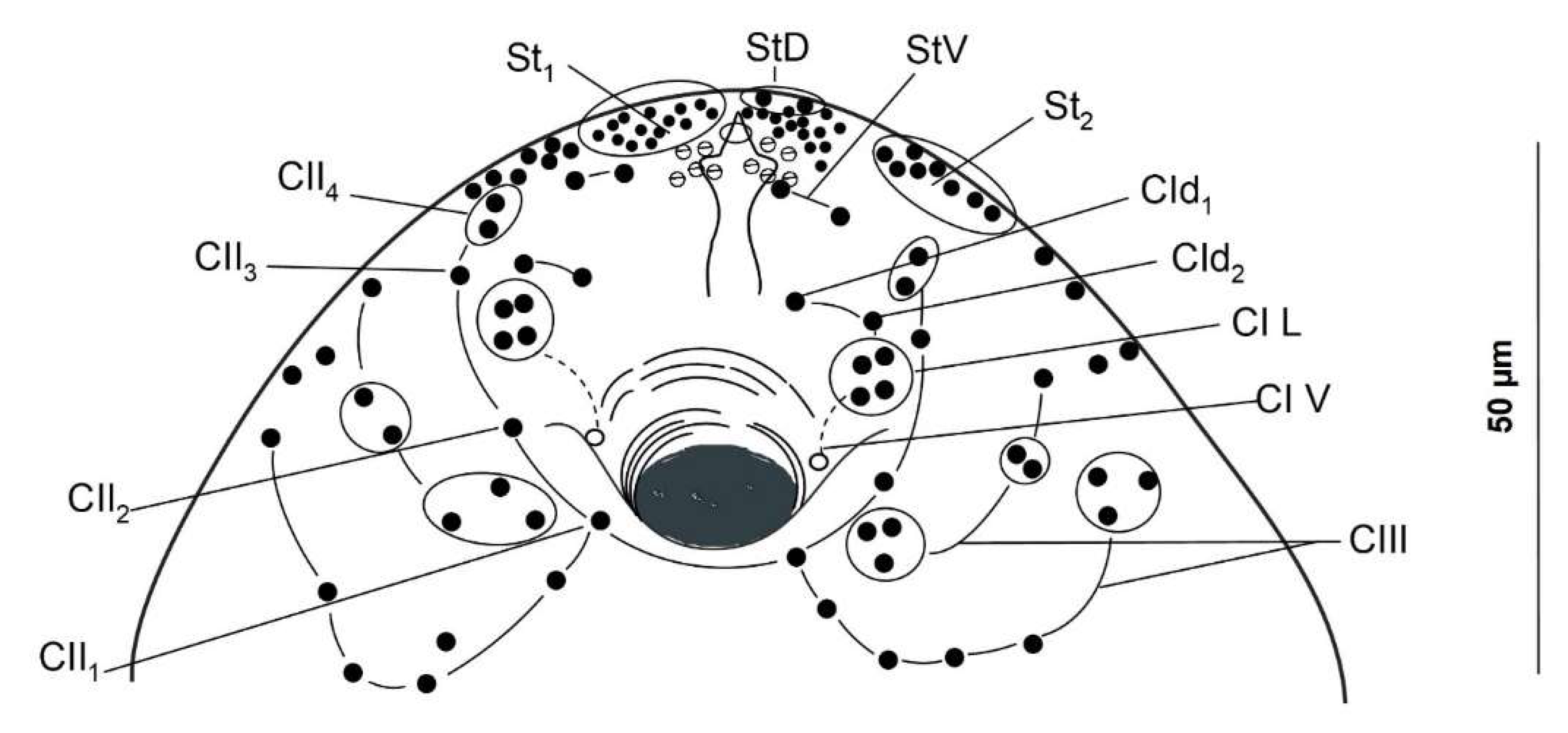
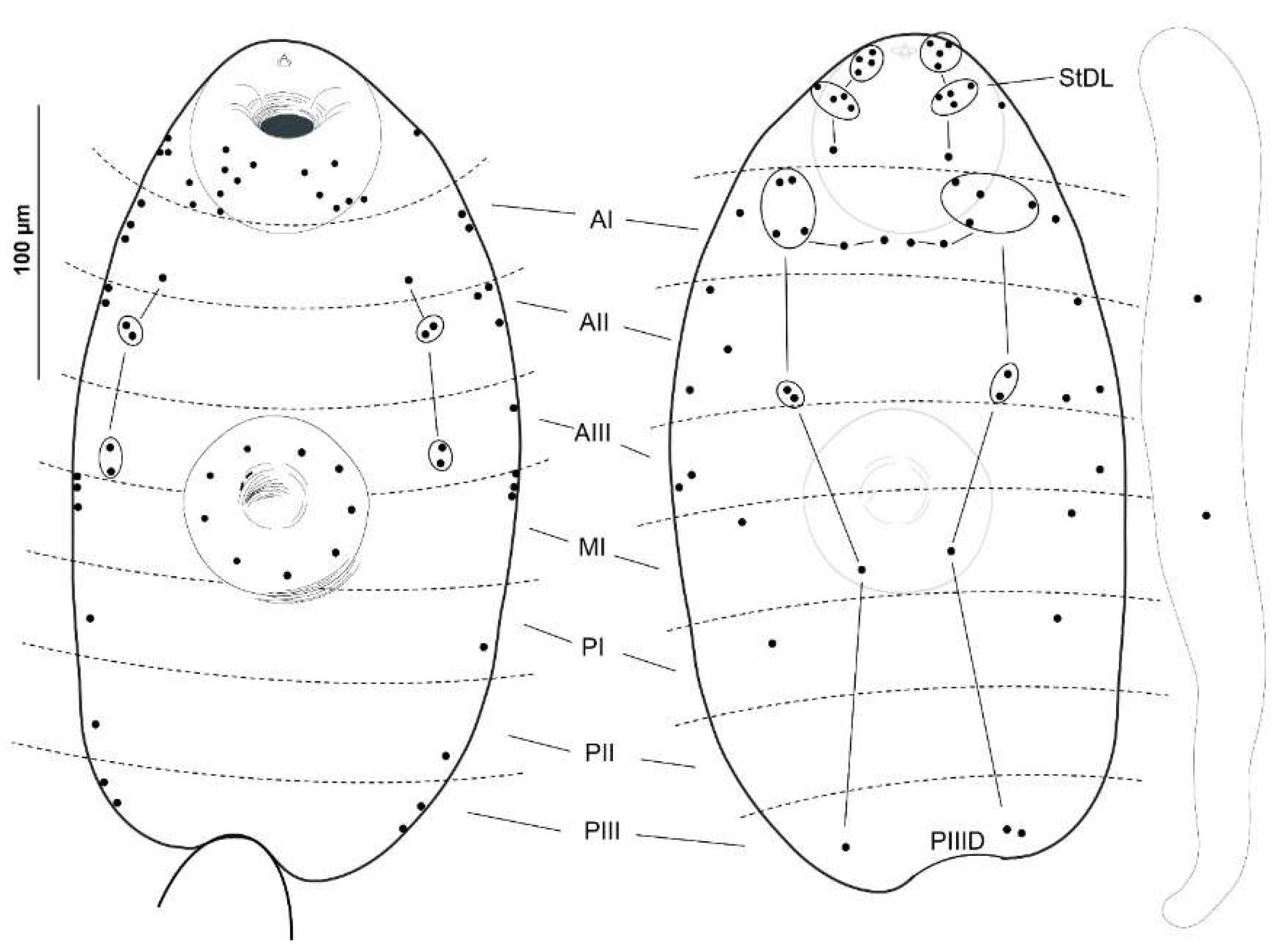
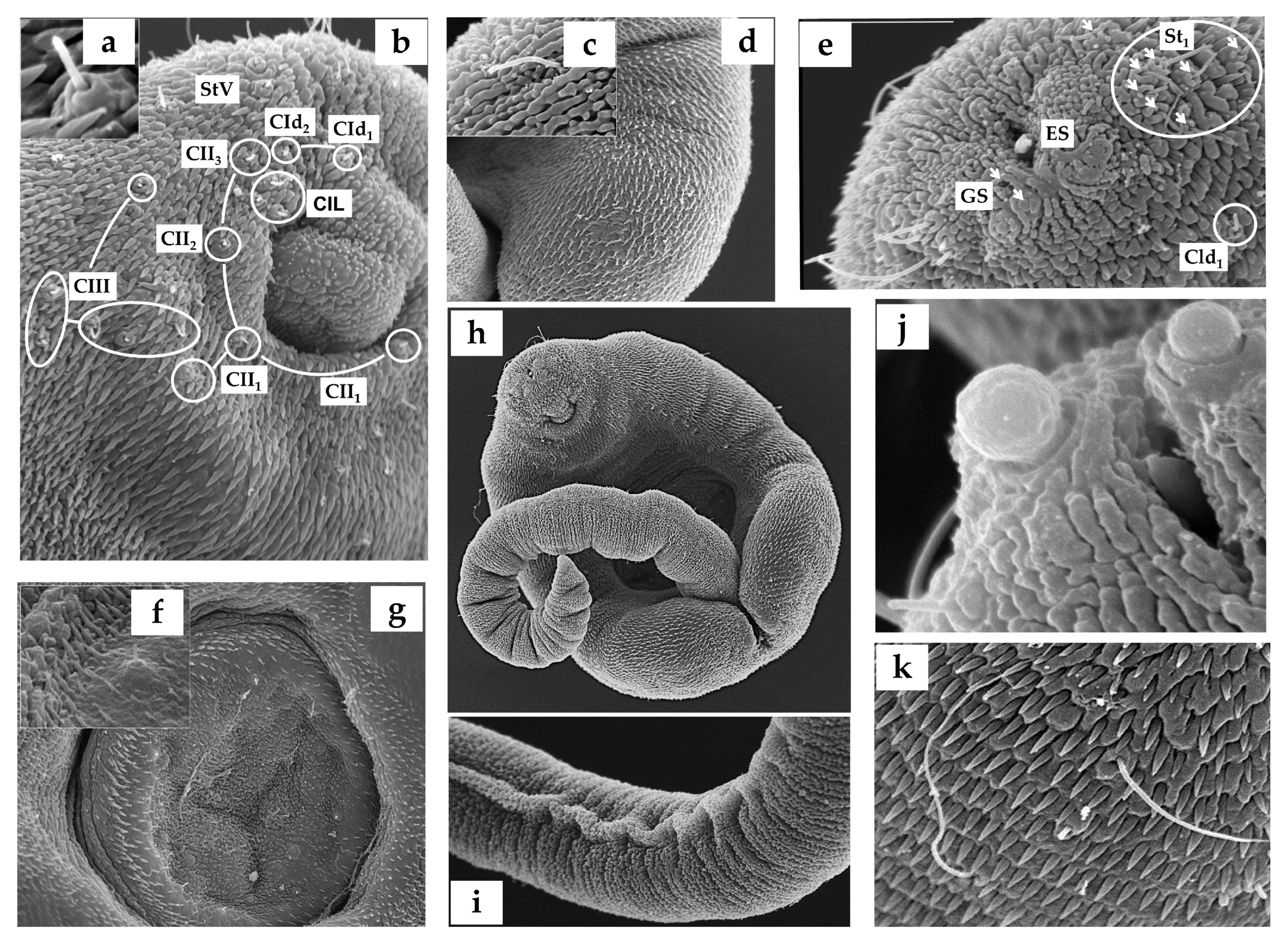

| Present Study | Grabda-Kazubska and Bayssade-Dufour (1999) Poland | |||||
|---|---|---|---|---|---|---|
| L. n. | L. n. | M. g | M. l. | P. b. | P. p. | |
| Cephalic | ||||||
| CI | 11; 02; 43; 14; 15 | 11; 02; 43; 14; 15 | 11; 02; 43; 14; 15 | 1–21; 02; 4(3)3; 14; 15 | 1–21; 0–12; 43; 14; 15 | 1–21; 12; 43; 14; 15 |
| CII | 11; 12; 13; 24; 20–225 | 11; 32; 13; 24; 18–225 | 2–31; 22; 23; 24; 17–215 | 11; 2(3)2; 33; 24; 18–255 | 21; 22; 23; 24; 16–225 | 21; 2(3)2; 23; 24; 16–245 |
