Histopathological Lesions Caused by a Digenean Trematode in a Pest Apple Snail, Pomacea canaliculata, in Its Native Geographic Distribution Area
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Morphological Description
2.2. Histology
2.3. DNA Extraction, Amplification, and Sequencing
2.4. Sequence Alignments and Phylogenetic Analysis
3. Results
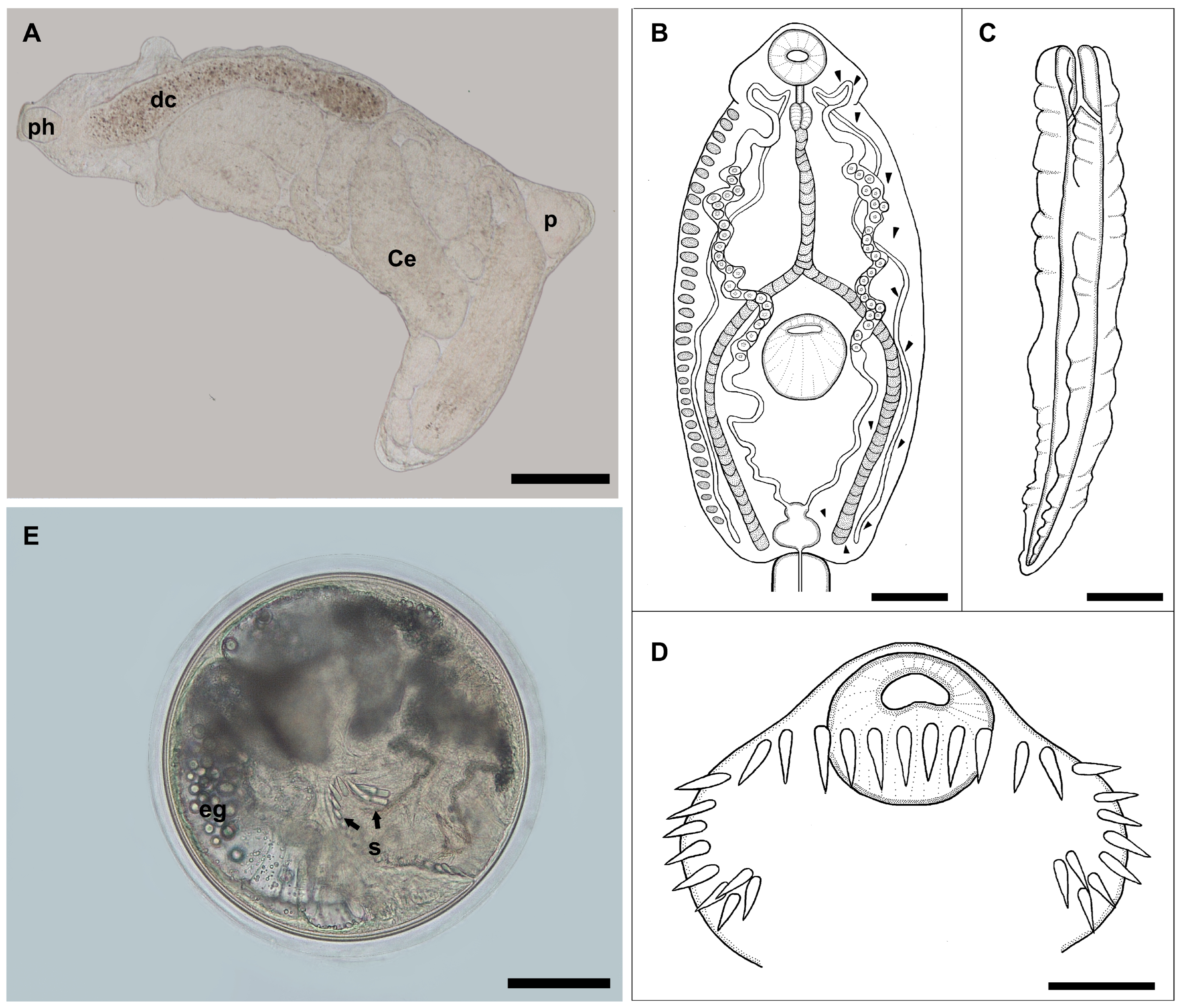
3.1. Description of Redia, Cercaria, and Metacercaria of Echinostomatidae gen. et sp.
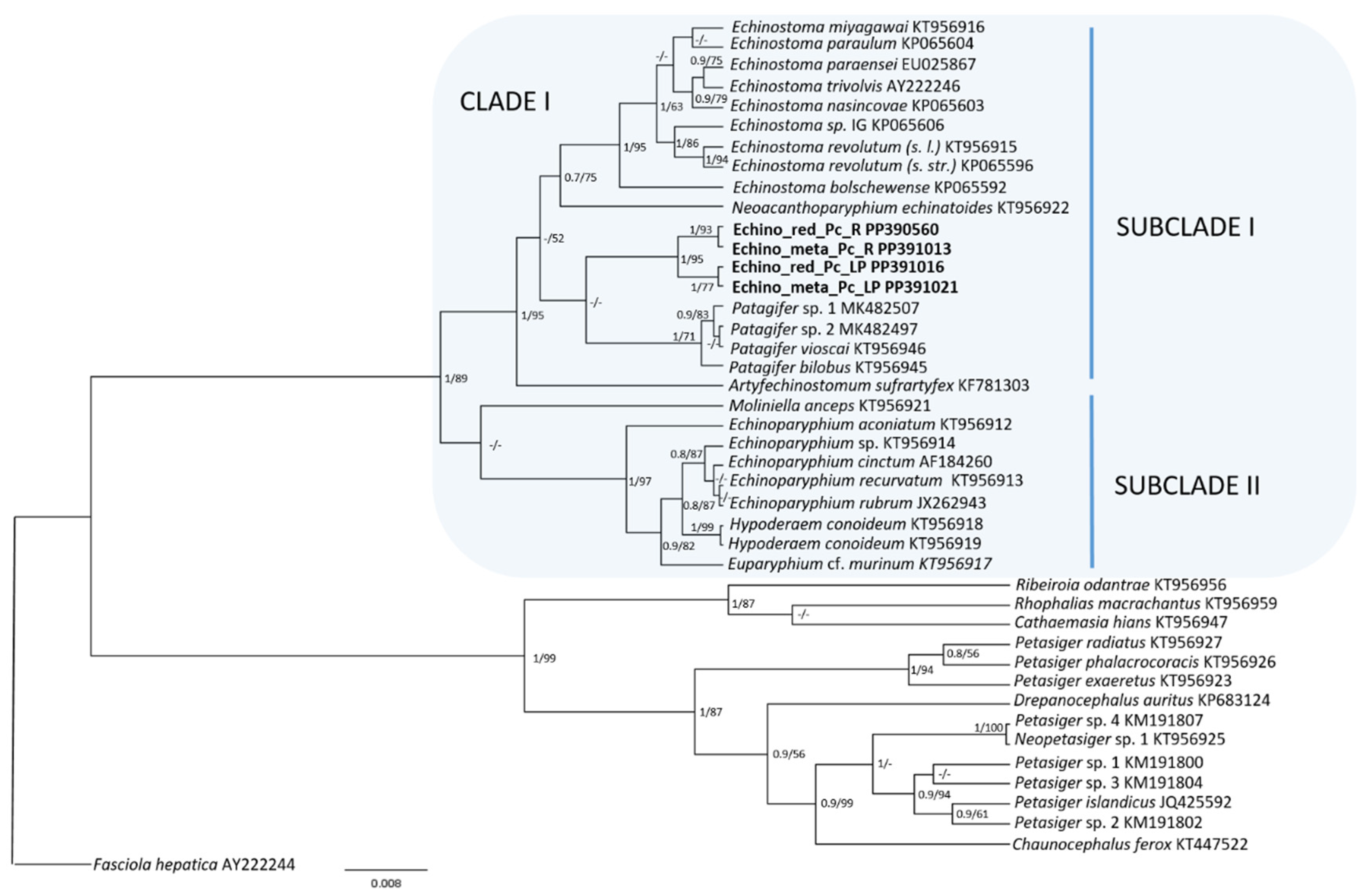
3.2. Molecular Analyses
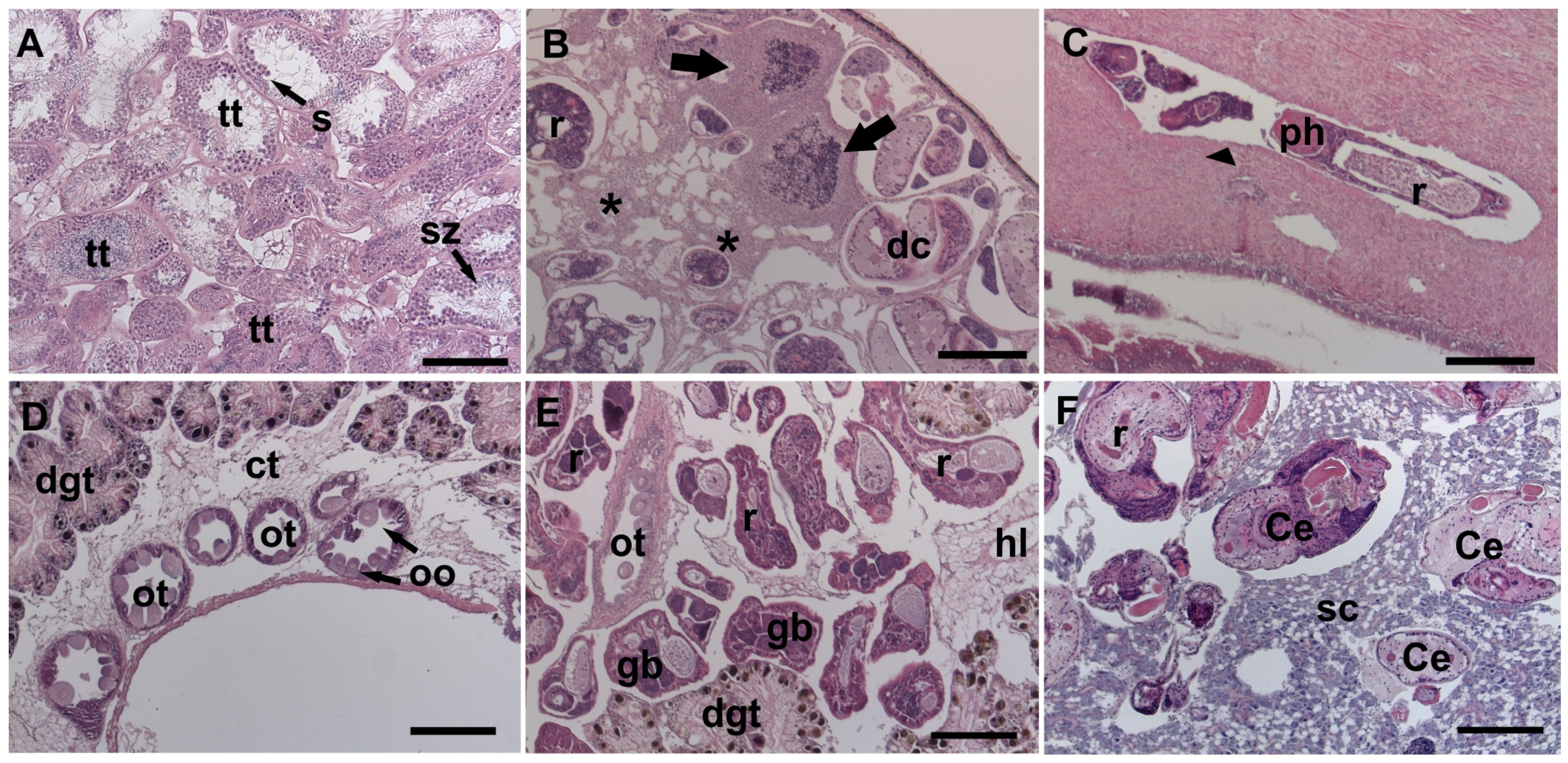
3.3. Sites of Infection and Host Tissue Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brito, F.C.; Joshi, C.R. The Golden Apple Snail Pomacea canaliculata: A Review on Invasion, Dispersion and control. Outlooks Pest Manag. 2016, 27, 157–163. [Google Scholar] [CrossRef]
- Seuffert, M.E.; Martín, P.R. Exceeding its own limits: Range expansion in Argentina of the globally invasive apple snail Pomacea canaliculata. Hydrobiología 2021, 848, 385–401. [Google Scholar] [CrossRef]
- Horgan, F.G.; Stuart, A.M.; Kudavidanage, E.P. Impact of invasive apple snails on the functioning and services of natural and managed wetlands. Acta Oecologica 2014, 54, 90–100. [Google Scholar] [CrossRef]
- Maldonado, M.A.; Martín, P.R. Dealing with a hyper-successful neighbor: Effects of the invasive apple snail Pomacea canaliculata on exotic and native snails in South America. Curr. Zool. 2019, 65, 225–235. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.B.S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species A Selection from the Global Invasive Species Database; The Invasive Species Specialist Group (ISSG) a Specialist Group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN): Auckland, New Zealand, 2000; p. 12. [Google Scholar]
- Damborenea, C.; Brusa, F.; Paola, A. Variation in worm assemblages associated with Pomacea canaliculata (Caenogastropoda, Ampullariidae) in sites near the Rio de la Plata estuary, Argentina. Biocell 2006, 30, 457–468. [Google Scholar] [CrossRef]
- Damborenea, M.; Brusa, F.; Negrete, J. Symbionts and diseases associated with invasive apple snails. In Biology and Management of Invasive Apple Snails; Joshi, R.C., Sebastian, L.S., Eds.; Philippine Rice Research Institute: Nueva Ecija, Philippines, 2017; pp. 73–97. [Google Scholar]
- Esch, G.W.; Barger, M.A.; Fellis, K.J. The Transmission of Digenetic Trematodes: Style, Elegance, Complexity. Integ. Comp. Biol. 2002, 42, 304–312. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Podvyaznaya, I.M.; Nikolaev, K.E.; Levakin, I.A. Self-sustaining infrapopulation or colony? Redial clonal groups of Himasthla elongata (Mehlis, 1831) (Trematoda: Echinostomatidae) in Littorina littorea (Linnaeus) (Gastropoda: Littorinidae) do not support the concept of eusocial colonies in trematodes. Folia Parasitol. 2015, 62, 067. [Google Scholar] [CrossRef][Green Version]
- Huffman, J.E.; Klockars, J.; Keeler, S.P.; Fried, B. Histopathological effects of the intramolluscan stages of Zygocotyle lunata, Echinostoma trivolvis, and Ribeiroia ondatrae on Helisoma trivolvis and observations on keratin in the trematode larvae. Parasitol. Res. 2009, 105, 1385–1389. [Google Scholar] [CrossRef]
