Potential of Wormwood and Oak Bark-Based Supplement in Health Improvement of Nosema ceranae-Infected Honey Bees
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Tested Supplement
2.2. Design of Laboratory Experiment
2.3. Inoculum Preparation and Experimental Infection
2.4. Nosema ceranae Spores Counting
2.5. Analyses of Oxidative Stress Parameters
2.6. Gene Expression Analyses
2.7. Statistical Analyses
3. Results
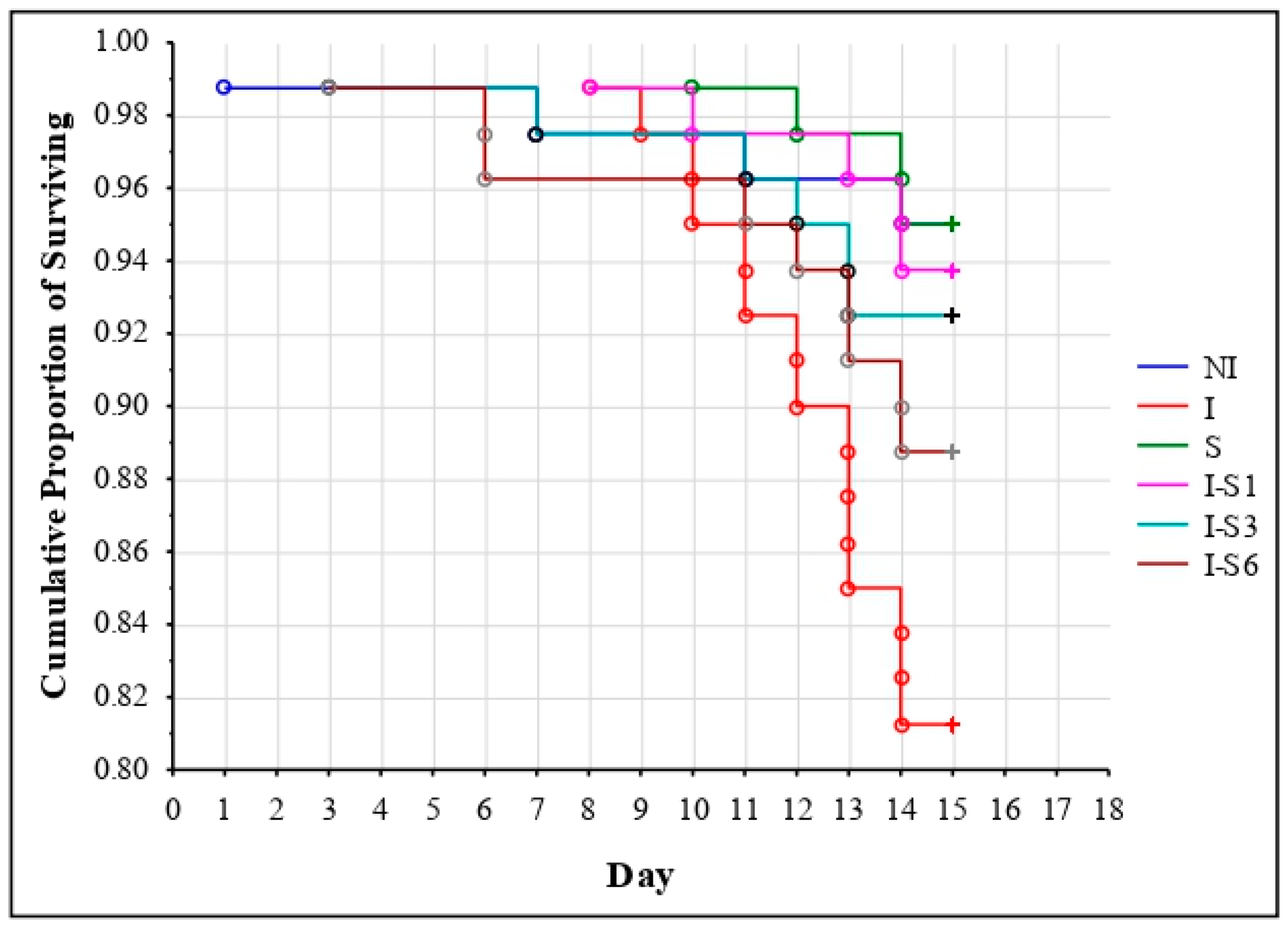
3.1. Bee Survival
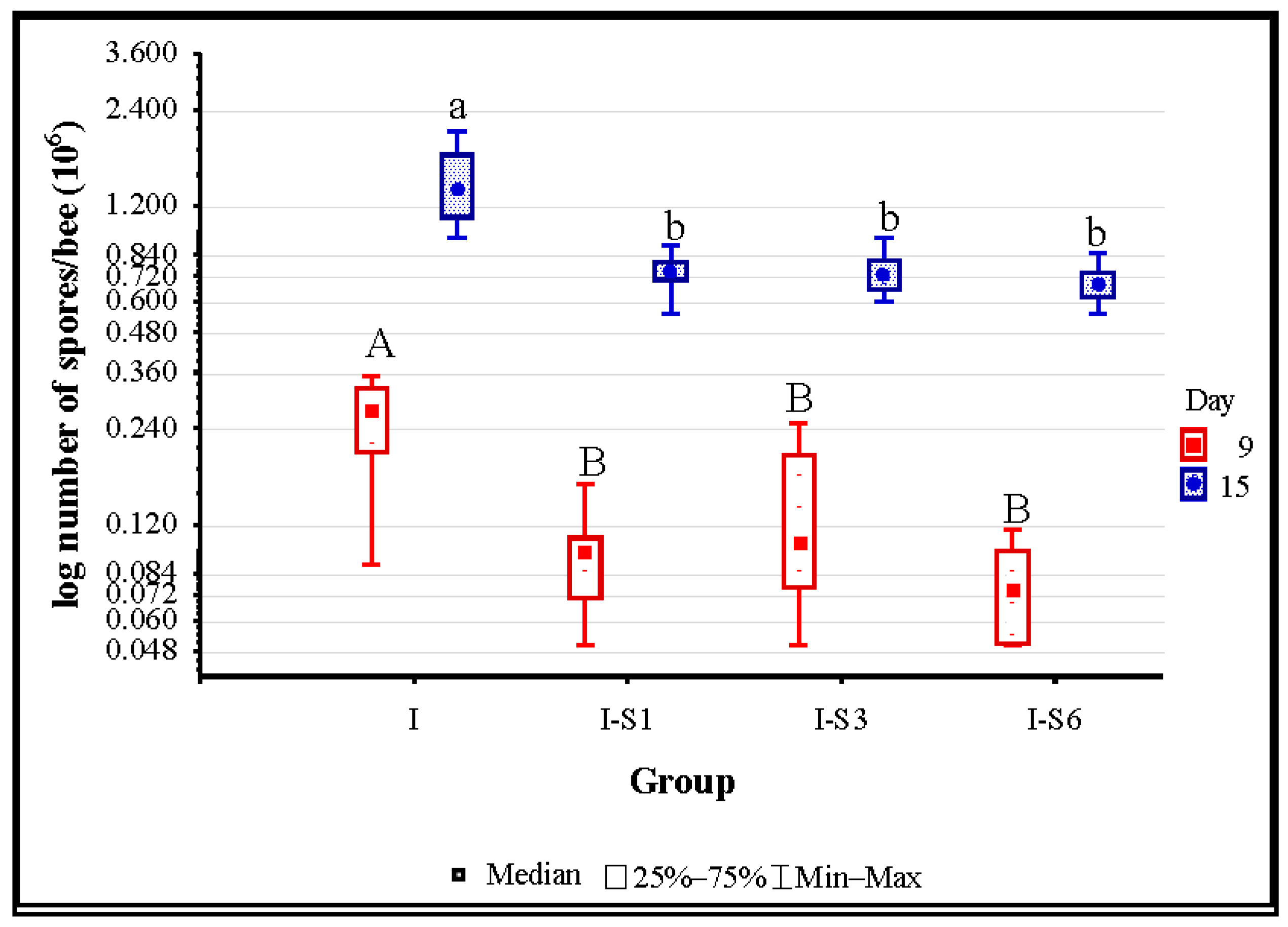
3.2. Number of Nosema ceranae Spores
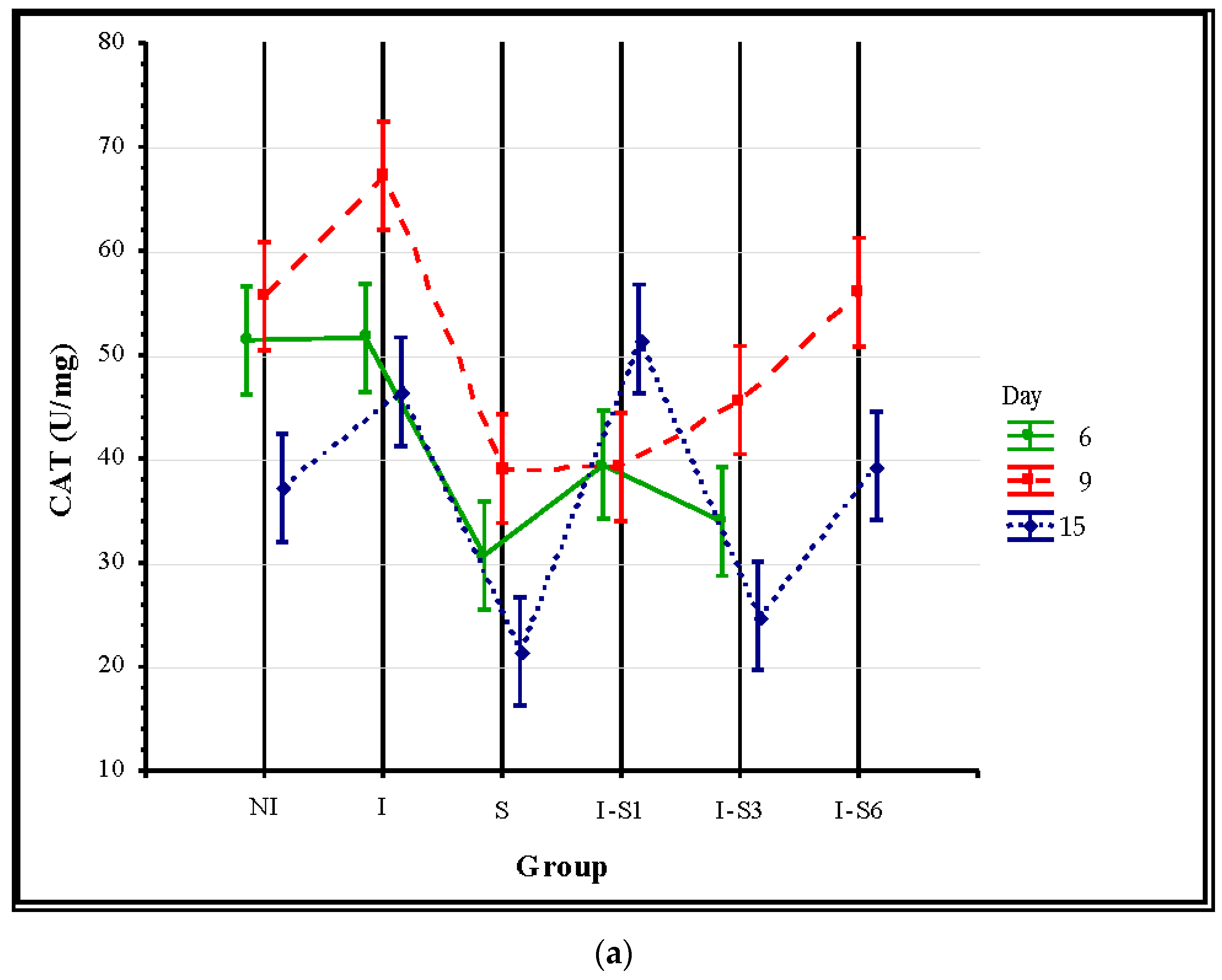
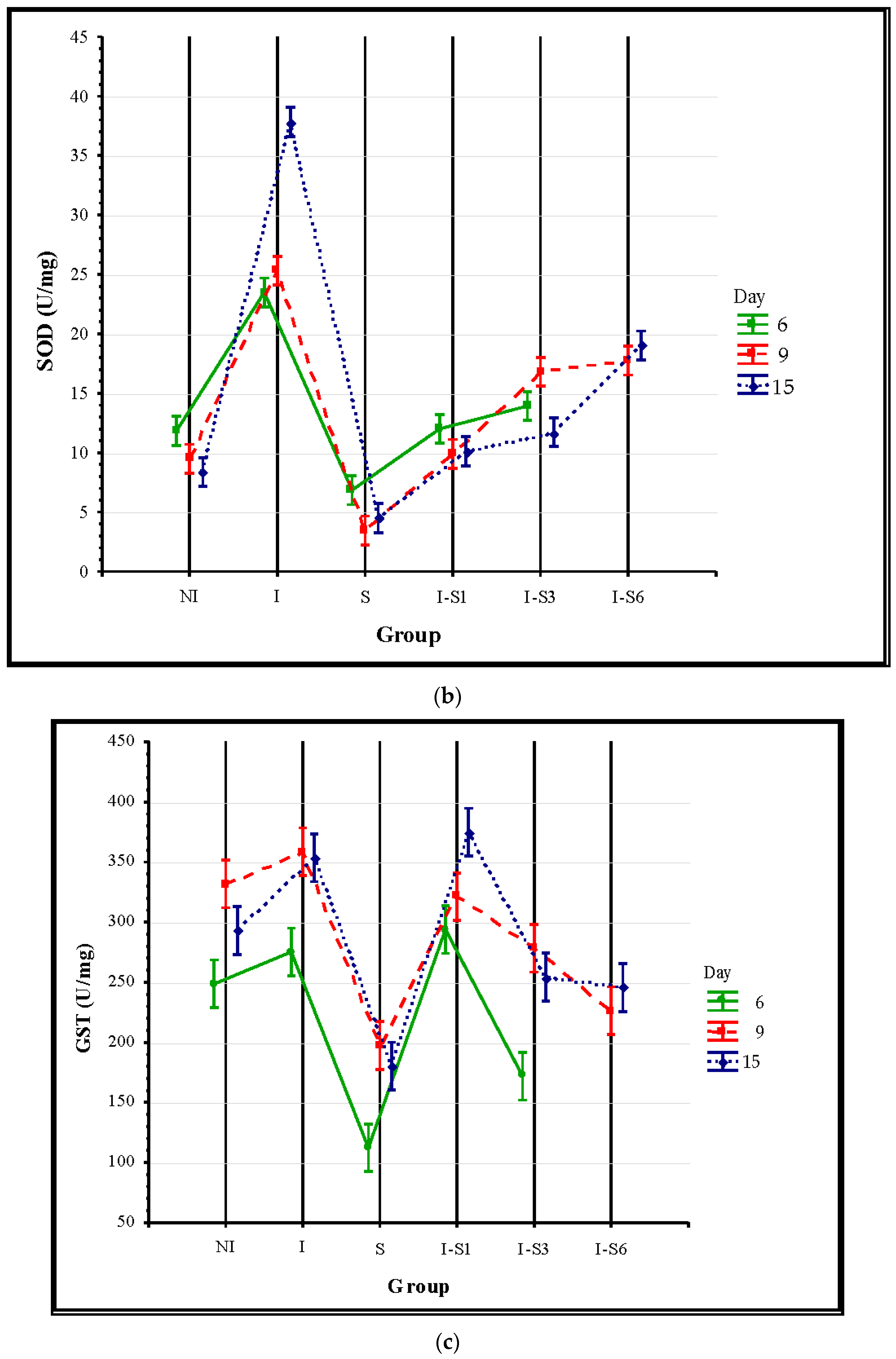
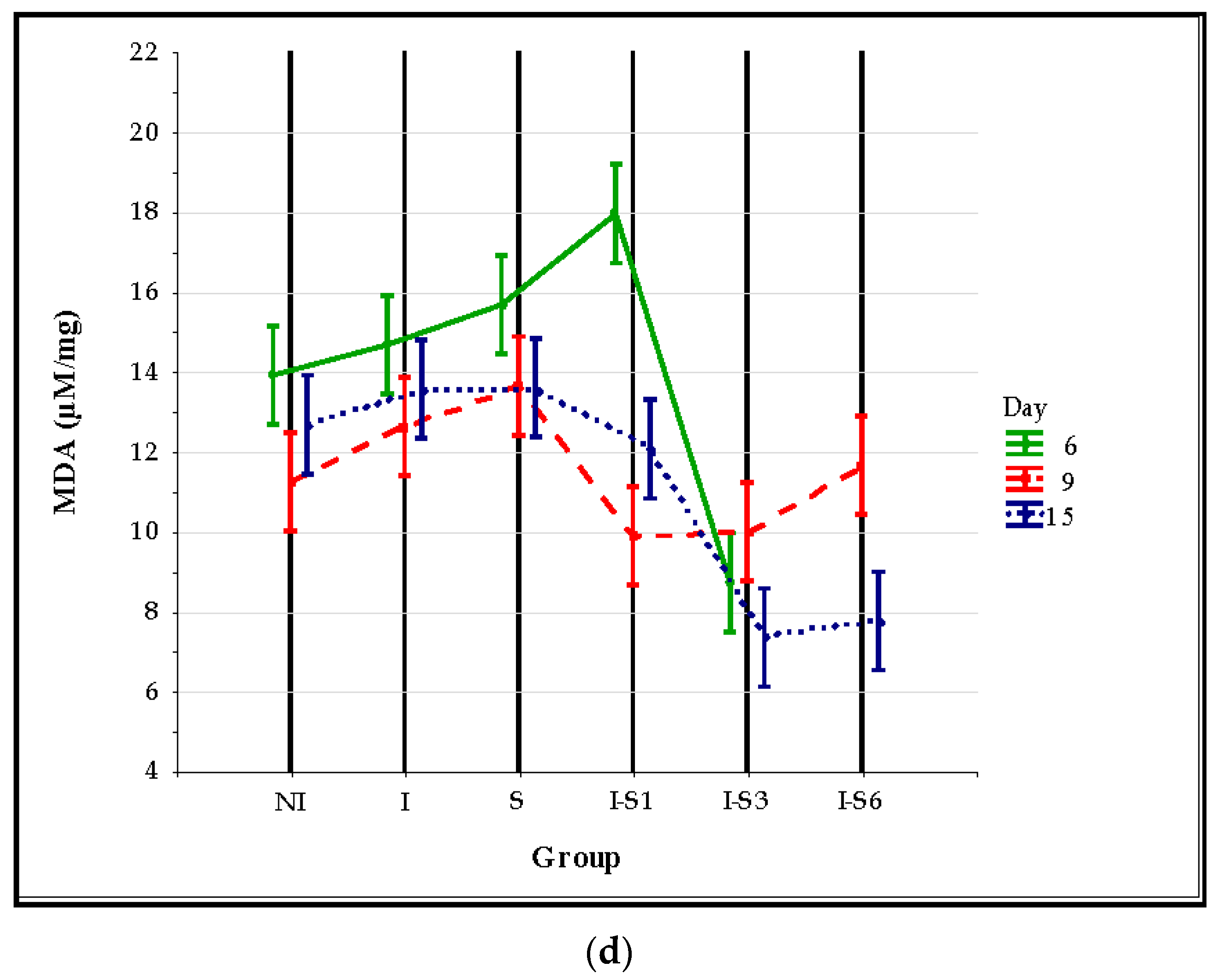
3.3. Oxidative Stress Parameters
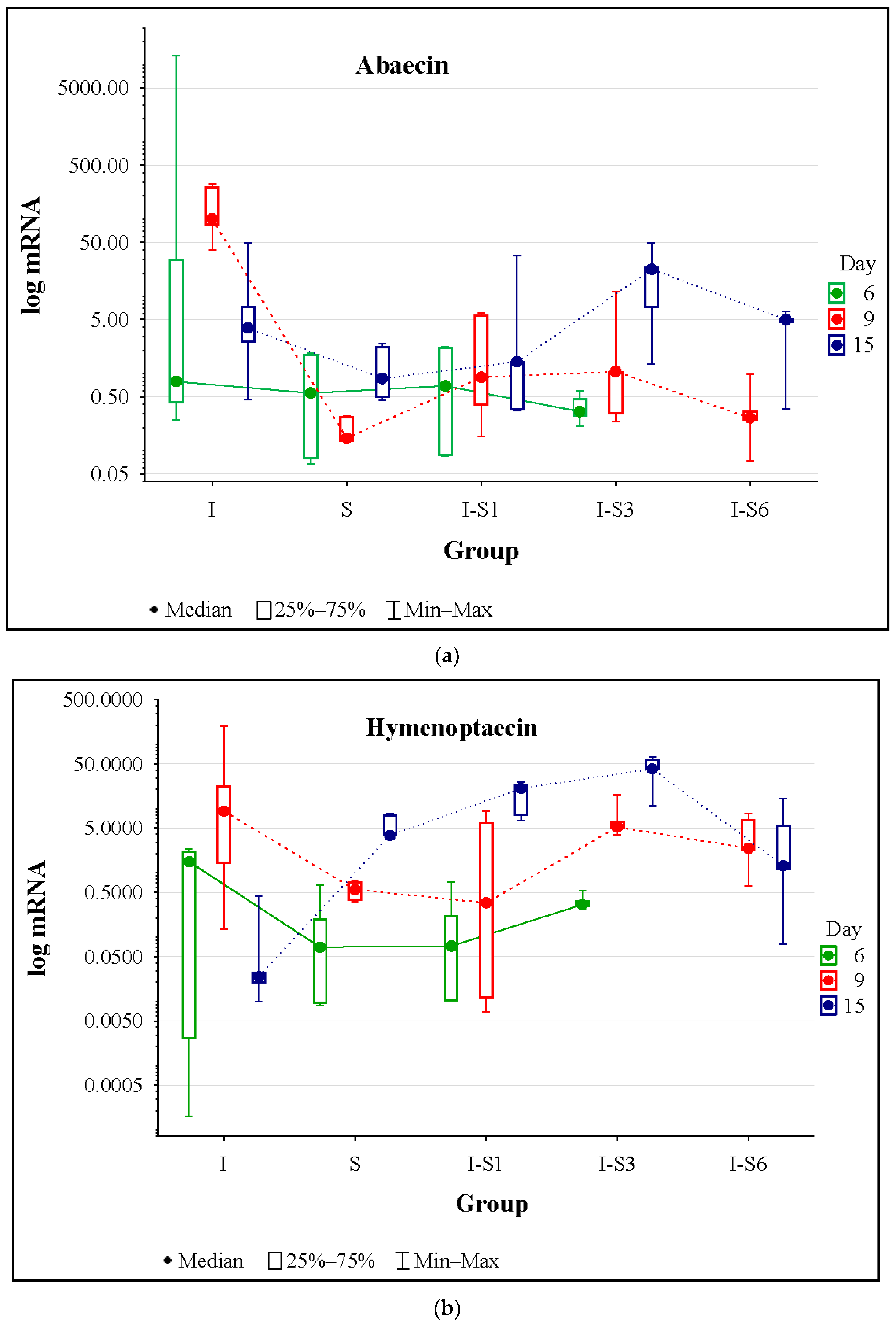
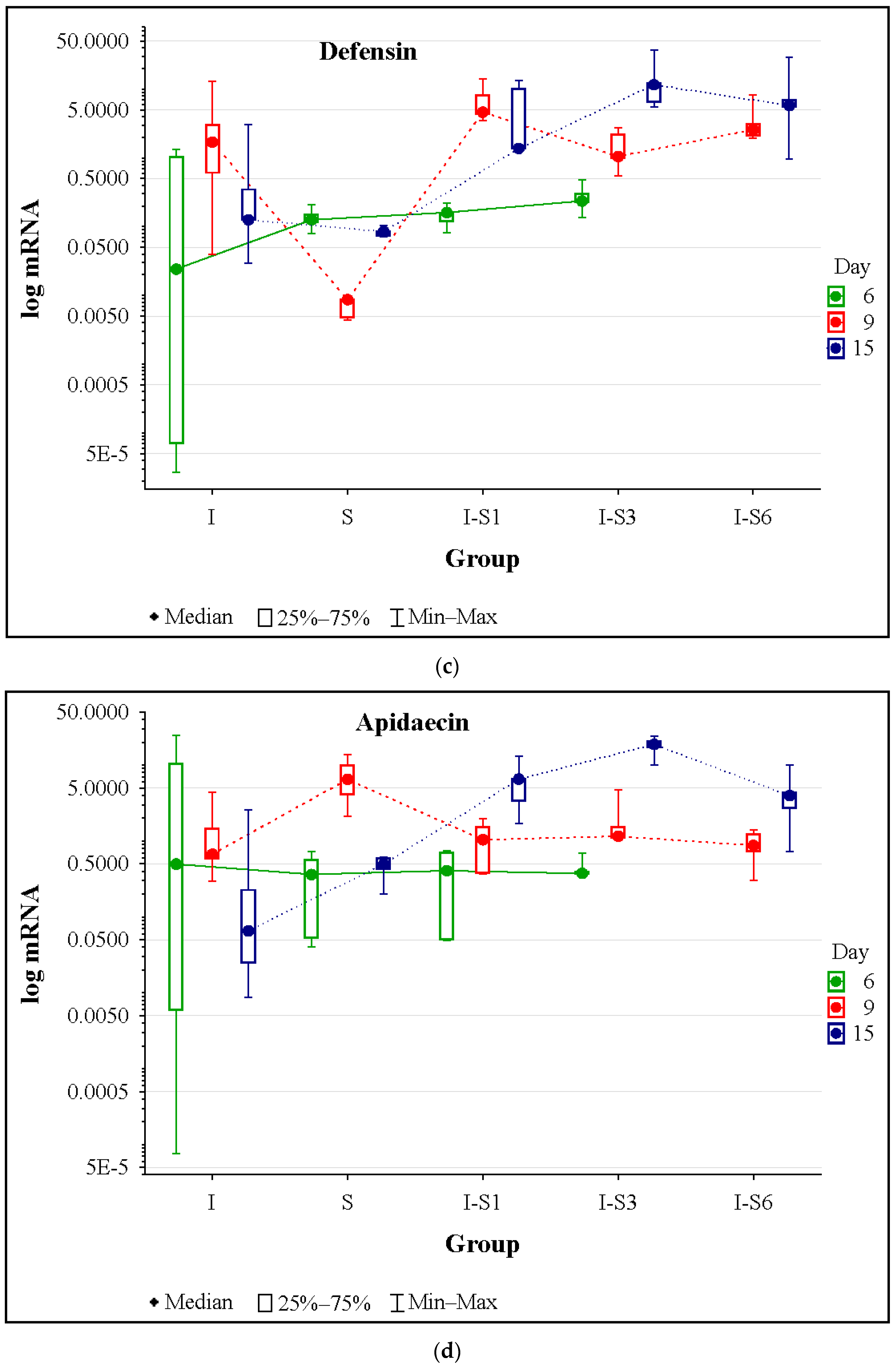
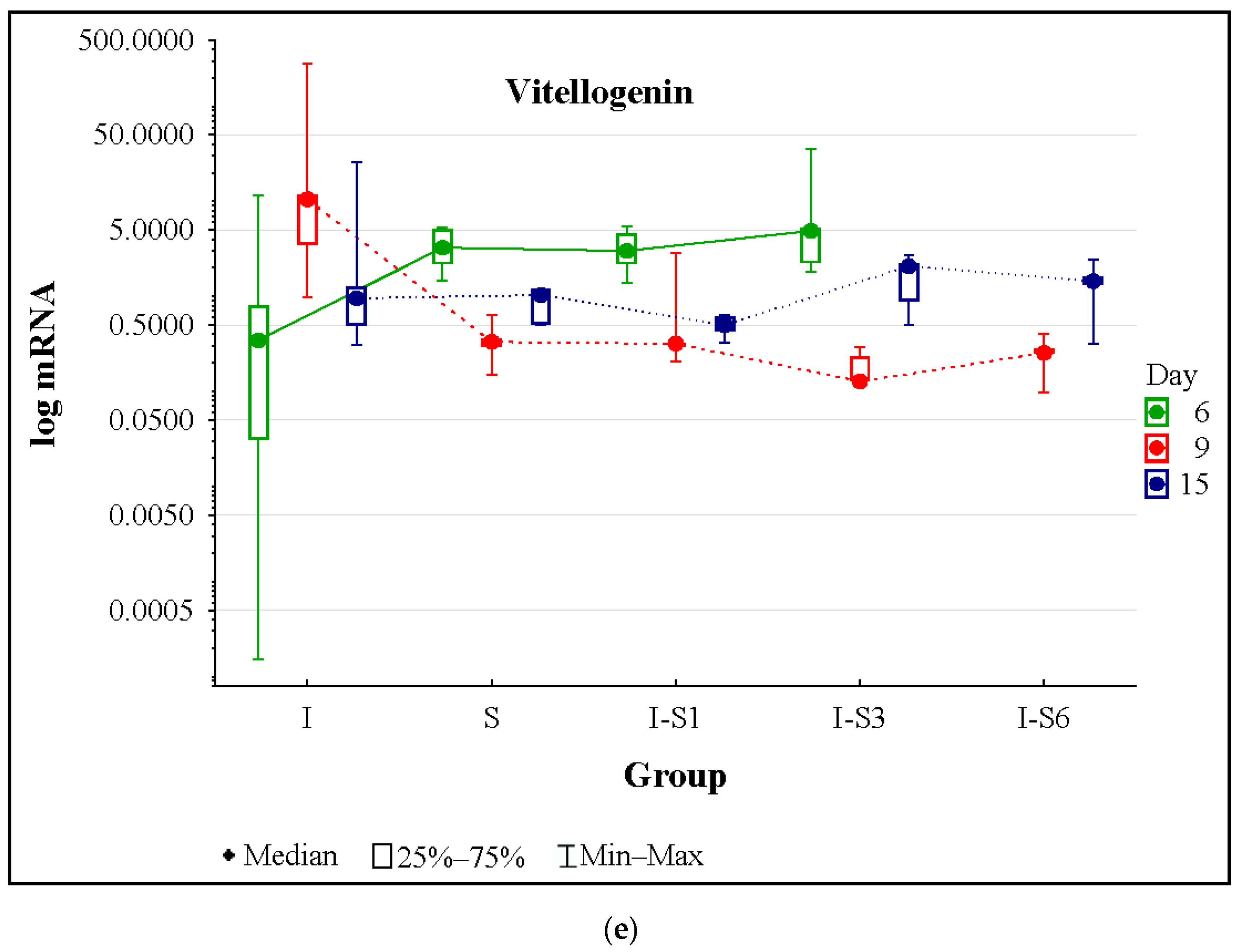
3.4. Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tokarev, Y.S.; Huang, W.F.; Solter, L.F.; Malysh, J.M.; Becnel, J.J.; Vossbrinck, C.R. A formal redefinition of the genera Nosema and Vairimorpha (Microsporidia: Nosematidae) and reassignment of species based on molecular phylogenetics. J. Invertebr. Pathol. 2020, 169, 107279. [Google Scholar] [CrossRef] [PubMed]
- Fries, I. Nosema ceranae in European honeybees (Apis mellifera). J. Invertebr. Pathol. 2010, 103, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Higes, M.; Martín-Hernández, R.; Meana, A. Nosema ceranae in Europe: An emergent type C nosemosis. Apidologie 2010, 41, 375–392. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Aleksić, N.; Jovanović, N.M.; Vejnović, B.; Stevanović, J. Looking for the causes of and solutions to the issue of honeybee colony losses. Acta Vet. 2019, 69, 1–31. [Google Scholar] [CrossRef]
- Kipkoech, A.; Okwaro, L.A.; Muli, E.; Lattorff, H.M.G. Occurrence and distribution of Nosema ceranae in honey bee colonies in the Comoros Islands. J. Apic. Res. 2023, 62, 1197–1206. [Google Scholar] [CrossRef]
- Blot, N.; Clémencet, J.; Jourda, C.; Lefeuvre, P.; Warrit, N.; Esnault, O.; Delatte, H. Geographic population structure of the honeybee microsporidian parasite Vairimorpha (Nosema) ceranae in the South West Indian Ocean. Sci. Rep. 2023, 13, 12122. [Google Scholar] [CrossRef] [PubMed]