| CIII | 21; 32; 33; 6–84; 35 | 21; 2–32; 23; 5–84; 35 | 21; 22; 23; 3–54; 3(4)5 | 21; 12; 23; 3–54; 45 | 21; 2(3)2; 2–33; 5–74; 3–45 | 2(1)1; 2(3)2; 2–43; 4–94; 35 |
| CIV | 21; 32; 13–4; 45 | 21; 22; 13–4; 45 | 21; 22; 2(0)3; 24; 3–45 | 21; 22; 23; 4–74; 45 | 2–31; 1–22; 1–33; 2–44; 45 | 1–31; 1–22; 2(3)3; 3–64; 45 |
| CIV-V | 14; 15 | 14; 15 | 24; 25 | 14; 05 | 1(2)4; 1(2)5 | 1(2)4; 1(2)5 |
| Body—Dorsal | ||||||
| AID | 2; 4 | 2; 4 | 2; 4 | 2; 4(3) | 2; 4 | 2; 4(3) |
| AIID | 2 | 2 | 1 | 1 | 2 | 2 |
| AIIID | - | - | 1 | 1 | - | - |
| PID | 1 (=MID) ** | 1 | 1 | 1 | 1 | 1 |
| PIID | - | - | - | - | - | - |
| PIIID | 1 | 1 | 1 | 1 | 1 | 1 |
| Body—Ventral | ||||||
| AIV | 1 | 1 | 1 | 1 | 1 | 1 |
| AIIV | 2 | 2 | 2 | 2 | 2 | 2 |