- Averbuj, A.; Cremonte, F. Parasitic castration of Buccinanops cochlidium (Gastropoda: Nassariidae) caused by a lepocreadiid digenean in San Jose Gulf, Argentina. J. Helminthol. 2010, 84, 381–389. [Google Scholar] [CrossRef]
- Cremonte, F. Enfermedades de moluscos bivalvos de interés comercial causadas por metazoos. In Enfermedades de Moluscos Bivalvos de Interés en Acuicultura; Figueras, A., Novoa, B., Eds.; Fundación Observatorio Español de Acuicultura: Madrid, Spain, 2011; pp. 333–396. [Google Scholar]
- Choubisa, S.L.; Sheikh, Z. Parasitic Castration in Freshwater Snail Melanoides tuberculatus (Mollusca: Gastropoda). Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2012, 83, 193–197. [Google Scholar] [CrossRef]
- Gilardoni, C.; Di Giorgio, G.; Ituarte, C.; Cremonte, F. Atypical lesions and infection sites of larval trematodes in marine gastropods from Argentina. Dis. Aquat. Organ. 2018, 130, 241–246. [Google Scholar] [CrossRef]
- Kuris, A.M. Trophic Interactions: Similarity of Parasitic Castrators to Parasitoids. Q. Rev. Biol. 1974, 49, 129–148. [Google Scholar] [CrossRef]
- Baudoin, M. Host castration as a parasitic strategy. Evolution 1975, 29, 335–352. [Google Scholar] [CrossRef]
- Dobson, A.P. The population biology of parasite-induced changes in host behavior. Q. Rev. Biol. 1988, 63, 139–165. [Google Scholar] [CrossRef]