- Botías, C.; Martín-Hernández, R.; Barrios, L.; Meana, A.; Higes, M. Nosema spp. infection and its negative effects on honeybees (Apis mellifera iberiensis) at the colony level. Vet. Res. 2013, 44, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, N.M.; Glavinic, U.; Delic, B.; Vejnovic, B.; Aleksic, N.; Mladjan, V.; Stanimirovic, Z. Plant-based supplement containing B-complex vitamins can improve bee health and increase colony performance. Prev. Vet. Med. 2021, 190, 105322. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Naug, D. Energetic stress in the honeybee Apis mellifera from Nosema ceranae infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Gage, S.L.; Kramer, C.; Calle, S.; Carroll, M.; Heien, M.; DeGrandi-Hoffman, G. Nosema ceranae parasitism impacts olfactory learning and memory and eurochemistry in honey bees (Apis mellifera). J. Exp. Biol. 2018, 221, 161489. [Google Scholar] [CrossRef]
- Goblirsch, M.; Huang, Z.Y.; Spivak, M. Physiological and behavioral changes in honey bees (Apis mellifera) induced by Nosema ceranae infection. PLoS ONE 2013, 8, e58165. [Google Scholar] [CrossRef]
- Li, Z.; He, J.; Yu, T.; Chen, Y.; Huang, W.F.; Huang, J.; Zhao, Y.; Nie, H.; Su, S. Transcriptional and physiological responses of hypopharyngeal glands in honeybees (Apis mellifera L.) infected by Nosema ceranae. Apidologie 2019, 50, 51–62. [Google Scholar] [CrossRef]
- Wolf, S.; McMahon, D.P.; Lim, K.S.; Pull, C.D.; Clark, S.J.; Paxton, R.J.; Osborne, J.L. So near and yet so Far: Harmonic radar reveals reduced homing ability of Nosema infected honeybees. PLoS ONE 2014, 9, e103989. [Google Scholar] [CrossRef] [PubMed]
- Dussaubat, C.; Maisonnasse, A.; Crauser, D.; Beslay, D.; Costagliola, G.; Soubeyrand, S.; Kretzchmar, A.; Le Conte, Y. Flight behavior and pheromone changes associated to Nosema ceranae infection of honey bee workers (Apis mellifera) in field conditions. J. Invertebr. Pathol. 2013, 113, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Glavinic, U.; Stankovic, B.; Draskovic, V.; Stevanovic, J.; Petrovic, T.; Lakic, N.; Stanimirovic, Z. Dietary amino acid and vitamin complex protects honey bee from immunosuppression caused by Nosema ceranae. PLoS ONE 2017, 12, e0187726. [Google Scholar] [CrossRef]
- Glavinic, U.; Stevanovic, J.; Ristanic, M.; Rajkovic, M.; Davitkov, D.; Lakic, N.; Stanimirovic, Z. Potential of fumagillin and Agaricus blazei mushroom extract to reduce Nosema ceranae in honeybees. Insects 2021, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Glavinic, U.; Rajkovic, M.; Vunduk, J.; Vejnovic, B.; Stevanovic, J.; Milenkovic, I.; Stanimirovic, Z. Effects of Agaricus bisporus mushroom extract on honeybees infected with Nosema ceranae. Insects 2021, 12, 915. [Google Scholar] [CrossRef] [PubMed]