| AIIIV | 2 | 2 | 2 | 1(2) | 2 | 2 |
| PIV | - * | 1 | 1 | 1 | 1 | 1 |
| PIIV | - * | 1 | 1 | - | (1) | (1) |
| PIIIV | - * | 1 | (1) | - | - | - |
| Body—Lateral | ||||||
| AI to PIII | 17–18 | 16–21 | 18–24 | 24–27 | 18–23 | 18–26 |
| Acetabulum | ||||||
| SI | 9 | 9 | 9 | 9 | 9 | 9–11 |
| SII | - | - | 5–6 | 0–4 | 0–3 | 0–6 |
| Caudal | ||||||
| UD | 2 | 2 | 2 | 2 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poonlaphdecha, S.; Ribas, A.; Martínez-Silvestre, A.; Villa, M. New Data on the Larval Stages of Leptophallus nigrovenosus (Digenea, Plagiorchiata). Animals 2024, 14, 1154. https://doi.org/10.3390/ani14081154
Poonlaphdecha S, Ribas A, Martínez-Silvestre A, Villa M. New Data on the Larval Stages of Leptophallus nigrovenosus (Digenea, Plagiorchiata). Animals. 2024; 14(8):1154. https://doi.org/10.3390/ani14081154
Chicago/Turabian StylePoonlaphdecha, Srisupaph, Alexis Ribas, Albert Martínez-Silvestre, and Mercedes Villa. 2024. "New Data on the Larval Stages of Leptophallus nigrovenosus (Digenea, Plagiorchiata)" Animals 14, no. 8: 1154. https://doi.org/10.3390/ani14081154
APA StylePoonlaphdecha, S., Ribas, A., Martínez-Silvestre, A., & Villa, M. (2024). New Data on the Larval Stages of Leptophallus nigrovenosus (Digenea, Plagiorchiata). Animals, 14(8), 1154. https://doi.org/10.3390/ani14081154