- Adema, C.M.; Loker, E.S. Digenean-gastropod host associations inform on aspects of specific immunity in snails. Dev. Comp. Immunol. 2015, 48, 275–283. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Dobrovolskij, A.A. An Essay on the Biology, Morphology, Life Cycles, Transmissions, and Evolution of Digenetic Trematodes. In The Biology and Evolution of Trematodes; Kluwer Academic Publishers: St. Petersburg, Russia, 2003; 592p. [Google Scholar]
- Vega, I.A.; Damborenea, M.C.; Gamarra-Luques, C.; Koch, E.; Cueto, J.A.; Castro-Vazquez, A. Facultative and obligate symbiotic associations of Pomacea canaliculata (Caenogastropoda, Ampullariidae). Biocell 2006, 30, 367–375. [Google Scholar]
- Martorelli, S.R. Estudios parasitológicos en biotopos lenticos de la República Argentina. IV: El ciclo biológico de Echinostoma parcespinosum Lutz, 1924 (Digenea) parásito de Rallus maculatus maculatus y Rallus sanguinolentus sanguinolentus (Aves: Rallidae). Rev. Mus. Plata Zool. 1987, 14, 48–59. [Google Scholar]
- Howard, D.; Lewis, J.; Keller, J.; Smith, C. Histological Techniques for Marine Bivalve Mollusks and Crustaceans; NOAA Technical Memorandum; NCCOS: Silver Spring, MD, USA, 2004; Volume 5. [Google Scholar]
- Cremonte, F.; Gilardoni, C.; Pina, S.; Rodrigues, P.; Ituarte, C. Revision of the family Gymnophallidae Odhner, 1905 (Digenea) based on morphological and molecular data. Parasitol. Int. 2015, 64, 202–210. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Nguyen, L.T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Drummond, A.; Rambaut, A.; Suchard, M. BEAST v. 1.4. Bayesian Evolutionary Analysis Sampling Trees. 2006. Available online: http://beast.bio.ed.ac.uk (accessed on 23 February 2024).