- Glavinic, U.; Blagojevic, J.; Ristanic, M.; Stevanovic, J.; Lakic, N.; Mirilovic, M.; Stanimirovic, Z. Use of thymol in Nosema ceranae control and health improvement of infected honeybees. Insects 2022, 13, 574. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, N.M.; Glavinic, U.; Ristanic, M.; Vejnovic, B.; Ilic, T.; Stevanovic, J.; Stanimirovic, Z. Effects of plant-based supplement on oxidative stress of honey bees (Apis mellifera) infected with Nosema ceranae. Animals 2023, 13, 3543. [Google Scholar] [CrossRef]
- Cotter, S.C.; Simpson, S.J.; Raubenheimer, D.; Wilson, K. Macronutrient balance mediates trade-offs between immune function and life history traits. Funct. Ecol. 2011, 25, 186–198. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Mott, B.M.; Maes, P.W.; Floyd, A.S.; Fitz, W.; Copeland, D.C.; Meikle, W.G.; Anderson, K.E. Honeybee colony performance and health are enhanced by apiary proximity to US Conservation Reserve Program (CRP) lands. Sci. Rep. 2019, 9, 4894. [Google Scholar] [CrossRef] [PubMed]
- Formato, G.; Rivera-Gomis, J.; Bubnic, J.; Martín-Hernández, R.; Milito, M.; Croppi, S.; Higes, M. Nosemosis prevention and control. Appl. Sci. 2022, 12, 783. [Google Scholar] [CrossRef]
- Chaimanee, V.; Kasem, A.; Nuanjohn, T.; Boonmee, T.; Siangsuepchart, A.; Malaithong, W.; Sinpoo, C.; Disayathanoowat, T.; Pettis, J.S. Natural extracts as potential control agents for Nosema ceranae infection in honeybees, Apis mellifera. J. Invertebr. Pathol. 2021, 186, 107688. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, J.; Stanimirovic, Z.; Simeunovic, P.; Lakic, N.; Radovic, I.; Sokovic, M.; Griensven, L.J. The effect of Agaricus brasiliensis extract supplementation on honey bee colonies. An. Acad. Bras. Cienc. 2018, 90, 219–229. [Google Scholar] [CrossRef]
- Shumkova, R.; Balkanska, R.; Hristov, P. The herbal supplements NOZEMAT HERB® and NOZEMAT HERB PLUS®: An alternative therapy for N. ceranae infection and its effects on honey bee strength and production traits. Pathogens 2021, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Shumkova, R.; Balkanska, R.; Salkova, D.; Hristov, P. Impact of the plant-based natural supplement immunostart herb on honey bee colony performance. Acta Vet. 2022, 72, 348–361. [Google Scholar] [CrossRef]
- Stanimirović, Z.; Glavinić, U.; Ristanić, M.; Jelisić, S.; Vejnović, B.; Niketić, M.; Stevanović, J. Diet supplementation helps honey bee colonies in combat infections by enhancing their hygienic behaviour. Acta Vet. 2022, 72, 145–166. [Google Scholar] [CrossRef]
- Jehlík, T.; Kodrík, D.; Krištůfek, V.; Koubová, J.; Sábová, M.; Danihlík, J.; Tomčala, A.; Čapková Frydrychová, R. Effects of Chlorella sp. on biological characteristics of the honey bee Apis mellifera. Apidologie 2019, 50, 564–577. [Google Scholar] [CrossRef]
