Fatty Acid Profile and Dietary Value of Thigh Meat of Broiler Chickens Receiving Mineral or Organic Forms of Zn
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
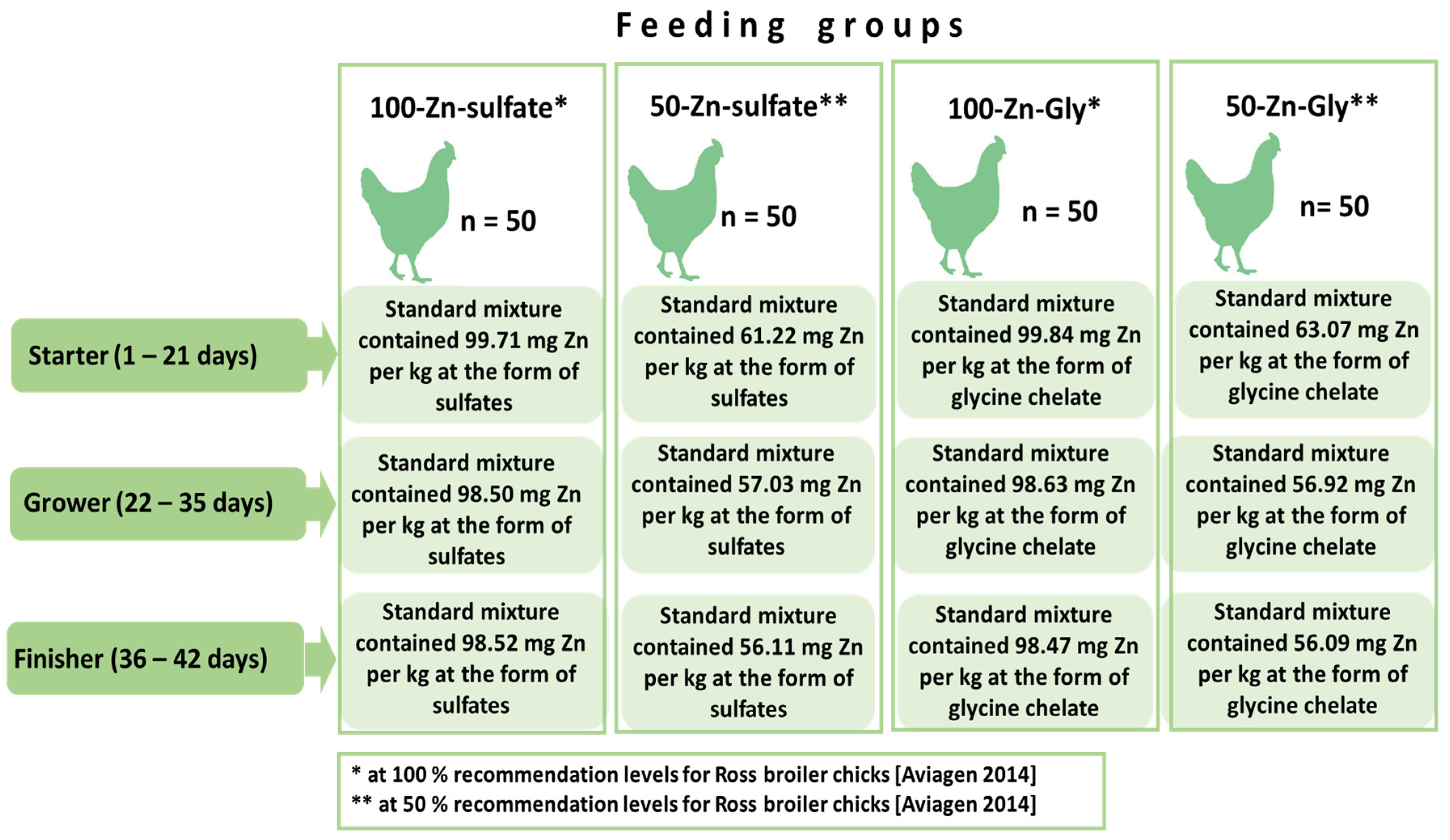
2.1. Experimental Factor
2.2. Description of the Experiment
- −
- Group 1: 100-Zn-sulphate, where chickens received Zn sulphate with feed covering 100% of the requirement.
