Preliminary Results on the Effects of Soybean Isoflavones on Growth Performance and Ruminal Microbiota in Fattening Goats
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Animal Management
2.2. Growth and Feed Intake Data
2.3. Body Measurements
2.4. Slaughter Procedure, Carcass and Non-Carcass Morphometric Measurements, and Butchering
2.5. Meat Quality Parameters
2.6. Blood Parameters
2.7. Ruminal Microbiota Sampling and Microbial Diversity Analyses
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
3.2. Biometrics and Morphometrics
3.3. Mass of Non-Carcass Components
3.4. Meat Quality
3.5. Biochemical Indicators in Serum
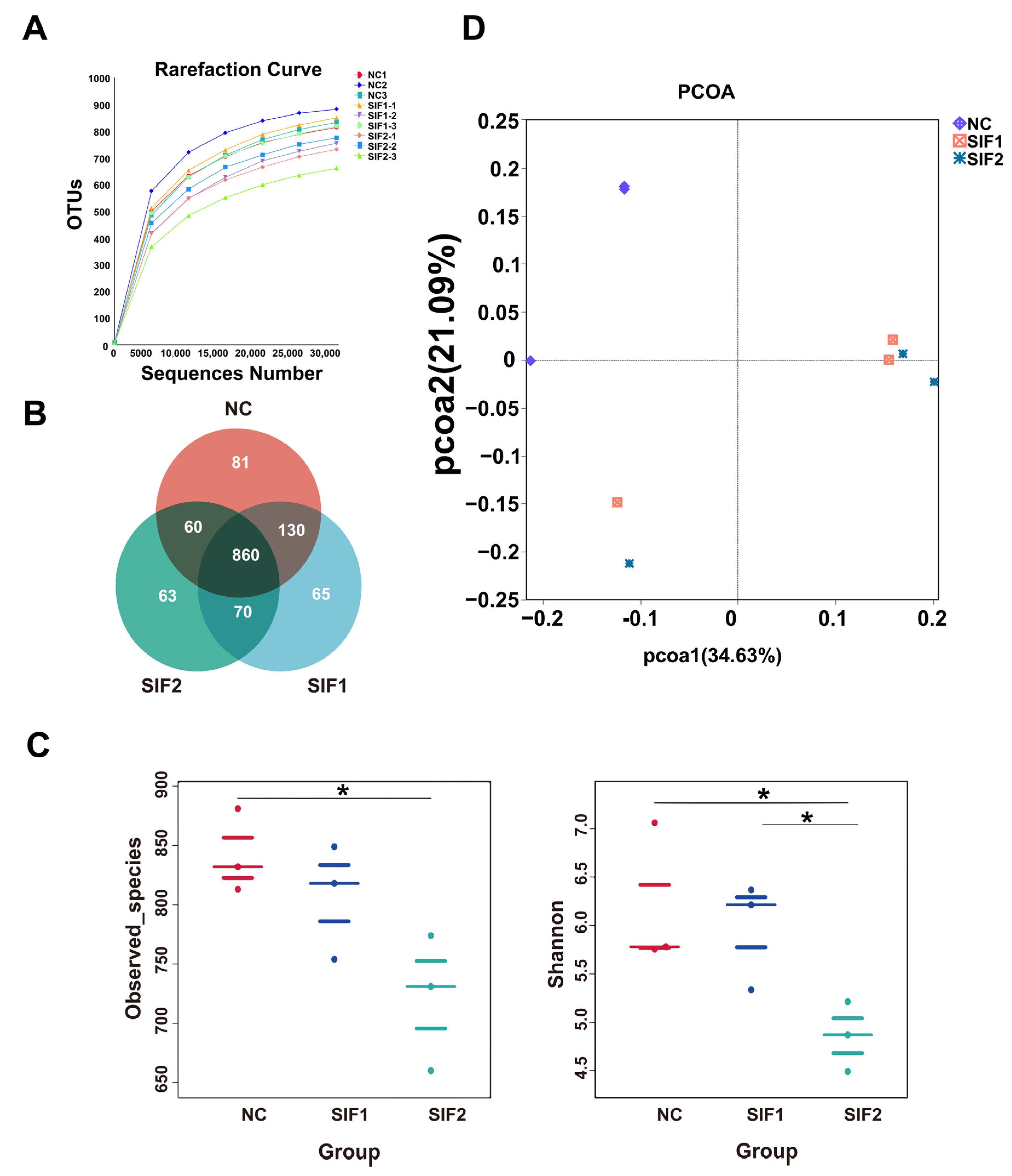
3.6. Ruminal Microbiota Diversity
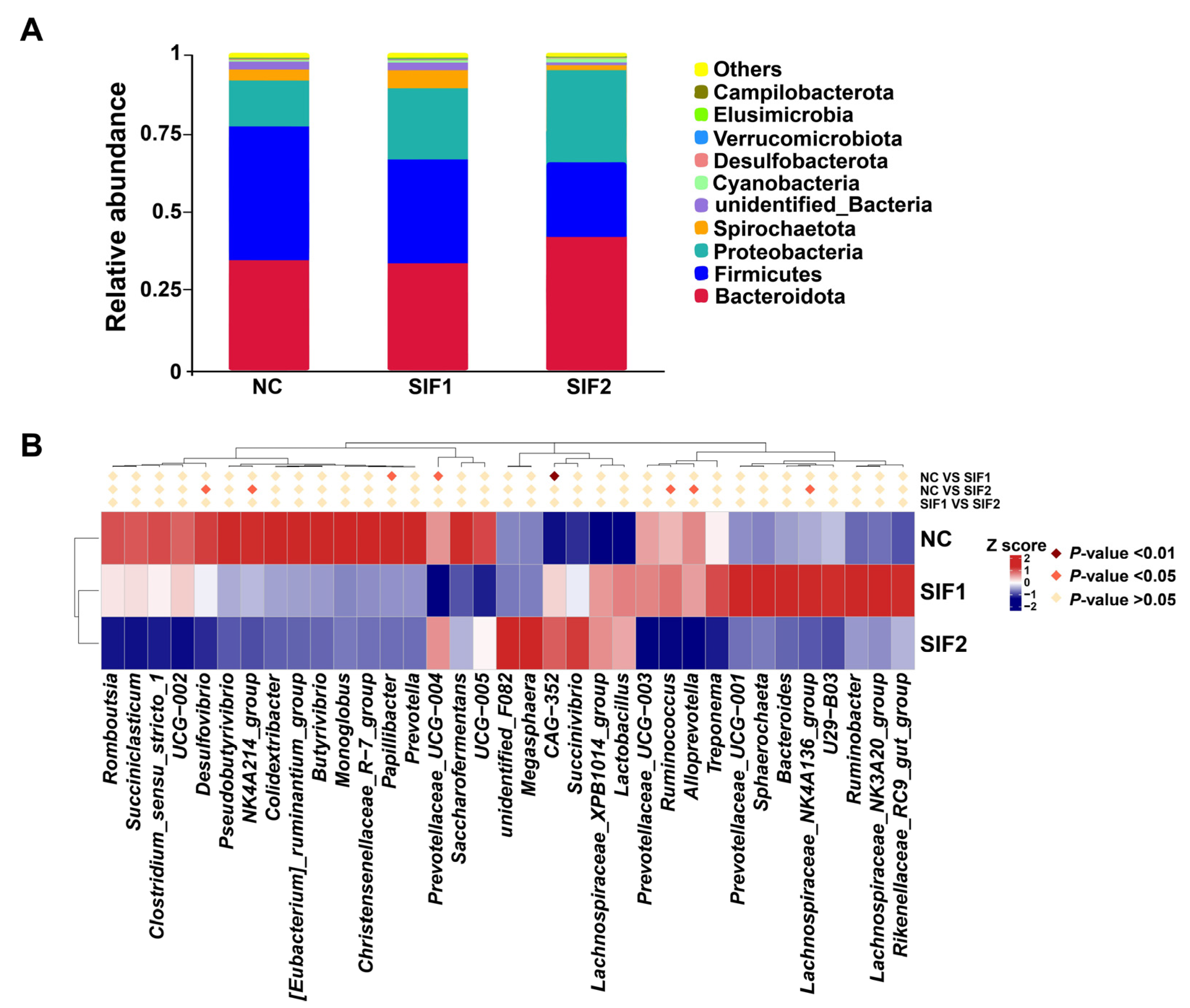
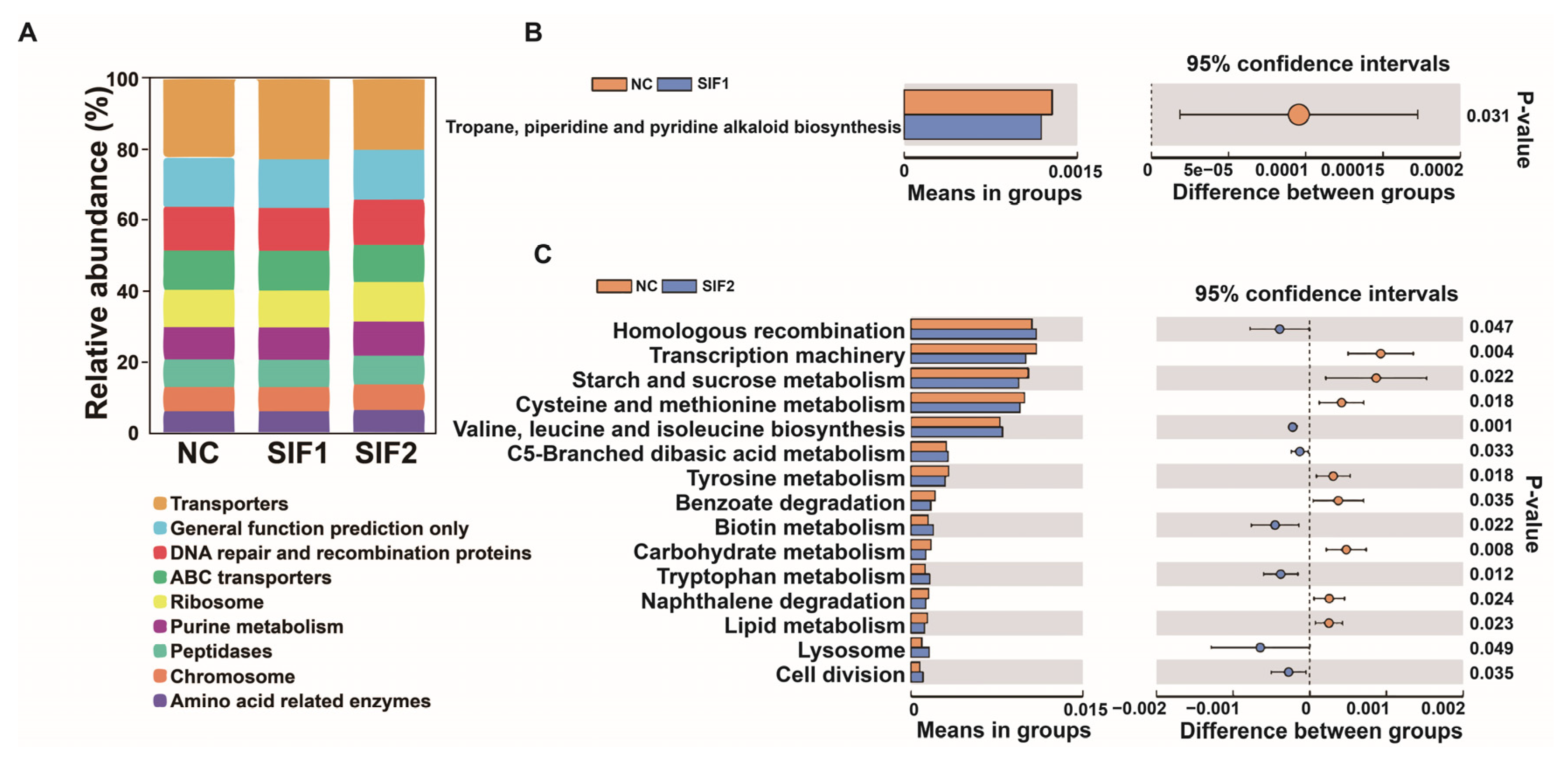
3.7. Ruminal Microbiota Composition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korma, S.A.; Li, L.; Wei, W.; Liu, P.; Zhang, X.; Bakry, I.A.; An, P.; Abdrabo, K.A.E.; Manzoor, M.F.; Umair, M.; et al. A Comparative Study of Milk Fat Extracted from the Milk of Different Goat Breeds in China: Fatty Acids, Triacylglycerols and Thermal and Spectroscopic Characterization. Biomolecules 2022, 12, 730. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, R.; Zhao, Z.; Liu, N.; Cheng, J.; Guo, M. Proteomic characterization and comparison of milk fat globule membrane proteins of Saanen goat milk from 3 habitats in China using SWATH-MS technique. J. Dairy Sci. 2023, 106, 2289–2302. [Google Scholar] [CrossRef] [PubMed]
- Szabò, S.; Barth, K.; Graml, C.; Futschik, A.; Palme, R.; Waiblinger, S. Introducing young dairy goats into the adult herd after parturition reduces social stress. J. Dairy Sci. 2013, 96, 5644–5655. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.L.; Yang, X.; Zhang, J.; Wang, W.; Liu, D.; Hou, B.; Bai, T.; Zhang, R.; Zhang, Y.; Liu, H.; et al. Effects of forage type on the rumen microbiota, growth performance, carcass traits, and meat quality in fattening goats. Front. Vet. Sci. 2023, 10, 1147685. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.S.; Noor, N.N.M.; Nasruddin, N.N.A.B.M. Evaluation of growth parameters and body condition score on weaning stages of Saanen goats. J. Adv. Vet. Anim. Res. 2022, 9, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Mojapelo, M.M.; Lehloenya, K.C. Effect of selenium supplementation on attainment of puberty in Saanen male goat kids. Theriogenology 2019, 138, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Lv, Z.; Gan, L.; Guo, Y. Transcriptomics-Related Mechanisms of Supplementing Laying Broiler Breeder Hens with Dietary Daidzein to Improve the Immune Function and Growth Performance of Offspring. J. Agric. Food Chem. 2018, 66, 2049–2060. [Google Scholar] [CrossRef] [PubMed]