- Charistos, L.; Parashos, N.; Hatjina, F. Long term effects of a food supplement HiveAlive™ on honey bee colony strength and Nosema ceranae spore counts. J. Apic. Res. 2015, 54, 420–426. [Google Scholar] [CrossRef]
- Roussel, M.; Villay, A.; Delbac, F.; Michaud, P.; Laroche, C.; Roriz, D.; El Alaoui, H.; Diogon, M. Antimicrosporidian activity of sulphated polysaccharides from algae and their potential to control honeybee nosemosis. Carbohydr. Polym. 2015, 133, 213–220. [Google Scholar] [CrossRef]
- Bravo, J.; Carbonell, V.; Sepúlveda, B.; Delporte, C.; Valdovinos, C.E.; Martín-Hernández, R.; Higes, M. Antifungal activity of the essential oil obtained from Cryptocarya alba against infection in honey bees by Nosema ceranae. J. Invertebr. Pathol. 2017, 149, 141–147. [Google Scholar] [CrossRef]
- Arredondo, D.; Castelli, L.; Porrini, M.P.; Garrido, P.M.; Eguaras, M.J.; Zunino, P.; Antúnez, K. Lactobacillus kunkeei strains decreased the infection by honey bee pathogens Paenibacillus larvae and Nosema ceranae. Benef. Microbes. 2018, 9, 279–290. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, S.; Rousseau, A.; Lecoeur, A.; Cheaib, B.; Bouslama, S.; Mercier, P.L.; Demey, V.; Castex, M.; Giovenazzo, P.; Derom, N. Deleterious interaction between honeybees (Apis mellifera) and its microsporidian intracellular parasite Nosema ceranae was mitigated by administrating either endogenous or allochthonous gut microbiota strains. Front. Ecol. Evol. 2018, 6, 58. [Google Scholar] [CrossRef]
- Michalczyk, M.; Sokół, R. Estimation of the influence of selected products on co-infection with N. apis/N. ceranae in Apis mellifera using real-time PCR. Invertebr. Reprod. Dev. 2018, 62, 92–97. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Trytek, M.; Borsuk, G.; Buczek, K.; Rybicka- Jasińska, K.; Gryko, D. Porphyrins inactivate Nosema spp. microsporidia. Sci. Rep. 2018, 8, 5523. [Google Scholar] [CrossRef] [PubMed]
- Ptaszynska, A.A.; Zaluski, D. Extracts from Eleutherococcus senticosus (rupr. et Maxim.) maxim. roots: A new hope against honeybee death caused by nosemosis. Molecules 2020, 25, 4452. [Google Scholar] [CrossRef]
- Kim, I.H.; Kim, D.J.; Gwak, W.S.; Woo, S.D. Increased survival of the honey bee Apis mellifera infected with the microsporidian Nosema ceranae by effective gene silencing. Arch. Insect Biochem. Physiol. 2020, 105, e21734. [Google Scholar] [CrossRef]