- −
- Group 2: 50-Zn-sulphate, where chickens received Zn sulphate with feed covering 50% of the requirement.
- −
- Group 3: 100-Zn-Gly, where chickens received Zn glycine chelate with feed covering 100% of the requirement.
- −
- Group 4: 50-Zn-Gly, where chickens received Zn glycine chelate with feed covering 50% of the requirement.
2.3. Muscle Samples
2.4. Chemical Analyses
2.5. Calculations
2.5.1. Total Fatty Acid Content
2.5.2. Dietary Value of Meat
2.5.3. Statistical Analysis
3. Results
3.1. Chemical Composition and pH Value of Thigh Meat
3.2. Fatty Acids Profile
3.3. Dietary Value of Thigh Meat
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bird, J.K.; Calder, P.C.; Eggersdorfer, M. The role of n-3 long chain polyunsaturated fatty acids in cardiovascular disease prevention, and interactions with statins. Nutrients 2018, 10, 775. [Google Scholar] [CrossRef]
- Ristić-Medić, D.; Vučić, V.; Takić, M.; Karadžić, I.; Glibetić, M. Polyunsaturated fatty acids in health and disease. J. Serb. Chem. Soc. 2013, 78, 1269–1289. [Google Scholar] [CrossRef]
- Jachimowicz, K.; Winiarska-Mieczan, A.; Tomaszewska, E. The impact of herbal additives for poultry feed on the fatty acid profile of meat. Animals 2022, 12, 1054. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, Y.; Xiao, Y.; Chen, H.; Zhao, L.; Huang, M.; Zhou, G. Differences in physicochemical and nutritional properties of breast and thigh meat from crossbred chickens, commercial broilers, and spent hens. Asian-Australas. J. Anim. Sci. 2016, 29, 855–864. [Google Scholar] [CrossRef]
- Kwiecień, M.; Winiarska-Mieczan, A.; Milczarek, A.; Klebaniuk, R. Biological response of broiler chickens to decreasing dietary inclusion levels of zinc glycine chelate. Biol. Trace Elem. Res. 2017, 175, 204–213. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Kwiecień, M.; Winiarska-Mieczan, A.; Świetlicka, I.; Wawrzyniak, A. Effect of zinc level and source (zinc oxide vs. zinc glycine) on bone mechanical and geometric parameters, and histomorphology in male ross 308 broiler chicken. Braz. J. Poult. Sci. 2017, 19, 159–170. [Google Scholar] [CrossRef]
- Aviagen. Ross 308 Broiler Nutrition Specification; Aviagen Incorporated Publishing: Huntsville, AL, USA, 2014; Available online: www.aviagen.com (accessed on 14 February 2024).
- Monteiro, J.P.; Fuzo, C.A.; Ued, F.V.; Kaput, J. Dietary patterns related to zinc and polyunsaturated fatty acids intake are associated with serum linoleic/dihomo-γ-linolenic ratio in NHANES males and females. Sci. Rep. 2021, 11, 12215. [Google Scholar] [CrossRef]
- Chimhashu, T.; Malan, L.; Baumgartner, J.; van Jaarsveld, P.J.; Galetti, V.; Moretti, D.; Smuts, C.M.; Zimmermann, M.B. Sensitivity of fatty acid desaturation and elongation to plasma zinc concentration: A randomised controlled trial in Beninese children. Br. J. Nutr. 2018, 1196, 610–619. [Google Scholar] [CrossRef]
