Phosphatidylethanolamine Improves Postnatal Growth Retardation by Regulating Mucus Secretion of Intestinal Goblet Cells in Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Tissue Collection and Processing
2.3. Serum Biochemical Index Assay
2.4. PE Concentration Analysis
2.5. Intestinal Morphology Detection
2.6. Immunofluorescence Staining
2.7. RNA Extraction and Real-Time PCR
2.8. Western Blot Analysis
2.9. TUNEL Assay
2.10. Statistics
3. Results
3.1. PE Oral Administration Improved the Growth Performance of PGR Piglets
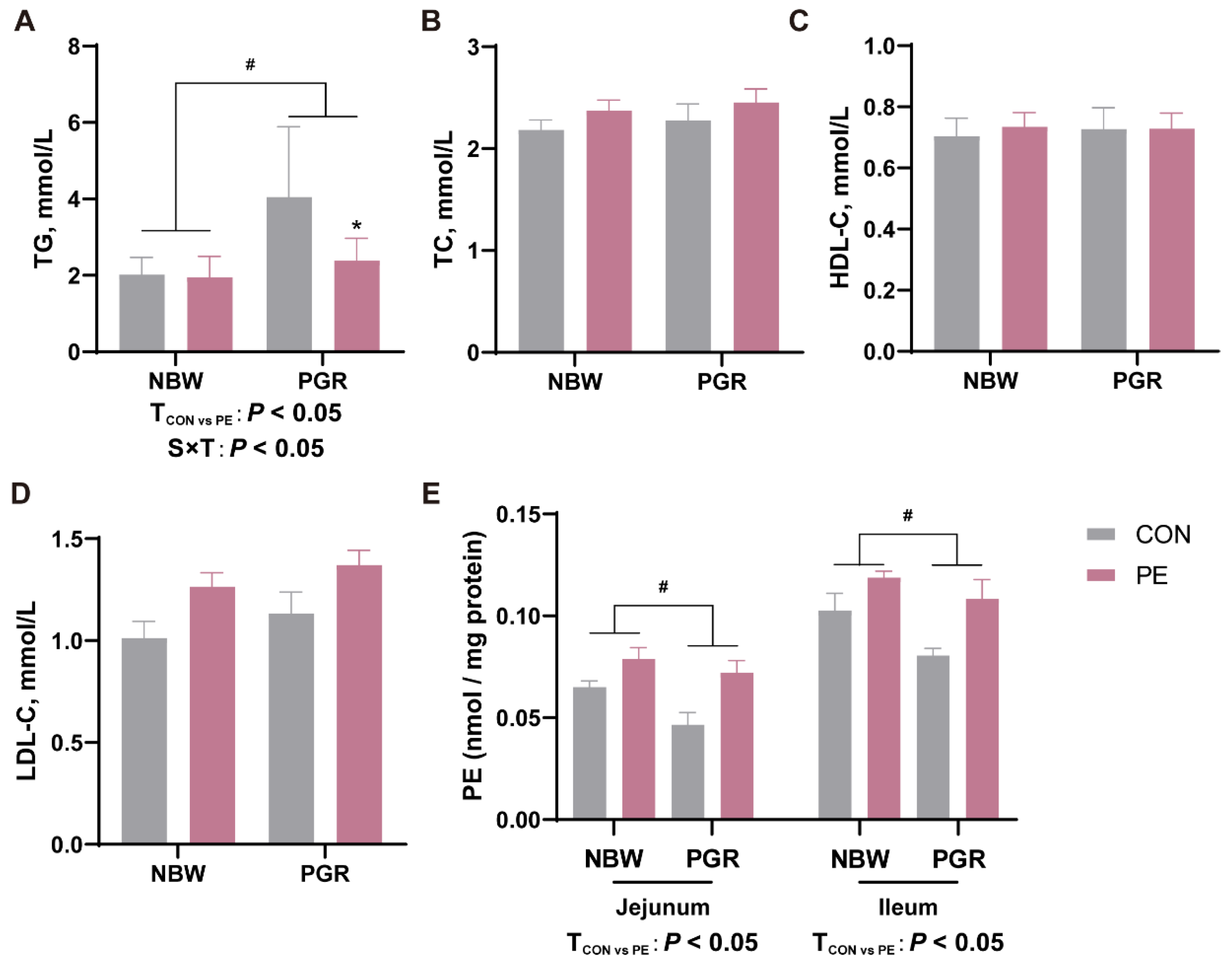
3.2. PE Oral Administration Affected the Serum Lipid and Intestinal PE Levels in Piglets
3.3. PE Supplementation Alleviated the Intestinal Morphological Injury of PGR Piglets
3.4. PE Supplementation Alleviated Intestinal Inflammation of PGR Piglets
3.5. PE Supplementation Increased the Number of Small Intestinal Goblet Cells and MUC2 Secretion of PGR Piglets
3.6. PE Supplementation Promoted the Differentation of Small Intestinal Goblet Cells of PGR Piglets
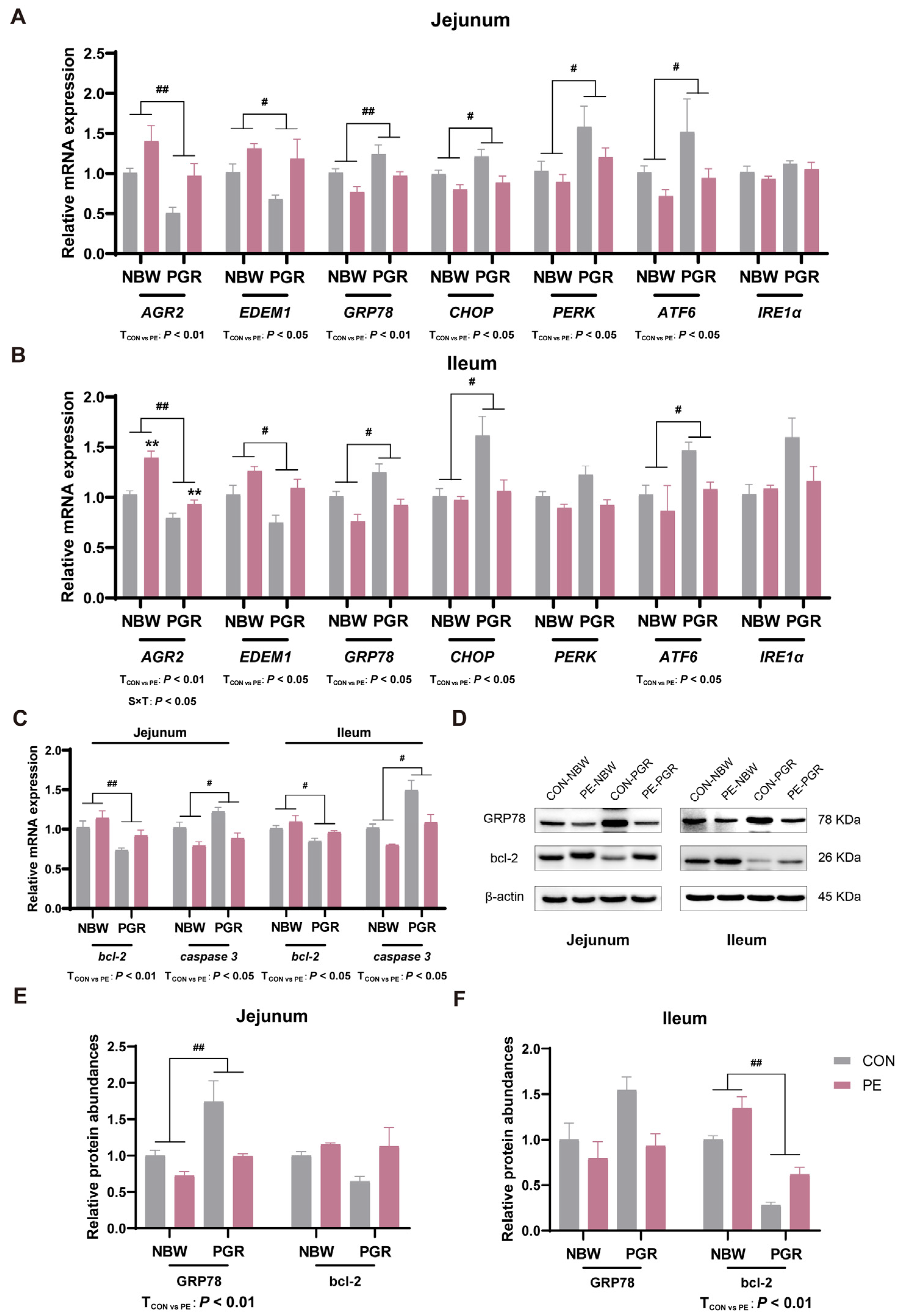
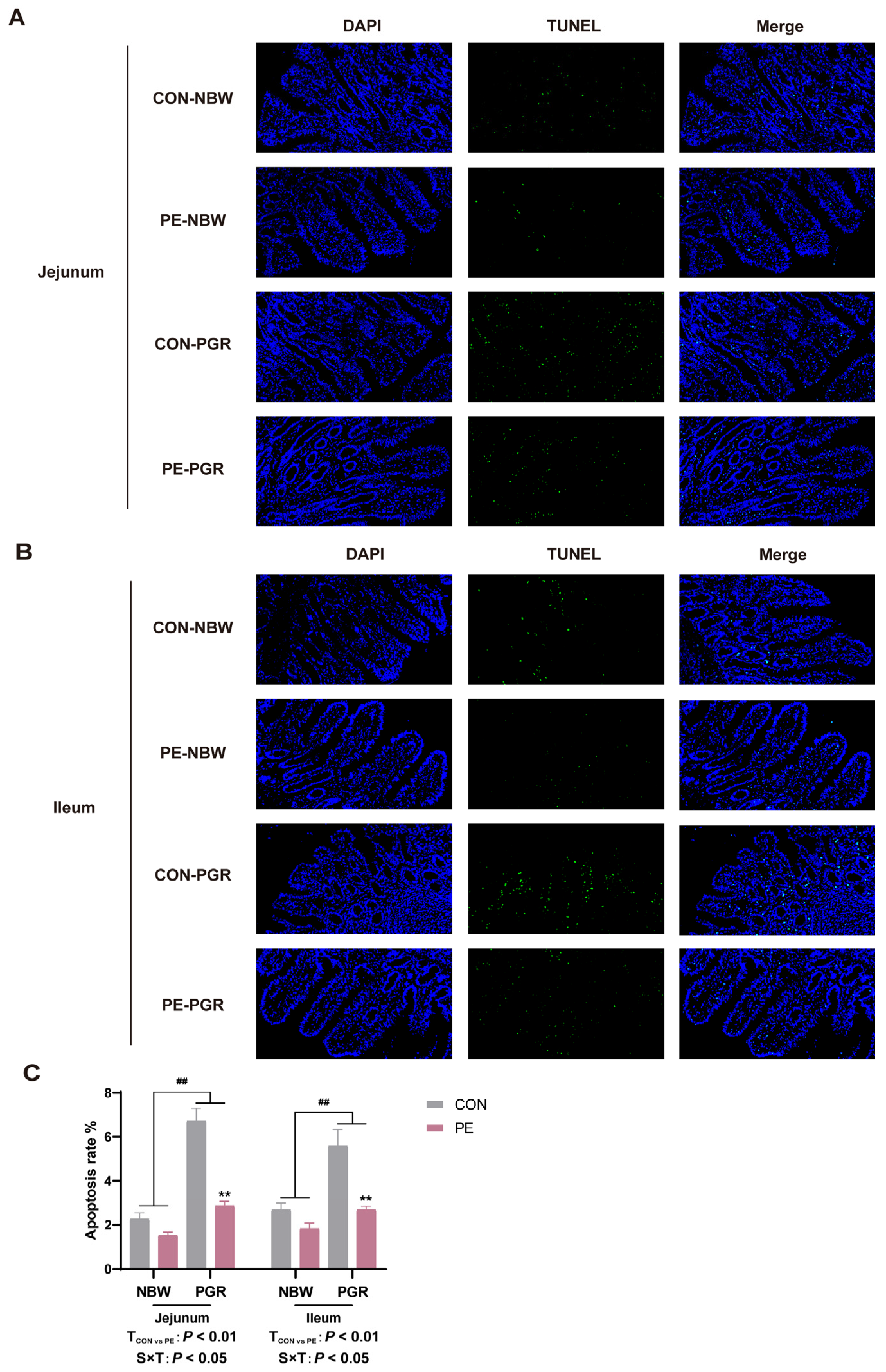
3.7. PE Supplementation Alleviated the Endoplasmic Reticulum Stress and Apoptosis in the Small Intestine of PGR Piglets
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Cowardin, C.A.; Syed, S.; Iqbal, N.; Jamil, Z.; Sadiq, K.; Iqbal, J.; Moore, S.R. Environmental enteric dysfunction: Gut and microbiota adaptation in pregnancy and infancy. Nat. Rev. Gastroenterol. Hepatol. 2022, 20, 223–237. [Google Scholar] [CrossRef] [PubMed]
- de Nies, L.; Kobras, C.M.; Stracy, M. Antibiotic-induced collateral damage to the microbiota and associated infections. Nat. Rev. Microbiol. 2023, 21, 789–804. [Google Scholar] [CrossRef]
- Lei, L.; Yang, J.; Zhang, J.; Zhang, G. The lipid peroxidation product EKODE exacerbates colonic inflammation and colon tumorigenesis. Redox Biol. 2021, 42, 101880. [Google Scholar] [CrossRef]
- Wells, J.C.; Sawaya, A.L.; Wibaek, R.; Mwangome, M.; Poullas, M.S.; Yajnik, C.S.; Demaio, A. The double burden of malnutrition: Aetiological pathways and consequences for health. Lancet 2020, 395, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.; Middelkoop, A.; de Souza, J.G.; van Veen, L.A.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.A.; Kleerebezem, A. Impact of early-life feeding on local intestinal microbiota and digestive system development in piglets. Sci. Rep. 2021, 11, 4213. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, X.; Han, D.; Pi, Y.; Tao, S.; Zhang, S.; Wang, S.; Zhao, J.; Chen, L.; Wang, J. Early life administration of milk fat globule membrane promoted SCFA-producing bacteria colonization, intestinal barriers and growth performance of neonatal piglets. Anim. Nutr. 2021, 7, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Vance, J.E. Phospholipid Synthesis and Transport in Mammalian Cells. Traffic 2015, 16, 1–18. [Google Scholar] [CrossRef]
- Boldyreva, L.V.; Morozova, M.V.; Saydakova, S.S.; Kozhevnikova, E.N. Fat of the Gut: Epithelial Phospholipids in Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 11682. [Google Scholar] [CrossRef]
- Ross, R.G.; Hunter, S.K.; Hoffman, M.C.; McCarthy, L.; Chambers, B.M.; Law, A.J.; Freedman, R. Perinatal Phosphatidylcholine Supplementation and Early Childhood Behavior Problems: Evidence forCHRNA7Moderation. Am. J. Psychiatry 2016, 173, 509–516. [Google Scholar] [CrossRef]
- Patel, D.; Witt, S.N. Ethanolamine and Phosphatidylethanolamine: Partners in Health and Disease. Oxid. Med. Cell Longev. 2017, 2017, 4829180. [Google Scholar] [CrossRef]
- Fang, W.; Liu, Y.; Chen, Q.; Xu, D.; Liu, Q.; Cao, X.; Hao, T.; Zhang, L.; Mai, K.; Ai, Q. Palmitic acid induces intestinal lipid metabolism disorder, endoplasmic reticulum stress and inflammation by affecting phosphatidylethanolamine content in large yellow croaker Larimichthys crocea. Front. Immunol. 2022, 13, 984508. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Guy, C.S.; Chapman, N.M.; Palacios, G.; Wei, J.; Zhou, P.; Long, L.; Wang, Y.-D.; Qian, C.; Dhungana, Y.; et al. Metabolic control of TFH cells and humoral immunity by phosphatidylethanolamine. Nature 2021, 595, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Calzada, E.; Onguka, O.; Claypool, S.M. Phosphatidylethanolamine Metabolism in Health and Disease. Int. Rev. Cell Mol. Biol. 2016, 321, 29–88. [Google Scholar] [CrossRef] [PubMed]
- Rockenfeller, P.; Koska, M.; Pietrocola, F.; Minois, N.; Knittelfelder, O.; Sica, V.; Franz, J.; Carmona-Gutierrez, D.; Kroemer, G.; Madeo, F. Phosphatidylethanolamine positively regulates autophagy and longevity. Cell Death Differ. 2015, 22, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xiong, X.; Wang, K.; Zou, L.; Lv, D.; Yin, Y. Ethanolamine Metabolism in the Mammalian Gastrointestinal Tract: Mechanisms, Patterns, and Importance. Curr. Mol. Med. 2017, 17, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Huang, R.; Wang, N.; Deng, Y.; Tan, B.; Yin, Y.; Qi, M.; Wang, J. Ellagic Acid Alleviates Oxidative Stress by Mediating Nrf2 Signaling Pathways and Protects against Paraquat-Induced Intestinal Injury in Piglets. Antioxidants 2022, 11, 252. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, N.; Deng, Y.; Zha, A.; Li, J.; Tan, B.; Qi, M.; Wang, J.; Yin, Y. β-hydroxybutyrate administration improves liver injury and metabolic abnormality in postnatal growth retardation piglets. Front. Vet. Sci. 2023, 10, 1294095. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.S.; Xiong, X.; Wen, Q.Q.; Yin, Y.L. Effects of dietary supplementation with ethanolamine on intestine development and growth performance of weaned piglets. J. Anim. Sci. 2016, 94 (Suppl. S3), 79–81. [Google Scholar] [CrossRef]
- Liu, Y.; Azad, A.K.; Ding, S.; Zhu, Q.; Blachier, F.; Yu, Z.; Gao, H.; Kong, X. Dietary bile acid supplementation in weaned piglets with intrauterine growth retardation improves colonic microbiota, metabolic activity, and epithelial function. J. Anim. Sci. Biotechnol. 2023, 14, 99. [Google Scholar] [CrossRef]
- Wang, J.; Wang, N.; Qi, M.; Li, J.; Tan, B. Glutamine, glutamate, and aspartate differently modulate energy homeostasis of small intestine under normal or low energy status in piglets. Anim. Nutr. 2022, 8, 216–226. [Google Scholar] [CrossRef]
- Zha, A.; Yuan, D.; Cui, Z.; Qi, M.; Liao, S.; Liao, P.; Tan, B. The Evaluation of the Antioxidant and Intestinal Protective Effects of Baicalin-Copper in Deoxynivalenol-Challenged Piglets. Oxid. Med. Cell Longev. 2020, 2020, 5363546. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Liao, S.; Wang, J.; Deng, Y.; Zha, A.; Shao, Y.; Cui, Z.; Song, T.; Tang, Y.; Tan, B.; et al. MyD88 deficiency ameliorates weight loss caused by intestinal oxidative injury in an autophagy-dependent mechanism. J. Cachexia Sarcopenia Muscle 2021, 13, 677–695. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Wang, N.; Xiao, Y.; Deng, Y.; Zha, A.; Tan, B.; Wang, J.; Yin, Y.; Liao, P. Ellagic acid ameliorates paraquat-induced liver injury associated with improved gut microbial profile. Environ. Pollut. 2022, 293, 118572. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Begum, S.; Moreau, F.; Gorman, H.; Chadee, K. Autophagy is required during high MUC2 mucin biosynthesis in colonic goblet cells to contend metabolic stress. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 321, G489–G499. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Tan, B.; Wang, J.; Liao, S.; Deng, Y.; Ji, P.; Song, T.; Zha, A.; Yin, Y. The microbiota–gut–brain axis: A novel nutritional therapeutic target for growth retardation. Crit. Rev. Food Sci. Nutr. 2021, 62, 4867–4892. [Google Scholar] [CrossRef] [PubMed]