- Hoang, D.T.; Chernomor, O.; von Haeseler, A.; Quang Minh, B.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Meth. 2012, 9, 772. [Google Scholar] [CrossRef]
- Tkach, V.V.; Kudlai, O.; Kostadinova, A. Molecular phylogeny and systematics of the Echinostomatoidea Looss, 1899 (Platyhelminthes: Digenea). Int. J. Parasitol. 2016, 46, 171–185. [Google Scholar] [CrossRef]
- Georgieva, S.; Faltýnková, A.; Brown, R.; Blasco-Costa, I.; Soldánová, M.; Sitko, J.; Scholz, T.; Kostadinova, A. Echinostoma ‘revolutum’ (Digenea: Echinostomatidae) species complex revisited: Species delimitation based on novel molecular and morphological data gathered in Europe. Parasites Vectors 2014, 7, 520. [Google Scholar]
- Lotfy, W.M.; Brant, S.V.; DeJong, R.J.; Hoa Le, T.; Demiaszkiewicz, J.; Aleksander, D.; Rajapakse, R.P.V.; Perera, V.B.V.P.; Laursen, J.R.; Loker, E.S. Evolutionary Origins, Diversification, and Biogeography of Liver Flukes (Digenea, Fasciolidae). Am. J. Trop. Med. Hyg. 2008, 79, 248–255. [Google Scholar] [CrossRef]
- Olson, P.D.; Cribb, T.H.; Tkach, V.V.; Bray, R.A.; Littlewood, D.T. Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). Int. J. Parasitol. 2003, 33, 733–755. [Google Scholar] [CrossRef]
- Tkach, V.V.; Pawlowski, J.; Mariaux, J.; Swiderski, Z. Molecular phylogeny of the suborder Plagiorchiata and its position in the system of Digenea. In Interrelationships of the Platyhelminthes; CRC Press: Boca Raton, FL, USA, 2014; pp. 186–193. [Google Scholar]
- Tkach, V.V.; Schroeder, J.A.; Greiman, S.E.; Vaughan, J.A. New genetic lineages, host associations and circulation pathways of Neorickettsia endosymbionts of digeneans. Acta Parasitol. 2012, 57, 285–292. [Google Scholar] [CrossRef]
- Kudlai, O.; Kostadinova, A.; Pulis, E.E.; Tkach, V.V. A new species of Drepanocephalus Dietz, 1909 (Digenea: Echinostomatidae) from the double-crested cormorant Phalacrocorax auritus (Lesson) (Aves: Phalacrocoracidae) in North America. Syst. Parasitol. 2015, 90, 221–230. [Google Scholar] [CrossRef]
- Selbach, C.; Soldanova, M.; Georgieva, S.; Kostadinova, A.; Kalbe, M.; Sures, B. Morphological and molecular data for larval stages of four species of Petasiger Dietz, 1909 (Digenea: Echinostomatidae) with an updated key to the known cercariae from the Palaearctic. Syst. Parasitol. 2014, 89, 153–166. [Google Scholar] [CrossRef]
- Laidemitt, M.R.; Brant, S.V.; Mutuku, M.W.; Mkoji, G.M.; Loker, E.S. The diverse echinostomes from East Africa: With a focus on species that use Biomphalaria and Bulinus as intermediate hosts. Acta Trop. 2019, 193, 38–49. [Google Scholar] [CrossRef]
- Pinto, H.A.; De Melo, A.L. A checklist of cercariae (Trematoda: Digenea) in molluscs from Brazil. Zootaxa 2013, 3666, 449–475. [Google Scholar] [CrossRef]
- Lafferty, K.D. Environmental Parasitology: What can Parasites tell us about Human Impacts on the Environment? Parasitol. Today 1997, 13, 251–255. [Google Scholar] [CrossRef]
- Whitney, K.L.; Hechinger, R.F.; Kuris, A.M.; Lafferty, K.D. Endangered light-footed clapper rail affects parasite community structure in coastal wetlands. Ecol. Appl. 2007, 17, 1694–1702. [Google Scholar] [CrossRef]
- Etchegoin, J.A.; Merlo, M.J.; Parietti, M. The role of the invasive polychaete Ficopomatus enigmaticus (Fauvel, 1923) (Serpulidae) as facilitator of parasite transmission in Mar Chiquita coastal lagoon (Buenos Aires, Argentina). Parasitology 2012, 139, 1506–1512. [Google Scholar] [CrossRef]
- Digiani, M.C. Digeneans and cestodes parasitic in the white-faced ibis Plegadis chihi (Aves: Threskiornithidae) from Argentina. Folia Parasitol. 2000, 47, 195–204. [Google Scholar] [CrossRef]
- Bertolero, A.; Navarro, J. A native bird as a predator for the invasive apple snail, a novel rice field invader in Europe. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1099–1104. [Google Scholar] [CrossRef]
- Lauckner, G. Diseases of mollusca: Bivalvia. In Diseases of Marine Animals; Biologische Anstalt Helgoland: Hamburg, Germany, 1983; Volume 2. [Google Scholar]