- Nakamura, M.T.; Nara, T.Y. Structure, function, and dietary regulation of delta-6, delta-5, and delta-9 desaturases. Annu. Rev. Nutr. 2004, 24, 345–376. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Kwiecień, M.; Kwiatkowska, K.; Baranowska-Wójcik, E.; Szwajgier, D.; Zaricka, E. Fatty acid profile, antioxidative status and dietary value of the breast muscle of broiler chickens receiving glycine-Zn chelates. Anim. Prod. Sci. 2020, 60, 1095–1102. [Google Scholar] [CrossRef]
- Takic, M.; Zekovic, M.; Terzic, B.; Stojsavljevic, A.; Mijuskovic, M.; Radjen, S.; Ristic-Medic, D. Zinc deficiency, plasma fatty acid profile and desaturase activities in hemodialysis patients: Is supplementation necessary? Front. Nutr. 2021, 8, 700450. [Google Scholar] [CrossRef]
- Knez, M.; Stangoulis, J.C.R.; Glibetic, M.; Tako, E. The linoleic acid: Dihomo-γ-linolenic acid ratio (LA:DGLA)-An emerging biomarker of Zn status. Nutrients 2017, 9, 825. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, A.J. Explaining longevity of different animals: Is membrane fatty acid composition the missing link? Age 2008, 30, 89–97. [Google Scholar] [CrossRef]
- Salim, H.M.; Jo, C.; Lee, B.D. Zinc in broiler feeding and nutrition. Avian Biol. Res. 2008, 1, 5–18. [Google Scholar] [CrossRef]
- Vieira, M.M.; Ribeiro, A.M.L.; Kessler, A.M.; Moraes, M.L.; Kunrath, M.A.; Ledur, V.S. Different sources of dietary zinc for broilers submitted to immunological, nutritional, and environmental challenge. J. App. Poulty Res. 2013, 22, 855–861. [Google Scholar] [CrossRef]
- Petrovič, V.; Nollet, L.; Kováč, G. Effect of dietary supplementation of trace elements on the growth performance and their distribution in the breast and thigh muscles depending on the age of broiler chickens. Acta Vet. Brno 2010, 79, 203–209. [Google Scholar] [CrossRef]
- NRC, National Research Council. Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- AOAC. Official Methods of Analysis, 17th ed.; AOAC International: Gaithersburg, MA, USA, 2000. [Google Scholar]
- Winiarska-Mieczan, A.; Kwiecień, M. The effects of copper-glycine complexes on chemical composition and sensory attributes of raw, cooked and grilled chicken meat. J. Food Sci. Technol. Mys. 2015, 52, 4226–4235. [Google Scholar] [CrossRef]