- Black, M.M.; Trude, A.C.B.; Lutter, C.K. All Children Thrive: Integration of Nutrition and Early Childhood Development. Annu. Rev. Nutr. 2020, 40, 375–406. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zou, H.; Wang, Z.; Cao, B.; Peng, Q.; Jing, X.; Wang, Y.; Shao, Y.; Pei, Z.; Zhang, X.; et al. Nutritional Interventions Improved Rumen Functions and Promoted Compensatory Growth of Growth-Retarded Yaks as Revealed by Integrated Transcripts and Microbiome Analyses. Front. Microbiol. 2019, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Tan, B.; Wang, J.; Liao, S.; Li, J.; Liu, Y.; Yin, Y. Post-natal Growth Retardation Associated With Impaired Gut Hormone Profiles, Immune and Antioxidant Function in Pigs. Front. Endocrinol. 2019, 10, 660. [Google Scholar] [CrossRef] [PubMed]
- Ko, C.-W.; Qu, J.; Black, D.D.; Tso, P. Regulation of intestinal lipid metabolism: Current concepts and relevance to disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 169–183. [Google Scholar] [CrossRef]
- Cikes, D.; Elsayad, K.; Sezgin, E.; Koitai, E.; Torma, F.; Orthofer, M.; Yarwood, R.; Heinz, L.X.; Sedlyarov, V.; Miranda, N.D.; et al. PCYT2-regulated lipid biosynthesis is critical to muscle health and ageing. Nat. Metab. 2023, 5, 495–515. [Google Scholar] [CrossRef]
- Qi, M.; Tan, B.; Wang, J.; Liao, S.; Li, J.; Cui, Z.; Shao, Y.; Ji, P.; Yin, Y. Postnatal growth retardation is associated with deteriorated intestinal mucosal barrier function using a porcine model. J. Cell Physiol. 2020, 236, 2631–2648. [Google Scholar] [CrossRef]
- Mehandru, S.; Colombel, J.-F. The intestinal barrier, an arbitrator turned provocateur in IBD. Nat. Rev. Gastroenterol. Hepatol. 2020, 18, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Rescigno, M. The intestinal epithelial barrier in the control of homeostasis and immunity. Trends Immunol. 2011, 32, 256–264. [Google Scholar] [CrossRef]
- Cui, C.; Wu, C.; Wang, J.; Ma, Z.; Zheng, X.; Zhu, P.; Wang, N.; Zhu, Y.; Guan, W.; Chen, F. Restored intestinal integrity, nutrients transporters, energy metabolism, antioxidative capacity and decreased harmful microbiota were associated with IUGR piglet’s catch-up growth before weanling. J. Anim. Sci. Biotechnol. 2022, 13, 129. [Google Scholar] [CrossRef] [PubMed]