- Choubisa, S.L.; Sheikh, Z.; Jaroli, V.J. Histopathological effects of larval trematodes on the digestive gland of freshwater snail species, Vivipara bengalensis and Lymnaea acuminata. J. Parasit. Dis. 2012, 36, 283–286. [Google Scholar] [CrossRef]
- Choubisa, S.L. Histological and histochemical observation on the digestive gland of Melanoides tuberculatus (Gastropoda) infected with certain larval trematodes and focus on their mode of nutrition. Proc. Indian Acad. Sci. 1988, 97, 251–262. [Google Scholar] [CrossRef]
- Cremonte, F.; Figueras, A.; Burreson, E.M. A histopathological survey of some commercially exploited bivalve molluscs in northern Patagonia, Argentina. Aquaculture 2005, 249, 23–33. [Google Scholar] [CrossRef]
- Rato, M.; Russel-Pinto, F.; Barroso, C. Assessment of digenean parasitism in Nassarius reticulatus (L.) Along the portuguese coast: Evaluation of possible impacts on reproduction and imposex expression. J. Parasitol. 2009, 95, 327–336. [Google Scholar] [CrossRef]
- Gamarra-Luques, C.; Giraud-Billoud, M.; Castro-Vazquez, A. Reproductive organogenesis in the apple snail Pomacea canaliculata (Lamarck, 1822), with reference to the effects of xenobiotics. J. Molluscan Stud. 2013, 79, 147–162. [Google Scholar] [CrossRef]
- Winstead, J.T.; Volety, A.K.; Gregory, T.S. Parasitic and symbiotic fauna in oysters (Crassostrea virginica) collected from the Caloosahatchee River and estuary in Florida. J. Shellfish Res. 2004, 23, 831–841. [Google Scholar]
- Lafferty, K.D.; Kuris, A.M. Parasitic castration: The evolution and ecology of body snatchers. Trends Parasitol. 2009, 25, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Laruelle, F.M.; Daniel, P.; Roitman Vitali, A. Histological analysis of trematodes in Dreissena polymorpha: Their location, pathogenicity, and distinguishing morphological characteristic. J. Parasitol. 2002, 88, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Demian, E.S. The respiratory system and the mechanism of respiration in Marisa cornuarietis (L.). Ark. För Zool. 1965, 17, 539–560. [Google Scholar]
- Santos, C.A.; Penteado, C.H.; Mendes, E.G. The respiratory responses of an amphibious snail Pomacea lineata (Spix, 1827), to temperature and oxygen tension variations. Comp. Biochem. Physiol. 1987, 86, 409–415. [Google Scholar] [CrossRef]
- Rodriguez, C.; Prieto, G.I.; Vega, I.A.; Castro-Vazquez, A. Assessment of the kidney and lung as immune barriers and hematopoietic sites in the invasive apple snail Pomacea canaliculata. PeerJ 2018, 6, e5789. [Google Scholar] [CrossRef]
- Vazquez, N.; Glasinovich, N.; Ortiz, N.; Gestal, C.; Pontones, J.; Gilardoni, C.; Cremonte, F. Host-parasite relationship Octopus tehuelchus—Aggregata valdessensis in Patagonian coast, southwestern Atlantic Ocean. J. Invertebr. Pathol. 2023, 201, 107993. [Google Scholar] [CrossRef]
- Cribb, T.H. Digenea (endoparasitic flukes). In Marine Parasitology; Collingwood, K.R., Ed.; CSIRO Publishing (Commonwealth Scientific and Industral Rsearch Organization): Collingwood, VIC, Australia, 2005. [Google Scholar]

| Taxon | Life Cycle Stage | Host | Country | GenBank N° | References |
|---|---|---|---|---|---|
| Family Echinostomatidae Looss, 1899 | |||||
| Subfamily Echinostomatinae Looss, 1899 | |||||
| Echinostomatidae gen. et sp. (red_Pc_R) | red | Pomacea canaliculata (Lamarck) | Argentina | PP390560 | Present study |
| Echinostomatidae gen. et sp. (meta_Pc_R) | meta | Pomacea canaliculata | Argentina | PP391013 | Present study |
| Echinostomatidae gen. et sp. (red_Pc_LP) | red | Pomacea canaliculata | Argentina | PP391016 | Present study |
| Echinostomatidae gen. et sp. (meta_Pc_LP) | meta | Pomacea canaliculata | Argentina | PP391021 | Present study |
| Echinostoma miyagawai Ishii, 1932 | ad | Anas platyrhynchos L. | Ukraine | KT956916 | [29] |
| Echinostoma paraulum Dietz, 1909 | cer | Lymnaea stagnalis (L.) | Germany | KP065604 | [30] |