- Valizadeh, P.; Guzman-Novoa, E.; Goodwin, P.H. Effect of immune inducers on Nosema ceranae multiplication and their Impact on honey bee (Apis mellifera L.) survivorship and behaviors. Insects 2020, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Mura, A.; Pusceddu, M.; Theodorou, P.; Angioni, A.; Floris, I.; Paxton, R.J.; Satta, A. Propolis consumption reduces Nosema ceranae infection of European honey bees (Apis mellifera). Insects 2020, 11, 124. [Google Scholar] [CrossRef]
- Cristina, R.T.; Kovacevic, Z.; Cincovic, M.; Dumitrescu, E.; Muselin, F.; Imre, K.; Militaru, D.; Mederle, N.; Radulov, I.; Hădărugă, N. Composition and efficacy of a natural phytotherapeutic blend against Nosemosis in honey bees. Sustainability 2020, 12, 5868. [Google Scholar] [CrossRef]
- Burnham, A.J.; De Jong, E.; Jones, J.A.; Lehman, H.K. North American propolis extracts from upstate New York decrease Nosema ceranae (Microsporidia) spore levels in honey bees (Apis mellifera). Front. Microbiol. 2020, 11, 1719. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P.H. Control of the microsporidian parasite Nosema ceranae in honey bees (Apis mellifera) using nutraceutical and immuno-stimulatory compounds. PLoS ONE 2020, 15, e0227484. [Google Scholar] [CrossRef] [PubMed]
- Borges, D.; Guzman-Novoa, E.; Goodwin, P.H. Effects of prebiotics and probiotics on honey bees (Apis mellifera) infected with the microsporidian parasite Nosema ceranae. Microorganisms 2021, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Nanetti, A.; Ugolini, L.; Cilia, G.; Pagnotta, E.; Malaguti, L.; Cardaio, I.; Matteo, R.; Lazzeri, L. Seed meals from Brassica nigra and Eruca sativa control artificial Nosema ceranae infections in Apis mellifera. Microorganisms 2021, 9, 949. [Google Scholar] [CrossRef] [PubMed]
- Maistrello, L.; Lodesani, M.; Costa, C.; Leonardi, F.; Marani, G.; Caldon, M.; Mutinelli, F.; Granato, A. Screening of natural compounds for the control of nosema disease in honeybees (Apis mellifera). Apidologie 2008, 39, 436–445. [Google Scholar] [CrossRef]
- Costa, C.; Lodesani, M.; Maistrello, L. Effect of thymol and resveratrol administered with candy or syrup on the development of Nosema ceranae and on the longevity of honeybees (Apis mellifera L.) in laboratory conditions. Apidologie 2010, 41, 141–150. [Google Scholar] [CrossRef]
- Cilia, G.; Fratini, F.; Tafi, E.; Turchi, B.; Mancini, S.; Sagona, S.; Nanetti, A.; Cerri, D.; Felicioli, A. Microbial profile of the ventriculum of honeybee (Apis mellifera ligustica Spinola, 1806) fed with veterinary drugs, dietary supplements and non-protein amino acids. Vet. Sci. 2020, 7, 76. [Google Scholar] [CrossRef]