- Dang, B.; Gao, Q.; Zhang, L.; Zhang, J.; Cai, H.; Zhu, Y.; Zhong, Q.; Liu, J.; Niu, Y.; Mao, K.; et al. The glycolysis/HIF-1α axis defines the inflammatory role of IL-4-primed macrophages. Cell Rep. 2023, 42, 112471. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, W.; O’Garra, A. IL-10 Family Cytokines IL-10 and IL-22, from Basic Science to Clinical Translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef] [PubMed]
- Woznicki, J.A.; Saini, N.; Flood, P.; Rajaram, S.; Lee, C.M.; Stamou, P.; Skowyra, A.; Bustamante-Garrido, M.; Regazzoni, K.; Crawford, N.; et al. TNF-α synergises with IFN-γ to induce caspase-8-JAK1/2-STAT1-dependent death of intestinal epithelial cells. Cell Death Dis. 2021, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Che, L.; Zhou, Q.; Liu, Y.; Hu, L.; Peng, X.; Wu, C.; Zhang, R.; Tang, J.; Wu, F.; Fang, Z.; et al. Flaxseed oil supplementation improves intestinal function and immunity, associated with altered intestinal microbiome and fatty acid profile in pigs with intrauterine growth retardation. Food Funct. 2019, 10, 8149–8160. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, N.; Liu, C.; Li, T.; Wang, W.; Jiang, L.; Li, Z.; Han, D.; Tao, S.; Wang, J. Characteristics of the gut microbiota colonization, inflammatory profile, and plasma metabolome in intrauterine growth restricted piglets during the first 12 hours after birth. J. Microbiol. 2019, 57, 748–758. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, W.; Azad, M.A.K.; Ma, C.; Zhu, Q.; Kong, X. Metabolome, microbiome, and gene expression alterations in the colon of newborn piglets with intrauterine growth restriction. Front. Microbiol. 2022, 13, 989060. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Oswald, I.P. Role of intestinal epithelial cells in the innate immune defence of the pig intestine. Vet. Res. 2006, 37, 359–368. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef] [PubMed]
- Sang, X.; Wang, Q.; Ning, Y.; Wang, H.; Zhang, R.; Li, Y.; Fang, B.; Lv, C.; Zhang, Y.; Wang, X.; et al. Age-Related Mucus Barrier Dysfunction in Mice Is Related to the Changes in Muc2 Mucin in the Colon. Nutrients 2023, 15, 1830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Li, Y.; Zhang, T.; Ying, Z.; Su, W.; Zhang, L.; Wang, T. l-Threonine improves intestinal mucin synthesis and immune function of intrauterine growth–retarded weanling piglets. Nutrition 2019, 59, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yu, M. Role of Goblet Cells in Intestinal Barrier and Mucosal Immunity. J. Inflamm. Res. 2021, 14, 3171–3183. [Google Scholar] [CrossRef] [PubMed]