| Echinostoma paraensei Lie and Basch, 1967 | ad | “hamster” | USA | EU025867 | [31] |
| Echinostoma trivolvis (Cort, 1914) | ad | Mesocricetus auratus Waterhouse (exp.) | USA | AY222246 | [32] |
| Echinostoma nasincovae Faltýnková et al., 2015 | cer | Planorbarius corneus (L.) | Czech Republic | KP065603 | [30] |
| Echinostoma sp. IG | cer | Radix auricularia (L.) | Germany | KP065606 | [30] |
| Echinostoma revolutum (Frölich, 1802) (sensu lato) | ad | Aythya collaris (Donovan) | USA | KT956915 | [29] |
| Echinostoma revolutum (Frölich, 1802) (sensu stricto) | ad | Aythya fuligula (L.) | Czech Republic | KP065596 | [30] |
| Echinostoma bolschewense (Kotova, 1939) | cer | Viviparus acerosus (Bourguignat) | Slovakia | KP065592 | [30] |
| Neoacanthoparyphium echinatoides (de Filippi, 1854) | cer | Viviparus acerosus (Bourguignat) | Slovakia | KT956922 | [29] |
| Moliniella anceps (Molin, 1859) | meta | Planorbarius corneus (L.) | Lithuania | KT956921 | [29] |
| Echinoparyphium aconiatum Dietz, 1909 | cer | Lymnaea stagnalis (L.) | Czech Republic | KT956912 | [29] |
| Echinoparyphium sp. | ad | Anas clypeata L. | USA | KT956914 | [29] |
| Echinoparyphium cinctum (Rudolphi, 1803) | ad | Anas platyrhynchos L. | Ukraine | AF184260 | [33] |
| Echinoparyphium recurvatum (von Linstow, 1873) | cer | Radix ovata (Draparnaud) | Slovakia | KT956913 | [29] |
| Echinoparyphium rubrum Cort, 1914 | cer | Planorbella trivolvis (Say) | USA | JX262943 | [34] |
| Hypoderaeum conoideum (Bloch, 1782) | ad | Anas platyrhynchos L. | Ukraine | KT956918 | [29] |
| Hypoderaeum conoideum (Bloch, 1782) | ad | Anas acuta L. | USA | KT956919 | [29] |
| Euparyphium cf. Murinum Tubangui, 1931 | ad | Malacomys longipes Milne-Edwards | Uganda | KT956917 | [29] |
| Petasiger radiatum Dujardin, 1845 | ad | Phalacrocorax carbo (L.) | Ukraine | KT956927 | [29] |
| Petasiger phalacrocoracis (Yamaguti, 1939) | ad | Phalacrocorax carbo (L.) | Ukraine | KT956926 | [29] |
| Petasiger exaeretus Dietz, 1909 | ad | Phalacrocorax carbo (L.) | Ukraine | KT956923 | [29] |
| Drepanocephalus auritus Kudlai et al., 2015 | ad | Phalacrocorax auritus (Lesson) | USA | KP683124 | [35] |
| Petasiger sp. 1 | cer | Planorbis planorbis (L.) | Czech Republic | KM191800 | [36] |
| Petasiger sp. 2 | cer | Gyraulus albus (O. F. Müller) | Germany | KM191802 | [36] |
| Petasiger sp. 3 | cer | Planorbis planorbis (L.) | Germany | KM191804 | [36] |
| Petasiger sp. 4 | meta | Gasterosteus aculeatus L. | Canada | KM191807 | [36] |
| Neopetasiger n. sp. | ad | Podiceps grisegena (Boddaert) | USA | KT956925 | [29] |
| Petasiger islandicus Kostadinova and Skírnisson, 2007 | ad | Aechmophorus occidentalis (Lawrence) | USA | KT956924 | [29] |
| Subfamily Nephrostominae Mendheim, 1943 | |||||
| Patagifer sp. 1 | cer | Biomphalaria sudanica (Marlens) | Kenia | MK482507 | [37] |
| Patagifer sp. 2 | cer | Biomphalaria pfeifferi | Kenia | MK482497 | [37] |
| Patagifer vioscai Lumsden, 1962 | ad | Eudocimus albus (L.) | USA | KT956946 | [29] |
| Patagifer bilobus (Rudolphi, 1819) | ad | Plegadis falcinellus (L.) | Ukraine | KT956945 | [29] |
| Subfamily Himasthlinae Odhner, 1910 | |||||
| Artyfechinostomum sufrartyfex Lane, 1915 | ad | Sus scrofa dom. | India | KF781303 | - |
| Subfamily Chaunocephalinae Travassos, 1922 | |||||
| Chaunocephalus ferox (Rudolphi, 1795) | ad | Ciconia nigra (L.) | Ukraine | KT447522 | - |
| Family Psilostomidae Looss, 1900 | |||||
| Subfamily Ribeiroiinae Travassos, 1951 | |||||
| Ribeiroia ondatrae (Price, 1931) | ad | Pelecanus erythrorhynchos Gmelin | USA | KT956956 | [29] |
| Family Cathaemasiidae Fuhrmann, 1928 | |||||
| Subfamily Cathaemasiinae Fuhrmann, 1928 | |||||
| Cathaemasia hians (Rudolphi, 1809) | cer | Planorbis planorbis (L.) | Czech Republic | KT956947 | [29] |
| Family Rhopaliidae Looss, 1899 | |||||
| Rhopalias macracanthus Chandler, 1932 | ad | Didelphis virginiana (Kerr) | USA | KT956959 | [29] |
| Family Fasciolidae Railliet, 1895 | |||||
| Subfamily Fasciolinae Railliet, 1895 | |||||