- Cilia, G.; Garrido, C.; Bonetto, M.; Tesoriero, D.; Nanetti, A. Effect of Api-Bioxal® and ApiHerb® treatments against Nosema ceranae infection in Apis mellifera investigated by two qPCR methods. Vet. Sci. 2020, 7, 125. [Google Scholar] [CrossRef]
- Alberoni, D.; Baffoni, L.; Braglia, C.; Gaggìa, F.; Di Gioia, D. Honeybees exposure to natural feed additives: Hows the gut microbiota affected? Microorganisms 2021, 9, 1009. [Google Scholar] [CrossRef]
- Gajger, I.T.; Vugrek, O.; Pinter, L.; Petrinec, Z. “Nozevit patties” treatment of honey bees (Apis mellifera) for the control of Nosema ceranae disease. J. Apic. Res. 2009, 149, 1053–1056. [Google Scholar]
- Gajger, I.T.; Petrinec, Z.; Pinter, L.; Kozarić, Z. Experimental treatment of nosema disease with “Nozevit” phyto-pharmacological preparation. Am. Bee J. 2010, 149, 485–490. [Google Scholar]
- Cantwell, G.E. Standard methods for counting Nosema spores. Am. Bee J. 1970, 110, 222–223. [Google Scholar] [CrossRef]
- Stevanovic, J.; Stanimirovic, Z.; Genersch, E.; Kovacevic, S.R.; Ljubenkovic, J.; Radakovic, M.; Aleksic, N. Dominance of Nosema ceranae in honey bees in the Balkan countries in the absence of symptoms of colony collapse disorder. Apidologie 2011, 42, 49–58. [Google Scholar] [CrossRef]
- Williams, G.R.; Alaux, C.; Costa, C.; Csaki, T.; Doublet, V.; Eisenhardt, D. Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apicult. Res. 2013, 52, 1–36. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Özkırım, A.; Küçüközmen, B. Application of herbal essential oil extract mixture for honey bees (Apis mellifera L.) Against Nosema ceranae and Nosema apis. J. Apic. Sci. 2021, 65, 163–175. [Google Scholar] [CrossRef]
- Huang, W.F.; Solter, L.F.; Yau, P.M.; Imai, B.S. Nosema ceranae escapes fumagillin control in honey bees. PLoS Pathog. 2013, 9, e1003185. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, J.; Simeunovic, P.; Gajic, B.; Lakic, N.; Radovic, D.; Fries, I.; Stanimirovic, Z. Characteristics of Nosema ceranae infection in Serbian honey bee colonies. Apidologie 2013, 44, 522–536. [Google Scholar] [CrossRef]
- Gajger, I.; Kozaric, Z.; Berta, D.; Nejedli, S.; Petrinec, Z. Effect of the Herbal preparation Nozevit® on the mid-gut structure of honeybees (Apis melífera) infected with Nosema sp. spores. Vet. Med. 2011, 56, 344–351. [Google Scholar] [CrossRef]
- Pohorecka, K. Laboratory studies on the effect of standardized Artemisia absinthium L. extract on Nosema apis infection in the worker Apis mellifera. J. Apic. Sci. 2004, 48, 131–136. [Google Scholar] [CrossRef]