- Sousa, A.B.B.; de Oliveira Santos Júnior, O.; Visentainer, J.V.; de Almeida, N.M. Total lipid nutritional quality of the adipose tissue from the orbital cavity in Nile tilapia from continental aquaculture. Acta Sci. Anim. Sci. 2017, 39, 335–341. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Cartoni Mancinelli, A.; Vaudo, G.; Cavallo, M.; Castellini, C.; Mattioli, S. Indexing of fatty acids in poultry meat for its characterization in healthy human nutrition: A comprehensive application of the scientific literature and new proposals. Nutrients 2022, 14, 3110. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bobe, G.; Zimmerman, S.; Hammond, E.G.; Luhman, C.M.; Boylston, T.D.; Freeman, A.E.; Beitz, D.C. Physical and sensory properties of dairy products from cows with various milk fatty acid compositions. J. Agric. Food Chem. 2004, 52, 3422–3428. [Google Scholar] [CrossRef] [PubMed]
- Winiarska-Mieczan, A.; Jachimowicz, K.; Kwiecień, M.; Kislova, S.; Baranowska-Wójcik, E.; Zasadna, Z.; Yanovych, D.; Kowalczuk-Vasilev, E. The Impact of Zn, Cu and Fe chelates on the fatty-acid profile and dietary value of broiler-chicken thigh meat. Animals 2021, 11, 3115. [Google Scholar] [CrossRef]
- Wołoszyn, J.; Haraf, G.; Okruszek, A.; Wereńska, M.; Goluch, Z.; Teleszko, M. Fatty acid profiles and health lipid indices in the breast muscles of local Polish goose varieties. Poulty Sci. 2020, 99, 1216–1224. [Google Scholar] [CrossRef]
- Reed, S.; Qin, X.; Ran-Ressler, R.; Brenna, J.T.; Glahn, R.P.; Tako, E. Dietary zinc deficiency affects blood linoleic acid: Dihomo-γ-linolenic acid (LA:DGLA) ratio; a sensitive physiological marker of zinc status in vivo (Gallus gallus). Nutrients 2014, 6, 1164–1180. [Google Scholar] [CrossRef]
- Li, Y.; Monroig, O.; Zhang, L.; Wang, S.; Zheng, X.; Dick, J.R.; You, C.; Tocher, D.R. Vertebrate fatty acyl desaturase with Δ4 activity. Proc. Natl. Acad. Sci. USA 2010, 107, 16840–16845. [Google Scholar] [CrossRef]
- Ayala, S.; Brenner, R.R. Essential fatty acid status in zinc deficiency. Effect on lipid and fatty acid composition, desaturation activity and structure of microsomal membranes of rat liver and testes. Acta Physiol. Lat. Am. 1983, 33, 193–204. [Google Scholar]
- Gonzalez-Soto, M.; Mutch, D.M. Diet regulation of long-chain PUFA synthesis: Role of macronutrients, micronutrients, and polyphenols on Δ-5/Δ-6 desaturases and elongases 2/5. Adv. Nutr. 2021, 12, 980–994. [Google Scholar] [CrossRef] [PubMed]
- Cunnane, S.C.; Yang, J. Zinc deficiency impairs whole-body accumulation of polyunsaturates and increases the utilisation of linoleate for de novo synthesis in pregnant rats. J. Physiol. Pharmacol. 1995, 73, 1246–1252. [Google Scholar] [CrossRef]
- Knez, M.; Pantovic, A.; Zekovic, M.; Pavlovic, Z.; Glibetic, M.; Zec, M. Is there a link between zinc intake and status with plasma fatty acid profile and desaturase activities in dyslipidemic subjects? Nutrients 2019, 12, 93. [Google Scholar] [CrossRef]