- Kasparovska, J.; Pecinkova, M.; Dadakova, K.; Krizova, L.; Hadrova, S.; Lexa, M.; Lochman, J.; Kasparovsky, T. Effects of Isoflavone-Enriched Feed on the Rumen Microbiota in Dairy Cows. PLoS ONE 2016, 11, e0154642. [Google Scholar] [CrossRef] [PubMed]
- Wyse, J.; Latif, S.; Gurusinghe, S.; McCormick, J.; Weston, L.A.; Stephen, C.P. Phytoestrogens: A Review of Their Impacts on Reproductive Physiology and Other Effects upon Grazing Livestock. Animals 2022, 12, 2709. [Google Scholar] [CrossRef] [PubMed]
- Ohishi, T.; Miyoshi, N.; Mori, M.; Sagara, M.; Yamori, Y. Health Effects of Soy Isoflavones and Green Tea Catechins on Cancer and Cardiovascular Diseases Based on Urinary Biomarker Levels. Molecules 2022, 27, 8899. [Google Scholar] [CrossRef]
- Ahn, H.; Park, Y.K. Soy isoflavone supplementation improves longitudinal bone growth and bone quality in growing female rats. Nutrition 2017, 37, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Li, F.; Xiong, X.; Kong, X.; Zhang, B.; Yuan, X.; Fan, J.; Duan, Y.; Geng, M.; Li, L.; et al. Soy isoflavones modulate adipokines and myokines to regulate lipid metabolism in adipose tissue, skeletal muscle and liver of male Huanjiang mini-pigs. Mol. Cell Endocrinol. 2013, 365, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Tsugami, Y.; Matsunaga, K.; Suzuki, T.; Nishimura, T.; Kobayashi, K. Isoflavones and their metabolites influence the milk component synthesis ability of mammary epithelial cells through prolactin/STAT5 signaling. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Tsugami, Y.; Suzuki, N.; Suzuki, T.; Nishimura, T.; Kobayashi, K. Regulatory Effects of Soy Isoflavones and Their Metabolites in Milk Production via Different Ways in Mice. J. Agric. Food Chem. 2020, 68, 5847–5853. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Aschenbach, J.R. Ureases in the gastrointestinal tracts of ruminant and monogastric animals and their implication in urea-N/ammonia metabolism: A review. J. Adv. Res. 2018, 13, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Plaizier, J.C.; Danesh Mesgaran, M.; Derakhshani, H.; Golder, H.; Khafipour, E.; Kleen, J.L.; Lean, I.; Loor, J.; Penner, G.; Zebeli, Q. Review: Enhancing gastrointestinal health in dairy cows. Animal 2018, 12, s399–s418. [Google Scholar] [CrossRef] [PubMed]