- Porrini, M.P.; Fernández, N.J.; Garrido, P.M.; Gende, L.B.; Medici, S.K.; Eguaras, M.J. In vivo evaluation of antiparasitic activity of plant extracts on Nosema ceranae (Microsporidia). Apidologie 2011, 42, 700–707. [Google Scholar] [CrossRef]
- Michalczyk, M.; Sokół, R.; Koziatek, S. Evaluation of the effectiveness of selected treatments of Nosema spp. infection by the hemocytometric method and duplex PCR. Acta Vet. 2016, 66, 115–124. [Google Scholar] [CrossRef][Green Version]
- Kim, J.H.; Park, J.K.; Lee, J.K. Evaluation of antimicrosporidian activity of plant extracts on Nosema ceranae. J. Apic. Sci. 2016, 60, 167–178. [Google Scholar] [CrossRef]
- Lee, J.K.; Kim, J.H.; Jo, M.; Rangachari, B.; Park, J.K. Anti-Nosemosis activity of Aster scaber and Artemisia dubia aqueous extracts. J. Apic. Sci. 2018, 62, 27–38. [Google Scholar] [CrossRef]
- Yücel, B.; Doğaroğlu, M. The impact of Nosema apis Z. infestation of honey bee (Apis mellifera L.) colonies after using different treatment methods and their effects on the population levels of workers and honey production on consecutive years. Pak. J. Biol. Sci. 2005, 8, 1142–1145. [Google Scholar] [CrossRef][Green Version]
- Holt, H.L.; Aronstein, K.A.; Grozinger, C.M. Chronic parasitization by Nosema microsporidia causes global expression changes in core nutritional, metabolic and behavioral pathways in honey bee workers (Apis mellifera). BMC Genomics 2013, 14, 799. [Google Scholar] [CrossRef] [PubMed]
- Mayack, C.; Natsopoulou, M.E.; McMahon, D.P. Nosema ceranae alters a highly conserved hormonal stress pathway in honey bees. Insect. Mol. Biol. 2015, 24, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Paris, L.; El Alaoui, H.; Delbac, F.; Diogon, M. Effects of the gut parasite Nosema ceranae on honey bee physiology and behavior. Curr. Opin. Insect Sci. 2018, 26, 149–154. [Google Scholar] [CrossRef]
- Lourenço, A.P.; Guidugli-Lazzarini, K.R.; De Freitas, N.H.; Message, D.; Bitondi, M.M.; Simões, Z.L.; Teixeira, É.W. Immunity and physiological changes in adult honey bees (Apis mellifera) infected with Nosema ceranae: The natural colony environment. J. Insect. Physiol. 2021, 131, 104237. [Google Scholar] [CrossRef]
- Antunez, K.; Martín-Hernández, R.; Prieto, L.; Meana, A.; Zunino, P.; Higes, M. Immune-suppression in the honey bee (Apis mellifera) following infection by Nosema ceranae (Microsporidia). Environ. Microbiol. 2009, 11, 2284–2290. [Google Scholar] [CrossRef]
- Chaimanee, V.; Chantawannakul, P.; Chen, Y.; Evans, J.D.; Pettis, J.S. Differential expression of immune genes of adult honey bee (Apis mellifera) after inoculated by Nosema ceranae. J. Insect Physiol. 2012, 58, 1090–1095. [Google Scholar] [CrossRef] [PubMed]