- Reed, S.; Neuman, H.; Moscovich, S.; Glahn, R.P.; Koren, O.; Tako, E. Chronic zinc deficiency alters chick gut microbiota composition and function. Nutrients 2015, 7, 9768–9784. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.; Petkov, E.; Ignatova, M.; Lukic, M. Fatty acid composition of thigh meat in two lines of slow-growing chickens as affected by the access to pasture. Int. J. Innov. Appro. Agri. Res. 2018, 2, 123–132. [Google Scholar] [CrossRef]
- La Mantia, M.C.; Calì, M.; Petrocchi Jasinski, L.; Contò, M.; Meo Zilio, D.; Guarino Amato, M. Black Soldier Meal in Feed Could Adversely Affect Organic Broiler Meat Quality When Used for the Total or Half Replacement of Diet Proteins. Poultry 2024, 3, 66–84. [Google Scholar] [CrossRef]
- Qaid, M.M.; Al-Mufarrej, S.I.; Al-Garadi, M.A.; Al-Abdullatif, A.A.; Alqhtani, A.H.; Alhotan, R.A.; Alharthi, A.S.; BaZeyad, A.Y. Meat fatty acids profile including metabolic, qualitative, nutritional indices, and organoleptic evaluation as affected by Rumex nervosus leaves meal fortified broiler diets. Ital. J. Anim. Sci. 2023, 22, 1050–1066. [Google Scholar] [CrossRef]
- Saracila, M.; Untea, A.E.; Panaite, T.D.; Varzaru, I.; Oancea, A.; Turcu, R.P.; Vlaicu, P.A. Creeping wood sorrel and chromium picolinate effect on the nutritional composition and lipid oxidative stability of broiler meat. Antioxidants 2022, 11, 780. [Google Scholar] [CrossRef]
- Eide, D.J. Homeostatic and adaptive responses to zinc deficiency in Saccharomyces cerevisiae. J. Biol. Chem. 2009, 284, 18565–18569. [Google Scholar] [CrossRef]
| Starter, 1–21 Days | Grower, 22–35 Days | Finisher, 36–42 Days | |
|---|---|---|---|
| Ingredients (g·kg−1) | |||
| Maize | 244.4 | 400.0 | 400.0 |
| Wheat | 429.9 | 278.4 | 288.4 |
| Soybean meal (46% crude protein) | 250.0 | 249.7 | 228.7 |
| Soybean oil | 25.0 | 36.90 | 39.8 |
| Monocalcium phosphate | 9.00 | 9.00 | 8.10 |
| Limestone | 14.0 | 11.3 | 10.9 |
| Sodium bicarbonate | 0.80 | 0.80 | 0.80 |
| NaCl | 2.90 | 2.50 | 2.60 |
| Vitamin–mineral premix (without Zn) | 5.00 | 5.00 | 5.00 |
| Fat–protein concentrate | 10.0 | - | 10.0 |
| DL-methionine 99% | 3.00 | 2.30 | 2.30 |
| L-lysine HCl | 4.20 | 2.80 | 2.70 |
| L-threonine 99% | 1.80 | 1.30 | 0.70 |
| Nutrient value | |||
| Values calculated: | |||
| Metabolizable energy, MJ·kg−1 | 12.7 | 13.1 | 13.2 |
| Lysine, g·kg−1 | 12.9 | 11.3 | 10.9 |
| Methionine + cysteine, g·kg−1 | 9.30 | 8.30 | 8.1 |
| Values determined: | |||
| Crude protein, g·kg−1 | 202 | 182 | 181 |
| Crude fibre, g·kg−1 | 30.6 | 29.9 | 29.9 |
| Crude fat, g·kg−1 | 46.6 | 60.8 | 64.3 |
| Fatty acids profile, % | |||
| Myristic (14:0) | 0.03 | 0.07 | 0.07 |
| Palmitic (16:0) | 1.42 | 1.17 | 1.15 |
| Stearic (18:0) | 0.29 | 0.32 | 0.33 |
| Oleic (18:1n-9) | 2.25 | 2.24 | 2.19 |
| Linoleic (18:2n-6) | 4.72 | 4.95 | 4.96 |
| Linolenic (18:3n-3) | 1.18 | 0.86 | 0.89 |