- Birchenough, G.M.; Schroeder, B.O.; Sharba, S.; Arike, L.; Recktenwald, C.V.; Puértolas-Balint, F.; Subramani, M.V.; Hansson, K.T.; Yilmaz, B.; Lindén, S.K.; et al. Muc2-dependent microbial colonization of the jejunal mucus layer is diet sensitive and confers local resistance to enteric pathogen infection. Cell Rep. 2023, 42, 112084. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Liu, L.; Zhou, W.; Yang, C.; Mai, G.; Li, H.; Chen, Y. Gut microbiota-derived butyrate regulates gut mucus barrier repair by activating the macrophage/WNT/ERK signaling pathway. Clin. Sci. 2022, 136, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Nyström, E.E.L.; Martinez-Abad, B.; Arike, L.; Birchenough, G.M.H.; Nonnecke, E.B.; Castillo, P.A.; Svensson, F.; Bevins, C.L.; Hansson, G.C.; Johansson, M.E.V. An intercrypt subpopulation of goblet cells is essential for colonic mucus barrier function. Science 2021, 372, eabb1590. [Google Scholar] [CrossRef]
- Kennelly, J.P.; Carlin, S.; Ju, T.; van der Veen, J.N.; Nelson, R.C.; Buteau, J.; Thiesen, A.; Richard, C.; Willing, B.P.; Jacobs, R.L. Intestinal Phospholipid Disequilibrium Initiates an ER Stress Response That Drives Goblet Cell Necroptosis and Spontaneous Colitis in Mice. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 999–1021. [Google Scholar] [CrossRef]
- Hasnain, S.Z.; Tauro, S.; Das, I.; Tong, H.; Chen, A.C.; Jeffery, P.L.; McDonald, V.; Florin, T.H.; McGuckin, M.A. IL-10 Promotes Production of Intestinal Mucus by Suppressing Protein Misfolding and Endoplasmic Reticulum Stress in Goblet Cells. Gastroenterology 2013, 144, 357–368.e9. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Peng, X.; Li, Q.; Wang, P.; Lv, P.; Song, Q.; She, S.; Huang, S.; Chen, K.; Gong, W.; et al. FAM3D is essential for colon homeostasis and host defense against inflammation associated carcinogenesis. Nat. Commun. 2020, 11, 5912. [Google Scholar] [CrossRef] [PubMed]
- Engevik, M.A.; Herrmann, B.; Ruan, W.; Engevik, A.C.; Engevik, K.A.; Ihekweazu, F.; Shi, Z.; Luck, B.; Chang-Graham, A.L.; Esparza, M.; et al. Bifidobacterium dentium-derived y-glutamylcysteine suppresses ER-mediated goblet cell stress and reduces TNBS-driven colonic inflammation. Gut Microbes 2021, 13, 1902717. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaibi, A.A.; Abdel-Motal, U.M.; Hubrack, S.Z.; Bullock, A.N.; Al-Marri, A.A.; Agrebi, N.; Al-Subaiey, A.A.; Ibrahim, N.A.; Charles, A.K.; Elawad, M.; et al. Human AGR2 Deficiency Causes Mucus Barrier Dysfunction and Infantile Inflammatory Bowel Disease. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 1809–1830. [Google Scholar] [CrossRef] [PubMed]