- Gunnarsson, D.; Selstam, G.; Ridderstråle, Y.; Holm, L.; Ekstedt, E.; Madej, A. Effects of dietary phytoestrogens on plasma testosterone and triiodothyronine (T3) levels in male goat kids. Acta Vet. Scand. 2009, 51, 51. [Google Scholar] [CrossRef] [PubMed]
- Martins, S.R.; Chizzotti, M.L.; Yamamoto, S.M.; Rodrigues, R.T.; Busato, K.C.; Silva, T.S. Carcass and non-carcass component yields of crossbred Boer and Brazilian semiarid indigenous goats subjected to different feeding levels. Trop. Anim. Health Prod. 2014, 46, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Dieters, L.S.E.; Meale, S.J.; Quigley, S.P.; Hoffman, L.C. Meat quality characteristics of lot-fed Australian Rangeland goats are unaffected by live weight at slaughter. Meat Sci. 2021, 175, 108437. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Zhong, H.M.; An, Z.G.; Niu, K.F.; Zhang, X.X.; Yao, Z.Q.; Yuan, J.; Nie, P.; Yang, L.G. Dung treated by high-temperature composting is an optimal bedding material for suckling calves according to analyses of microbial composition, growth performance, health status, and behavior. J. Dairy Sci. 2023, 106, 4785–4798. [Google Scholar] [CrossRef]
- Villegas-Rivera, G.; Vargas-Cabrera, Y.; González-Silva, N.; Aguilera-García, F.; Gutiérrez-Vázquez, E.; Bravo-Patiño, A.; Cajero-Juárez, M.; Baizabal-Aguirre, V.M.; Valdez-Alarcón, J.J. Evaluation of DNA extraction methods of rumen microbial populations. World J. Microbiol. Biotechnol. 2013, 29, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Sotoca, A.M.; Ratman, D.; van der Saag, P.; Ström, A.; Gustafsson, J.A.; Vervoort, J.; Rietjens, I.M.; Murk, A.J. Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERalpha/ERbeta ratio. J. Steroid Biochem. Mol. Biol. 2008, 112, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Qi, H.; Gao, X. Daidzein promotes milk synthesis and proliferation of mammary epithelial cells via the estrogen receptor α-dependent NFκB1 activation. Anim. Biotechnol. 2022, 33, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Li, Y.; Du, W.; Zheng, N.; Wang, J.; Zhao, S. Effect of dietary biochanin A on lactation performance, antioxidant capacity, rumen fermentation and rumen microbiome of dairy goat. Front. Microbiol. 2023, 14, 1101849. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Jiang, X.R.; Wei, Z.X.; Cai, L.; Yin, J.D.; Li, X.L. Effects of soybean isoflavones on the growth performance, intestinal morphology and antioxidative properties in pigs. Animal 2020, 14, 2262–2270. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Khadijah, W.E.; Abdullah, R.B. Feeding soywaste or pellet on performance and carcass characteristics of post-weaning kids. Trop. Anim. Health Prod. 2016, 48, 1287–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, M.; Li, Q.; Gao, Y.; Li, Q.; Li, J.; Cao, Y. Transcriptome profiling of longissimus lumborum in Holstein bulls and steers with different beef qualities. PLoS ONE 2020, 15, e0235218. [Google Scholar] [CrossRef] [PubMed]
- Szmańko, T.; Lesiów, T.; Górecka, J. The water-holding capacity of meat: A reference analytical method. Food Chem. 2021, 357, 129727. [Google Scholar] [CrossRef] [PubMed]
- Gou, Z.Y.; Cui, X.Y.; Li, L.; Fan, Q.L.; Lin, X.J.; Wang, Y.B.; Jiang, Z.Y.; Jiang, S.Q. Effects of dietary incorporation of linseed oil with soybean isoflavone on fatty acid profiles and lipid metabolism-related gene expression in breast muscle of chickens. Animal 2020, 14, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.Y.; Jiang, S.Q.; Lin, Y.C.; Xi, P.B.; Yu, D.Q.; Wu, T.X. Effects of soybean isoflavone on growth performance, meat quality, and antioxidation in male broilers. Poult. Sci. 2007, 86, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.; Zhong, L.; Luo, H.; Meng, L.; Dong, Y.; Qi, Z.; Wang, H. A comparative analysis of carcass and meat traits, and rumen bacteria between Chinese Mongolian sheep and Dorper × Chinese Mongolian crossbred sheep. Animal 2022, 16, 100503. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yin, L.; Liu, L.; Lan, X.; He, J.; Wan, F.; Shen, W.; Tang, S.; Tan, Z.; Yang, Y. Tannic acid reduced apparent protein digestibility and induced oxidative stress and inflammatory response without altering growth performance and ruminal microbiota diversity of Xiangdong black goats. Front. Vet. Sci. 2022, 9, 1004841. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, P.; Zhang, K.; Zhang, L.; Wang, X.; Li, L.; Zhang, H. Distinct Stage Changes in Early-Life Colonization and Acquisition of the Gut Microbiota and Its Correlations with Volatile Fatty Acids in Goat Kids. Front. Microbiol. 2020, 11, 584742. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.R.G.; de Souza Duarte, M.; La Reau, A.J.; Chaves, I.Z.; de Oliveira Mendes, T.A.; Detmann, E.; Bento, C.B.P.; Mercadante, M.E.Z.; Bonilha, S.F.M.; Suen, G.; et al. Assessing the relationship between the rumen microbiota and feed efficiency in Nellore steers. J. Anim. Sci. Biotechnol. 2021, 12, 79. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Liu, H.; Gao, Z.; Xu, J.; Liu, B.; Guo, M.; Yang, X.; Niu, J.; Zhu, X.; Ma, S.; et al. Whole-plant corn silage improves rumen fermentation and growth performance of beef cattle by altering rumen microbiota. Appl. Microbiol. Biotechnol. 2022, 106, 4187–4198. [Google Scholar] [CrossRef]
- Fan, Q.; Cui, X.; Wang, Z.; Chang, S.; Wanapat, M.; Yan, T.; Hou, F. Rumen Microbiota of Tibetan Sheep (Ovis aries) Adaptation to Extremely Cold Season on the Qinghai-Tibetan Plateau. Front. Vet. Sci. 2021, 8, 673822. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xu, S.; Meng, J.; Li, L. Protective effect of nimbolide against streptozotocin induced gestational diabetes mellitus in rats via alteration of inflammatory reaction, oxidative stress, and gut microbiota. Environ. Toxicol. 2022, 37, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Qin, X.; Chen, L.; Chen, Z.; Hao, R.; Zhang, S.; Yang, S.; Wang, L.; Cui, Y.; Li, Y.; et al. Serum Biochemical Parameters, Rumen Fermentation, and Rumen Bacterial Communities Are Partly Driven by the Breed and Sex of Cattle When Fed High-Grain Diet. Microorganisms 2022, 10, 323. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, B.; Gao, J.; Zhao, G. Dietary Supplementation with Sodium Sulfate Improves Rumen Fermentation, Fiber Digestibility, and the Plasma Metabolome through Modulation of Rumen Bacterial Communities in Steers. Appl. Environ. Microbiol. 2020, 86, e01412-20. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, C.; Statello, R.; Carnevali, L.; Mancabelli, L.; Milani, C.; Mangifesta, M.; Duranti, S.; Lugli, G.A.; Jimenez, B.; Lodge, S.; et al. How to Feed the Mammalian Gut Microbiota: Bacterial and Metabolic Modulation by Dietary Fibers. Front. Microbiol. 2017, 8, 1749. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, H.; Liu, S.; Chai, S.; Meng, Q.; Zhou, Z. Dynamic Alterations in Yak Rumen Bacteria Community and Metabolome Characteristics in Response to Feed Type. Front. Microbiol. 2019, 10, 1116. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Yang, C.; Liang, Z.; Zhang, J.; Yang, Y.; Ahmad, A.A.; Yan, P.; Ding, X. Dietary Energy Levels Affect Carbohydrate Metabolism-Related Bacteria and Improve Meat Quality in the Longissimus Thoracis Muscle of Yak (Bos grunniens). Front. Vet. Sci. 2021, 8, 718036. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Liu, C.; Chen, X.; Yang, Z.; Hu, G.; Zhang, M.; Sun, L.; Su, L.; Zhao, L.; Jin, Y. Supplemental Clostridium butyricum modulates skeletal muscle development and meat quality by shaping the gut microbiota of lambs. Meat Sci. 2023, 204, 109235. [Google Scholar] [CrossRef] [PubMed]
- NRC (National Research Council). Nutrient Requirements of Small Ruminants, Sheep, Goats, Cervids, and New World Camelids, 3rd ed.; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
| Time | Control | SIF1 | SIF2 | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|
| Diet | Time | Diet × Time | |||||
| BW, kg | |||||||
| Day 0 | 18.32 | 18.38 | 18.16 | 0.35 | 0.976 | <0.01 | 0.149 |
| Day 14 | 20.34 | 20.64 | 20.06 | ||||
| Day 28 | 22.03 | 21.70 | 21.83 | ||||
| Day 42 | 24.84 | 25.24 | 24.71 | ||||
| Day 56 | 26.78 | 26.99 | 26.83 | ||||
| ADG, g | |||||||
| Days 0 to 14 | 44.84 | 161.11 | 163.77 | 6.17 | 0.085 | <0.01 | 0.147 |
| Days 14 to 28 | 138.77 | 105.95 | 157.65 | ||||
| Days 28 to 42 | 201.19 | 253.17 | 205.16 | ||||
| Days 42 to 56 | 138.49 | 133.48 | 177.14 | ||||
| Days 0 to 56 | 151.19 | 153.67 | 154.88 | ||||
| ADFI, kg | |||||||
| Days 0 to 14 | 1.05 | 1.07 | 1.03 | 0.09 | 0.012 | <0.01 | 0.511 |
| Days 14 to 28 | 1.12 | 1.14 | 1.11 | ||||
| Days 28 to 42 | 1.25 | 1.28 | 1.22 | ||||
| Days 42 to 56 | 1.19 | 1.22 | 1.18 | ||||
| Days 0 to 56 | 1.02 | 1.11 | 1.13 | ||||
| FCR | |||||||
| Days 0 to 14 | 6.81 | 6.78 | 7.20 | 0.66 | 0.004 | 0.002 | 0.083 |
| Days 14 to 28 | 8.42 b | 10.56 a | 7.25 b | ||||
| Days 28 to 42 | 6.29 | 5.46 | 6.27 | ||||
| Days 42 to 56 | 10.47 a | 10.07 a | 7.79 b | ||||
| Days 0 to 56 | 6.84 | 7.28 | 7.65 | ||||
| Item | Control | SIF1 | SIF2 | SEM | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| Diet | Time | Diet × Time | ||||||
| Head length | Day 0 | 12.47 | 12.23 | 12.32 | 0.14 | 0.856 | <0.01 | 0.184 |
| Day 28 | 13.63 | 13.93 | 13.97 | |||||
| Day 56 | 14.68 | 14.52 | 14.91 | |||||
| WH | Day 0 | 56.40 | 55.99 | 55.26 | 0.56 | 0.720 | <0.01 | 0.021 |
| Day 28 | 59.07 | 60.42 | 60.77 | |||||
| Day 56 | 66.71 | 66.74 | 64.27 | |||||
| RH | Day 0 | 54.90 | 54.23 | 54.09 | 0.38 | 0.855 | <0.01 | 0.189 |
| Day 28 | 57.02 | 57.36 | 57.61 | |||||