| 100-Zn-Sulphate n = 10 | 50-Zn-Sulphate n = 10 | 100-Zn-Gly n = 10 | 50-Zn-Gly n = 10 | SEM | p-Value | |
|---|---|---|---|---|---|---|
| pH15 | 6.220 ± 0.32 | 6.200 ± 0.44 | 6.221 ± 0.28 | 6.206 ± 0.51 | 1.06 | 0.081 |
| pH45 | 5.431 ± 0.12 | 5.429 ± 0.32 | 5.433 ± 0.51 | 5.434 ± 0.39 | 0.52 | 0.113 |
| Moisture, % | 72.91 ± 3.03 | 72.96 ± 6.08 | 72.81 ± 3.76 | 72.98 ± 5.51 | 6.97 | 0.108 |
| Crude ash, % | 1.767 b ± 0.11 | 1.702 a ± 0.06 | 1.963 d ± 0.09 | 1.927 c ± 0.12 | 0.09 | 0.023 |
| Crude protein, % | 19.06 ± 1.23 | 19.08 ± 1.54 | 18.97 ± 1.22 | 18.84 ± 1.49 | 2.05 | 0.089 |
| Crude fat, % | 6.263 ± 2.04 | 6.258 ± 0.55 | 6.257 ± 0.49 | 6.253 ± 0.41 | 0.47 | 0.102 |
| Cholesterol, mg | 89.55 ± 7.56 | 89.48 ± 6.81 | 89.47 ± 6.55 | 89.53 ± 3.98 | 7.71 | 0.125 |
| Zn, mg | 13.67 a ± 0.94 | 13.70 a ± 1.01 | 15.87 b ± 1.34 | 15.79 b ± 1.44 | 0.12 | 0.022 |
| 100-Zn-Sulphate vs. 50-Zn-Sulphate | 100-Zn-Sulphate vs. 100-Zn-Gly | 100-Zn-Gly vs. 50-Zn-Gly | 50-Zn-Sulphate vs. 50-Zn-Gly | |
|---|---|---|---|---|
| pH15 | NS | NS | NS | NS |
| pH45 | NS | NS | NS | NS |
| Moisture, % | NS | NS | NS | NS |
| Crude ash, % | 0.031 | 0.048 | 0.038 | 0.028 |
| Crude protein, % | NS | NS | NS | NS |
| Crude fat, % | NS | NS | NS | NS |
| Cholesterol, mg | NS | NS | NS | NS |
| Zn, mg | NS | 0.025 | NS | 0.012 |
| Fatty Acids | 100-Zn-Sulphate n = 10 | 50-Zn-Sulphate n = 10 | 100-Zn-Gly n = 10 | 50-Zn-Gly n = 10 | SEM | p-Value | |
|---|---|---|---|---|---|---|---|
| 6:0 | Caproic | 0.012 a ± 0.004 | 0.012 a ± 0.001 | 0.011 a ± 0.002 | 0.017 b ± 0.004 | 0.03 | 0.023 |
| 8:0 | Caprylic | 0.013 a ± 0.004 | 0.013 a ± 0.002 | 0.016 b ± 0.005 | 0.017 b ± 0.004 | 0.05 | 0.035 |
| 10:0 | Capric | 0.013 a ± 0.002 | 0.015 b ± 0.03 | 0.014 ab ± 0.002 | 0.013 a ± 0.003 | 0.05 | 0.041 |
| 12:0 | Lauric | 0.236 b ± 0.03 | 0.230 ab ± 0.01 | 0.224 a ± 0.02 | 0.222 a ± 0.03 | 0.09 | 0.047 |
| 14:0 | Myristic | 0.418 a ± 0.11 | 0.420 a ± 0.06 | 0.492 b ± 0.07 | 0.499 b ± 0.06 | 0.03 | 0.008 |
| 15:0 | Pentadecanoic | 0.114 a ± 0.02 | 0.111 a ± 0.01 | 0.123 b ± 0.02 | 0.124 b ± 0.02 | 0.03 | 0.029 |
| 16:0 | Palmitic | 22.26 ± 2.13 | 22.29 ± 1.34 | 22.27 ± 1.70 | 22.26 ± 2.27 | 1.67 | 0.077 |
| 17:0 | Margaric | 0.152 b ± 0.02 | 0.155 b ± 0.03 | 0.136 a ± 0.02 | 0.133 a ± 0.02 | 0.02 | 0.036 |
| 18:0 | Stearic | 6.544 b ± 0.68 | 6.639 c ± 0.99 | 6.392 a ± 0.53 | 6.410 a ± 0.59 | 0.18 | 0.006 |
| 20:0 | Arachidic | 0.144 b ± 0.05 | 0.138 ab ± 0.02 | 0.134 b ± 0.03 | 0.130 a ± 0.03 | 0.03 | 0.042 |
| 16:1n-7 | Palmitoleic | 2.681 b ± 0.35 | 2.682 b ± 0.43 | 2.563 a ± 0.67 | 2.567 a ± 0.65 | 0.45 | 0.039 |
| 17:1 | Heptadecenoic | 0.046 ± 0.02 | 0.044 ± 0.01 | 0.048 ± 0.02 | 0.050 ± 0.02 | 0.02 | 0.066 |