| Fasciola hepatica Linnaeus, 1758 | ad | Capra hircus (L.) | Saudi Arabia | AY222244 | [32] |
| Sampling Sites | Harbor Natural Reserve | Los Padres Lagoon |
|---|---|---|
| N total (stereoscope microscope examination) | 67 | 137 |
| Shell length mean (±SD) (cm) | 4.37 (0.7) | 4.87 (0.7) |
| Sex ratio (female/male) | 41/25 | 83/54 |
| Prevalence of rediae (%) | 15.1 | 0.72 |
| Prevalence of metacercariae (%) | 74.2 | 9.5 |
| Mean intensity of metacercariae | 298 (17−1912) | 41 (2−169) |
| N (histology subsample) | 31 | 67 |
| Shell length mean (±SD) (cm) | 4.34 (0.8) | 4.86 (0.8) |
| Sex ratio (female/male) | 19/31 | 41/67 |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. | ||||||||||||||||||
| 2. | 0.00000 | |||||||||||||||||
| 3. | 0.00600 | 0.00490 | ||||||||||||||||
| 4. | 0.00600 | 0.00490 | 0.00000 | |||||||||||||||
| 5. | 0.01918 | 0.01838 | 0.01542 | 0.01501 | ||||||||||||||
| 6. | 0.01799 | 0.01716 | 0.01423 | 0.01386 | 0.00115 | |||||||||||||
| 7. | 0.01679 | 0.01593 | 0.01305 | 0.01270 | 0.00231 | 0.00115 | ||||||||||||
| 8. | 0.01799 | 0.01716 | 0.01423 | 0.01386 | 0.00115 | 0.00000 | 0.00115 | |||||||||||
| 9. | 0.01559 | 0.01593 | 0.01661 | 0.01617 | 0.02076 | 0.01961 | 0.01845 | 0.01961 | ||||||||||
| 10. | 0.01799 | 0.01838 | 0.01898 | 0.01848 | 0.02307 | 0.02191 | 0.02076 | 0.02191 | 0.00461 | |||||||||
| 11. | 0.02638 | 0.02574 | 0.02728 | 0.02656 | 0.03114 | 0.02999 | 0.02884 | 0.02999 | 0.01269 | 0.01153 | ||||||||
| 12. | 0.02038 | 0.02083 | 0.02135 | 0.02194 | 0.02653 | 0.02537 | 0.02422 | 0.02537 | 0.00577 | 0.00807 | 0.01615 | |||||||
| 13. | 0.01918 | 0.01961 | 0.02017 | 0.01963 | 0.02422 | 0.02307 | 0.02191 | 0.02307 | 0.00346 | 0.00577 | 0.01153 | 0.00461 | ||||||
| 14. | 0.01687 | 0.01724 | 0.01788 | 0.01740 | 0.02202 | 0.02086 | 0.01970 | 0.02086 | 0.00348 | 0.00348 | 0.01159 | 0.00695 | 0.00463 | |||||
| 15. | 0.02558 | 0.02491 | 0.02651 | 0.02579 | 0.03044 | 0.02927 | 0.02810 | 0.02927 | 0.01171 | 0.01054 | 0.00468 | 0.01522 | 0.01288 | 0.01054 | ||||
| 16. | 0.02038 | 0.02083 | 0.02017 | 0.01963 | 0.02307 | 0.02191 | 0.02076 | 0.02191 | 0.01269 | 0.01038 | 0.02076 | 0.01384 | 0.01153 | 0.01159 | 0.01991 | |||
| 17. | 0.02158 | 0.02206 | 0.02254 | 0.02194 | 0.02653 | 0.02537 | 0.02422 | 0.02537 | 0.01038 | 0.00923 | 0.01384 | 0.01384 | 0.01153 | 0.00927 | 0.01288 | 0.01499 | ||
| 18. | 0.03234 | 0.03182 | 0.03081 | 0.02999 | 0.03114 | 0.02999 | 0.02884 | 0.02999 | 0.03345 | 0.03345 | 0.03922 | 0.03691 | 0.03460 | 0.03244 | 0.03864 | 0.03460 | 0.03460 | |
| 19. | 0.02278 | 0.02328 | 0.02135 | 0.02079 | 0.02540 | 0.02425 | 0.02309 | 0.02425 | 0.02425 | 0.02425 | 0.02771 | 0.02771 | 0.02309 | 0.02320 | 0.02931 | 0.02079 | 0.02656 | 0.03460 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, L.E.; Gilardoni, C.; Medina, C.D.; Cremonte, F.; Etchegoin, J.A. Histopathological Lesions Caused by a Digenean Trematode in a Pest Apple Snail, Pomacea canaliculata, in Its Native Geographic Distribution Area. Animals 2024, 14, 1191. https://doi.org/10.3390/ani14081191
Martinez LE, Gilardoni C, Medina CD, Cremonte F, Etchegoin JA. Histopathological Lesions Caused by a Digenean Trematode in a Pest Apple Snail, Pomacea canaliculata, in Its Native Geographic Distribution Area. Animals. 2024; 14(8):1191. https://doi.org/10.3390/ani14081191
Chicago/Turabian StyleMartinez, Lorena Evangelina, Carmen Gilardoni, Cintia Débora Medina, Florencia Cremonte, and Jorge Alejandro Etchegoin. 2024. "Histopathological Lesions Caused by a Digenean Trematode in a Pest Apple Snail, Pomacea canaliculata, in Its Native Geographic Distribution Area" Animals 14, no. 8: 1191. https://doi.org/10.3390/ani14081191
APA StyleMartinez, L. E., Gilardoni, C., Medina, C. D., Cremonte, F., & Etchegoin, J. A. (2024). Histopathological Lesions Caused by a Digenean Trematode in a Pest Apple Snail, Pomacea canaliculata, in Its Native Geographic Distribution Area. Animals, 14(8), 1191. https://doi.org/10.3390/ani14081191