| Day 56 | 59.30 | 60.08 | 61.24 | |||||
| BL | Day 0 | 51.24 | 51.46 | 50.07 | 0.50 | 0.580 | <0.01 | 0.680 |
| Day 28 | 56.01 | 54.71 | 54.90 | |||||
| Day 56 | 59.72 | 60.14 | 58.30 | |||||
| CG | Day 0 | 60.33 | 60.64 | 60.89 | 0.44 | 0.979 | <0.01 | 0.550 |
| Day 28 | 64.84 | 62.99 | 63.03 | |||||
| Day 56 | 66.72 | 66.79 | 66.99 | |||||
| CD | Day 0 | 26.09 | 25.74 | 25.73 | 0.13 | 0.957 | <0.01 | 0.153 |
| Day 28 | 27.42 | 26.82 | 26.85 | |||||
| Day 56 | 27.50 | 27.09 | 27.00 | |||||
| CBC | Day 0 | 7.42 | 7.43 | 7.23 | 0.07 | 0.574 | <0.01 | 0.453 |
| Day 28 | 7.76 | 7.66 | 7.65 | |||||
| Day 56 | 7.97 | 7.89 | 7.74 | |||||
| AG | Day 0 | 59.50 | 61.11 | 60.08 | 0.47 | 0.198 | <0.01 | 0.850 |
| Day 28 | 63.88 | 65.21 | 62.05 | |||||
| Day 56 | 66.84 | 67.51 | 66.22 | |||||
| Item | Control | SIF1 | SIF2 | SEM | p-Value |
|---|---|---|---|---|---|
| Slaughter body weight/kg | 24.53 | 26.47 | 25.13 | 0.62 | 0.486 |
| Carcass weight/kg | 12.55 | 12.53 | 12.76 | 0.32 | 0.960 |
| Carcass length/cm | 74.83 | 70.67 | 70.00 | 1.40 | 0.355 |
| Carcass depth/cm | 19.50 | 20.33 | 20.17 | 0.40 | 0.725 |
| GR value/cm | 0.92 | 1.37 | 1.03 | 0.13 | 0.410 |
| Net meat weight/kg | 8.57 | 9.02 | 8.76 | 0.20 | 0.705 |
| Bone/kg | 3.45 | 2.93 | 3.43 | 0.16 | 0.380 |
| Eye muscle area/cm2 | 13.18 | 9.66 | 7.09 | 1.19 | 0.089 |
| Dressing percentage/% | 51.13 | 47.40 | 50.81 | 0.95 | 0.227 |
| Neat percentage/% | 34.96 | 34.10 | 34.87 | 0.30 | 0.504 |
| Carcass neat percentage/% | 68.37 | 72.17 | 68.62 | 0.93 | 0.184 |
| Meat–bone ratio/% | 2.48 | 3.23 | 2.55 | 0.19 | 0.223 |
| Time | Control | SIF1 | SIF2 | SEM | p-Value |
|---|---|---|---|---|---|
| Head/kg | 1.50 | 1.60 | 1.50 | 0.03 | 0.296 |
| Four feet/kg | 0.64 | 0.65 | 0.72 | 0.02 | 0.055 |
| Pelt/kg | 1.43 | 1.63 | 1.63 | 0.05 | 0.125 |
| Heart/g | 124.00 | 116.17 | 122.83 | 2.14 | 0.308 |
| Liver/g | 555.17 | 625.17 | 596.00 | 19.60 | 0.392 |
| Spleen/g | 47.50 | 46.50 | 49.00 | 2.81 | 0.951 |
| Kidneys/g | 99.17 b | 112.50 a | 117.33 a | 3.25 | 0.027 |
| Fat/g | 327.33 | 389.67 | 384.50 | 51.65 | 0.961 |
| Rumen/g | 468.83 | 522.00 | 496.33 | 15.64 | 0.437 |
| Reticulum/g | 91.17 | 82.00 | 76.17 | 4.28 | 0.405 |
| Omasum/g | 80.17 | 76.83 | 70.00 | 3.05 | 0.439 |
| Abomasum/g | 143.83 | 169.67 | 177.00 | 7.58 | 0.093 |
| Large intestine/g | 363.83 b | 534.33 a | 532.50 a | 31.03 | 0.005 |
| Small intestine/g | 675.33 | 642.67 | 592.67 | 25.21 | 0.461 |