- Chiritoiu, M.; Chiritoiu, G.N.; Munteanu, C.V.A.; Pastrama, F.; Ivessa, N.E.; Petrescu, S.M. EDEM1 Drives Misfolded Protein Degradation via ERAD and Exploits ER-Phagy as Back-Up Mechanism When ERAD Is Impaired. Int. J. Mol. Sci. 2020, 21, 3468. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.S. Endoplasmic Reticulum Stress and Unfolded Protein Response in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 636–644. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhang, B.; Guan, H.; Jiao, X.; Yang, J.; Cai, J.; Liu, Q.; Zhang, Z. Selenium deficiency causes apoptosis through endoplasmic reticulum stress in swine small intestine. BioFactors 2021, 47, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xiong, X.; Li, T.; Yin, Y. Ethanolamine enhances the proliferation of intestinal epithelial cells via the mTOR signaling pathway and mitochondrial function. In Vitro Cell Dev. Biol. Anim. 2016, 52, 562–567. [Google Scholar] [CrossRef]
- Ahmadi, S.; Razazan, A.; Nagpal, R.; Jain, S.; Wang, B.; Mishra, S.P.; Wang, S.; Justice, J.; Ding, J.; McClain, D.A.; et al. Metformin reduces aging-related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin axis. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, e9–e21. [Google Scholar] [CrossRef]




| Gene | Gene Bank No. | Sequence (5′-3′) |
|---|---|---|
| β-actin | XM_0031242803 | F: CTGCGGCATCCACGAAACT R: AGGGCCGTGATCTCCTTCTG |
| ZO-1 | XM_021098896.1 | F: CCTGAGTTTGATAGTGGCGTTGA |
| R: AAATAGATTTCCTGCTCAATTCC | ||
| IL-4 | NM_214340.1 | F: CCCGAGTGTCAAGTGGCTTA R: TGATGATGCCGAAATAGCAG |
| IL-10 | NM_214041.1 | F: GGGCTATTTGTCCTGACTGC R: GGGCTCCCTAGTTTCTCTTCC |
| IFN-γ | NM_213948.1 | F: TTCAGCTTTGCGTGACTTTG R: GGTCCACCATTAGGTACATCTG |
| MUC2 | XM_021082584.1 | F: CTGTGTGGGGCCTGACAA R: AGTGCTTGCAGTCGAACTCA |
| spdef | XM_005665895.3 | F: GGCAGGGTTATGTGGGGAGTA R: GCTGTGTGAGGGGTGAGATAAT |
| AGR2 | XM_005674278.2 | F: AGCTCCTCCCTCTGTGTTAGG R: TGAGTATGTTCACCAGTGCCTT |
| EDEM1 | XM_021069286.1 | F: GGAAGGTCCCCAGCGTTTTA R: AAGACAAGCCACAGCACTCC |
| GRP78 | XM_001927795.7 | F: TATATAAGCGGAGCAGGCGAC R: TTCGCAAGCAAACCGATCAC |
| CHOP | XM_005674378.2 | F: TCTGGCTTGGCTGACTGAGGAG R: CCGTTTCCTGGGTCTTCTTTGGTC |