| 18:1n-9 | Oleic | 34.72 ± 1.34 | 32.87 ± 1.67 | 32.63 ± 2.12 | 33.54 ± 2.24 | 4.37 | 0.054 |
| 18:1n-11 | Vaccenic | 2.469 ± 1.17 | 2.475 ± 0.45 | 2.480 ± 0.52 | 2.264 ± 0.42 | 1.11 | 0.059 |
| 20:1n-7 | Paullinic | 0.073 a ± 0.02 | 0.073 a ± 0.01 | 0.087 ab ± 0.02 | 0.095 b ± 0.01 | 0.03 | 0.045 |
| 20:1n-9 | Gondoic | 0.022 a ± 0.02 | 0.028 a ± 0.01 | 0.030 ab ± 0.01 | 0.036 b ± 0.01 | 0.04 | 0.038 |
| 20:1n-11 | Cetoleic | 0.295 ± 0.05 | 0.305 ± 0.06 | 0.294 ± 0.08 | 0.298 ± 0.07 | 0.01 | 0.066 |
| 18:2n-6 | Linoleic | 25.53 c ± 1.47 | 25.16 b ± 1.12 | 24.70 a ± 1.68 | 24.62 a ± 1.62 | 2.27 | 0.033 |
| 20:2n-6 | Eicosadienoic | 0.346 ± 0.07 | 0.349 ± 0.07 | 0.345 ± 0.11 | 0.344 ± 0.01 | 0.06 | 0.082 |
| 18:3n-6 | Linolenic | 2.322 a ± 0.16 | 2.324 a ± 0.16 | 2.468 b ± 0.55 | 2.525 c ± 0.36 | 0.07 | 0.020 |
| 20:3n-3 | Eicosatrienoic | 0.164 a ± 0.04 | 0.173 ab ± 0.02 | 0.172 ab ± 0.02 | 0.184 b ± 0.01 | 0.01 | 0.041 |
| 20:4n-6 | Arachidonic | 0.118 ± 0.04 | 0.117 ± 0.02 | 0.116 ± 0.03 | 0.119 ± 0.03 | 0.02 | 0.076 |
| ΣSFA | 29.85 ± 2.07 | 30.02 ± 2.08 | 29.81 ± 1.98 | 29.82 ± 2.48 | 1.77 | 0.089 | |
| ΣMUFA | 40.26 b ± 1.41 | 38.43 ab ± 1.48 | 38.09 a ± 1.88 | 38.80 ab ± 1.95 | 1.04 | 0.043 | |
| ΣPUFA | 28.48 ± 1.55 | 28.12 ± 1.12 | 27.80 ± 1.68 | 27.79 ± 1.82 | 0.55 | 0.062 | |
| ΣUFA | 68.74 b ± 2.22 | 66.55 a ± 1.78 | 65.89 a ± 3.01 | 66.58 a ± 2.83 | 6.04 | 0.035 | |
| ΣPUFAn-3 | 2.486 a ± 0.18 | 2.497 a ± 0.17 | 2.640 b ± 0.53 | 2.709 c ± 0.35 | 0.03 | 0.020 | |
| ΣPUFAn-6 | 25.99 ± 1.47 | 25.63 ± 1.10 | 25.16 ± 1.67 | 25.08 ± 1.62 | 0.11 | 0.059 | |
| ΣPUFA/SFA | 0.960 b ± 0.11 | 0.941 a ± 0.08 | 0.938 a ± 0.11 | 0.941 a ± 0.13 | 0.03 | 0.009 | |
| ΣSFA/UFA | 0.426 ± 0.04 | 0.437 ± 0.04 | 0.444 ± 0.05 | 0.440 ± 0.05 | 0.03 | 0.067 | |
| n-6/n-3 | 10.49 c ± 0.77 | 10.30 c ± 0.85 | 10.04 b ± 3.04 | 9.360 a ± 1.04 | 0.10 | 0.033 | |
| 100-Zn-Sulphate vs. 50-Zn-Sulphate | 100-Zn-Sulphate vs. 100-Zn-Gly | 100-Zn-Gly vs. 50-Zn-Gly | 50-Zn-Sulphate vs. 50-Zn-Gly | ||
|---|---|---|---|---|---|
| 6:0 | Caproic | NS | NS | 0.018 | 0.041 |
| 8:0 | Caprylic | NS | NS | NS | 0.027 |
| 10:0 | Capric | 0.036 | NS | NS | 0.037 |
| 12:0 | Lauric | NS | 0.017 | NS | NS |
| 14:0 | Myristic | NS | 0.009 | NS | 0.040 |
| 15:0 | Pentadecanoic | NS | 0.029 | NS | 0.044 |
| 16:0 | Palmitic | NS | NS | NS | NS |
| 17:0 | Margaric | NS | 0.033 | NS | 0.024 |
| 18:0 | Stearic | 0.029 | 0.027 | NS | 0.046 |
| 20:0 | Arachidic | NS | NS | 0.018 | NS |
| 16:1n-7 | Palmitoleic | NS | 0.011 | NS | 0.019 |
| 17:1 | Heptadecenoic | NS | NS | NS | NS |
| 18:1n-9 | Oleic | NS | NS | NS | NS |
| 18:1n-11 | Vaccenic | NS | NS | NS | NS |
| 20:1n-7 | Paullinic | NS | NS | NS | 0.027 |
| 20:1n-9 | Gondoic | NS | NS | NS | 0.045 |