| Large intestine/cm | 438.50 | 599.67 | 582.67 | 34.59 | 0.093 |
| Small intestine/cm | 2002.00 | 2020.33 | 1966.83 | 52.78 | 0.935 |
| Time | Control | SIF1 | SIF2 | SEM | p-Value |
|---|---|---|---|---|---|
| Water loss/% | 10.81 | 5.91 | 6.86 | 1.17 | 0.167 |
| Shear force (N) | 58.84 | 44.64 | 50.62 | 2.67 | 0.068 |
| Cooking loss/% | 42.19 a | 31.87 b | 39.39 ab | 1.92 | 0.045 |
| Time | Control | SIF1 | SIF2 | SEM | p-Value | ||
|---|---|---|---|---|---|---|---|
| Diet | Time | Diet × Time | |||||
| SIFs/(pg/mL) | |||||||
| Day 0 | 29.77 | 31.29 | 33.94 | 1.92 | 0.133 | 0.09 | <0.01 |
| Day 28 | 28.78 | 29.53 | 24.77 | ||||
| Day 56 | 32.63 b | 55.74 a | 29.28 b | ||||
| GH/(μg/L) | |||||||
| Day 0 | 19.29 | 19.45 | 16.93 | 0.26 | 0.156 | 0.01 | 0.264 |
| Day 28 | 18.10 | 17.07 | 17.88 | ||||
| Day 56 | 16.71 | 16.98 | 15.89 | ||||
| Serum-[TP]/(μg/mL) | |||||||
| Day 0 | 342.48 | 337.97 | 334.45 | 7.85 | 0.368 | 0.02 | 0.882 |
| Day 28 | 295.31 | 276.23 | 299.82 | ||||
| Day 56 | 296.31 | 267.70 | 318.14 | ||||
| Serum-[TC]/(mmol/L) | |||||||
| Day 0 | 2.05 | 2.07 | 1.98 | 0.04 | 0.724 | <0.01 | 0.119 |
| Day 28 | 2.25 | 2.50 | 2.23 | ||||
| Day 56 | 2.14 | 2.25 | 2.39 | ||||
| Serum-[TAG]/(mmol/L) | |||||||
| Day 0 | 0.27 | 0.28 | 0.29 | 0.01 | 0.987 | <0.01 | 0.202 |
| Day 28 | 0.24 | 0.26 | 0.23 | ||||
| Day 56 | 0.30 | 0.28 | 0.29 | ||||
| BUN/(mmol/L) | |||||||
| Day 0 | 6.63 | 6.91 | 6.33 | 0.25 | 0.835 | <0.01 | 0.443 |
| Day 28 | 8.47 | 8.28 | 8.63 | ||||
| Day 56 | 8.56 | 8.92 | 8.08 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, Y.; Xu, J.; Wang, M.; Ren, Y.; Wei, M.; Tian, B.; Luo, J.; Loor, J.J.; Shi, H. Preliminary Results on the Effects of Soybean Isoflavones on Growth Performance and Ruminal Microbiota in Fattening Goats. Animals 2024, 14, 1188. https://doi.org/10.3390/ani14081188
Shao Y, Xu J, Wang M, Ren Y, Wei M, Tian B, Luo J, Loor JJ, Shi H. Preliminary Results on the Effects of Soybean Isoflavones on Growth Performance and Ruminal Microbiota in Fattening Goats. Animals. 2024; 14(8):1188. https://doi.org/10.3390/ani14081188
Chicago/Turabian StyleShao, Yuexin, Junhong Xu, Mengyu Wang, Yalun Ren, Manhong Wei, Bowen Tian, Jun Luo, Juan J. Loor, and Huaiping Shi. 2024. "Preliminary Results on the Effects of Soybean Isoflavones on Growth Performance and Ruminal Microbiota in Fattening Goats" Animals 14, no. 8: 1188. https://doi.org/10.3390/ani14081188