- Aufauvre, J.; Misme-Aucouturier, B.; Viguès, B.; Texier, C.; Delbac, F.; Blot, N. Transcriptome analyses of the honeybee response to Nosema ceranae and insecticides. PLoS ONE 2014, 9, e91686. [Google Scholar] [CrossRef] [PubMed]
- Badaoui, B.; Fougeroux, A.; Petit, F.; Anselmo, A.; Gorni, C.; Cucurachi, M.; Cersini, A.; Granato, A.; Cardeti, G.; Formato, G.; et al. RNA-sequence analysis of gene expression from honeybees (Apis mellifera) infected with Nosema ceranae. PLoS ONE 2017, 12, e0173438. [Google Scholar] [CrossRef] [PubMed]
- Martín-Hernández, R.; Bartolomé, C.; Chejanovsky, N.; Le Conte, Y.; Dalmon, A.; Dussaubat, C.; García-Palencia, P.; Meana, A.; Pinto, M.A.; Soroker, V.; et al. Nosema ceranae in Apis mellifera: A 12 years postdetection perspective. Environ. Microbiol. 2018, 20, 1302–1329. [Google Scholar] [CrossRef] [PubMed]
| Groups | Day after Emergence, When the Test Supplement Was Introduced into the Food | Day after Emergence, When the Infection with Nosema ceranae was Conducted | Sampling Day | ||
|---|---|---|---|---|---|
| NI | - | - | 6 | 9 | 15 |
| I | - | 3 | 6 | 9 | 15 |
| S | 1 | - | 6 | 9 | 15 |
| I-S1 | 1 | 3 | 6 | 9 | 15 |
| I-S3 | 3 | 3 | 6 | 9 | 15 |
| I-S6 | 6 | 3 | - | 9 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glavinic, U.; Jovanovic, N.M.; Dominikovic, N.; Lakic, N.; Ćosić, M.; Stevanovic, J.; Stanimirovic, Z. Potential of Wormwood and Oak Bark-Based Supplement in Health Improvement of Nosema ceranae-Infected Honey Bees. Animals 2024, 14, 1195. https://doi.org/10.3390/ani14081195
Glavinic U, Jovanovic NM, Dominikovic N, Lakic N, Ćosić M, Stevanovic J, Stanimirovic Z. Potential of Wormwood and Oak Bark-Based Supplement in Health Improvement of Nosema ceranae-Infected Honey Bees. Animals. 2024; 14(8):1195. https://doi.org/10.3390/ani14081195
Chicago/Turabian StyleGlavinic, Uros, Nemanja M. Jovanovic, Nina Dominikovic, Nada Lakic, Milivoje Ćosić, Jevrosima Stevanovic, and Zoran Stanimirovic. 2024. "Potential of Wormwood and Oak Bark-Based Supplement in Health Improvement of Nosema ceranae-Infected Honey Bees" Animals 14, no. 8: 1195. https://doi.org/10.3390/ani14081195
APA StyleGlavinic, U., Jovanovic, N. M., Dominikovic, N., Lakic, N., Ćosić, M., Stevanovic, J., & Stanimirovic, Z. (2024). Potential of Wormwood and Oak Bark-Based Supplement in Health Improvement of Nosema ceranae-Infected Honey Bees. Animals, 14(8), 1195. https://doi.org/10.3390/ani14081195