| 20:1n-11 | Cetoleic | NS | NS | NS | NS |
| 18:2n-6 | Linoleic | 0.039 | 0.009 | NS | 0.019 |
| 20:2n-6 | Eicosadienoic | NS | NS | NS | NS |
| 18:3n-6 | Linolenic | NS | 0.043 | 0.009 | 0.011 |
| 20:3n-3 | Eicosatrienoic | NS | NS | NS | NS |
| 20:4n-6 | Arachidonic | NS | NS | NS | NS |
| ΣSFA | NS | NS | NS | NS | |
| ΣMUFA | NS | 0.047 | NS | NS | |
| ΣPUFA | NS | NS | NS | NS | |
| ΣUFA | 0.029 | 0.039 | NS | NS | |
| ΣPUFAn-3 | NS | 0.038 | 0.030 | 0.008 | |
| ΣPUFAn-6 | NS | NS | NS | NS | |
| ΣPUFA/SFA | 0.037 | 0.029 | NS | NS | |
| ΣSFA/UFA | NS | NS | NS | NS | |
| PUFAn-6/PUFAn-3 | NS | 0.035 | 0.041 | 0.007 | |
| ΣSFA | ΣMUFA | ΣPUFA | ΣUFA | ΣPUFAn-3 | ΣPUFAn-6 | Cholesterol, mg | |
|---|---|---|---|---|---|---|---|
| Zn content, mg | −0.699 | −0.560 | −0.897 | −0.669 | −0.959 | −0.936 | −0.248 |
| p-value | 0.300 | 0.440 | 0.103 | 0.331 | 0.041 | 0.064 | 0.752 |
| 100-Zn-Sulphate n = 10 | 50-Zn-Sulphate n = 10 | 100-Zn-Gly n = 10 | 50-Zn-Gly n = 10 | SEM | p-Value | |
|---|---|---|---|---|---|---|
| AIs | 0.353 a ± 0.04 | 0.364 b ± 0.03 | 0.373 c ± 0.04 | 0.369 b ± 0.05 | 0.02 | 0.042 |
| TIs | 0.720 a ± 0.08 | 0.742 c ± 0.05 | 0.738 c ± 0.61 | 0.730 b ± 0.01 | 0.24 | 0.023 |
| H/H | 2.793 d ± 0.35 | 2.674 b ± 0.21 | 2.652 a ± 0.30 | 2.703 c ± 0.37 | 0.19 | 0.021 |
| HPI | 2.872 d ± 0.34 | 2.760 c ± 0.20 | 2.712 a ± 0.29 | 2.750 b ± 0.04 | 0.16 | 0.008 |
| 100-Zn-Sulphate vs. 50-Zn-Sulphate | 100-Zn-Sulphate vs. 100-Zn-Gly | 100-Zn-Gly vs. 50-Zn-Gly | 50-Zn-Sulphate vs. 50-Zn-Gly | |
|---|---|---|---|---|
| AIs | 0.025 | 0.019 | 0.044 | NS |
| TIs | 0.039 | 0.034 | 0.048 | 0.043 |
| H/H | 0.016 | 0.009 | 0.008 | 0.045 |
| HPI | 0.033 | 0.019 | 0.032 | 0.043 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winiarska-Mieczan, A.; Kwiecień, M.; Purwin, C.; Jachimowicz-Rogowska, K.; Borsuk-Stanulewicz, M.; Pogorzelska-Przybyłek, P.; Kiczorowska, B. Fatty Acid Profile and Dietary Value of Thigh Meat of Broiler Chickens Receiving Mineral or Organic Forms of Zn. Animals 2024, 14, 1156. https://doi.org/10.3390/ani14081156
Winiarska-Mieczan A, Kwiecień M, Purwin C, Jachimowicz-Rogowska K, Borsuk-Stanulewicz M, Pogorzelska-Przybyłek P, Kiczorowska B. Fatty Acid Profile and Dietary Value of Thigh Meat of Broiler Chickens Receiving Mineral or Organic Forms of Zn. Animals. 2024; 14(8):1156. https://doi.org/10.3390/ani14081156
Chicago/Turabian StyleWiniarska-Mieczan, Anna, Małgorzata Kwiecień, Cezary Purwin, Karolina Jachimowicz-Rogowska, Marta Borsuk-Stanulewicz, Paulina Pogorzelska-Przybyłek, and Bożena Kiczorowska. 2024. "Fatty Acid Profile and Dietary Value of Thigh Meat of Broiler Chickens Receiving Mineral or Organic Forms of Zn" Animals 14, no. 8: 1156. https://doi.org/10.3390/ani14081156