| PERK | XM_003124925.4 | F: ACTACAAGCGGGAAAGGAGC R: CACCAGTGCAAAAGGAGCAC |
| ATF6 | XM_021089516.1 | F: GCTCCTCCGTTCCTCCTTACCTC R: CTGACAACATGGGCTGCCTCTG |
| IRE1α | XM_005668695.3 | F: CTGCACTCCCTCAACATCGT R: GTAGGTGGGGTTCTCCTTGC |
| bcl-2 | NM_214285.1 | F: AGGGCATTCAGTGACCTGAC R: CGATCCGACTCACCAATACC |
| caspase 3 | NM_214131 | F: CGTGCTTCTAAGCCATGGTG R: GTCCCACTGTCCGTCTCAAT |
| Item | Treatments | Piglet Status | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| CON | PE | NBW | PGR | Treatment | Status | Treatment × Status | ||
| Average daily gain, g/d | 158.16 | 226.34 | 223.75 | 160.75 | 8.00 | <0.01 | <0.01 | 0.127 |
| Abdominal circumference, cm | 42.71 | 49.22 | 48.65 | 43.28 | 0.72 | <0.01 | <0.01 | 0.192 |
| Crown–rump length, cm | 49.10 | 55.63 | 55.06 | 48.66 | 0.76 | <0.01 | <0.01 | 0.124 |
| Body mass index, kg/m2 | 33.07 | 38.09 | 36.66 | 34.50 | 0.94 | 0.259 | 0.012 | 0.440 |
| Relative weight, g/kg | ||||||||
| Heart | 5.26 | 4.70 | 4.64 | 5.31 | 0.12 | 0.010 | 0.027 | 0.093 |
| Liver | 22.69 | 21.92 | 21.41 | 23.20 | 0.60 | 0.151 | 0.528 | 0.947 |
| Spleen | 1.89 | 1.67 | 1.68 | 1.89 | 0.08 | 0.185 | 0.171 | 0.527 |
| Lung | 17.83 | 13.73 | 15.57 | 16.02 | 0.89 | 0.799 | 0.026 | 0.421 |
| Kidney | 4.68 | 4.72 | 4.72 | 4.69 | 0.09 | 0.868 | 0.812 | 0.426 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Wang, C.; Qi, M.; Lin, X.; Zha, A.; Tan, B.; Yin, Y.; Wang, J. Phosphatidylethanolamine Improves Postnatal Growth Retardation by Regulating Mucus Secretion of Intestinal Goblet Cells in Piglets. Animals 2024, 14, 1193. https://doi.org/10.3390/ani14081193
Wang N, Wang C, Qi M, Lin X, Zha A, Tan B, Yin Y, Wang J. Phosphatidylethanolamine Improves Postnatal Growth Retardation by Regulating Mucus Secretion of Intestinal Goblet Cells in Piglets. Animals. 2024; 14(8):1193. https://doi.org/10.3390/ani14081193
Chicago/Turabian StyleWang, Nan, Chengming Wang, Ming Qi, Xingtong Lin, Andong Zha, Bie Tan, Yulong Yin, and Jing Wang. 2024. "Phosphatidylethanolamine Improves Postnatal Growth Retardation by Regulating Mucus Secretion of Intestinal Goblet Cells in Piglets" Animals 14, no. 8: 1193. https://doi.org/10.3390/ani14081193
APA StyleWang, N., Wang, C., Qi, M., Lin, X., Zha, A., Tan, B., Yin, Y., & Wang, J. (2024). Phosphatidylethanolamine Improves Postnatal Growth Retardation by Regulating Mucus Secretion of Intestinal Goblet Cells in Piglets. Animals, 14(8), 1193. https://doi.org/10.3390/ani14081193






