β-Sitosterol Reduces the Content of Triglyceride and Cholesterol in a High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease Zebrafish (Danio rerio) Model
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Zebrafish Stocks and Rearing Conditions
2.3. Preparation of Reagents
2.4. Preparation of HFD and Establishment of a Zebrafish Model of NAFLD
2.5. Oil Red O Staining of Zebrafish Larvae
2.6. Filipin Staining of Zebrafish Larvae
2.7. Zebrafish Eyeball Lens Extraction and Vascular Diameter Statistics
2.8. Quantification of Cholesterol, Glucose, and Triglyceride Levels in Hepatic Tissue of Adult Zebrafish
2.9. Enzyme-Linked Immunosorbent Assay (ELISA)
2.10. Lipidomics Sample Preparation and Analysis
2.11. Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
2.12. Statistical Analysis
3. Results
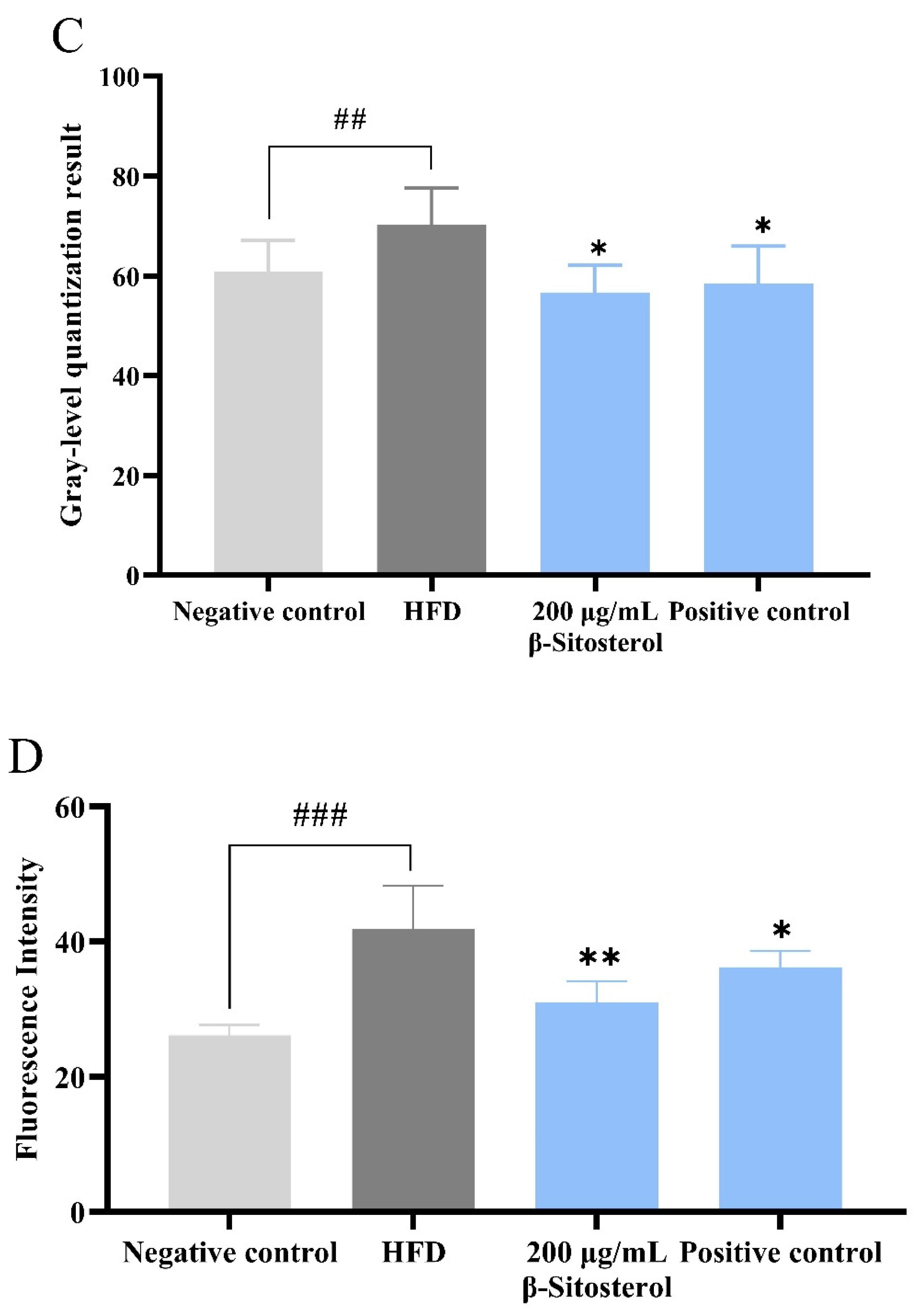
3.1. β-Sitosterol Reduces Triglyceride and Cholesterol Levels in Zebrafish
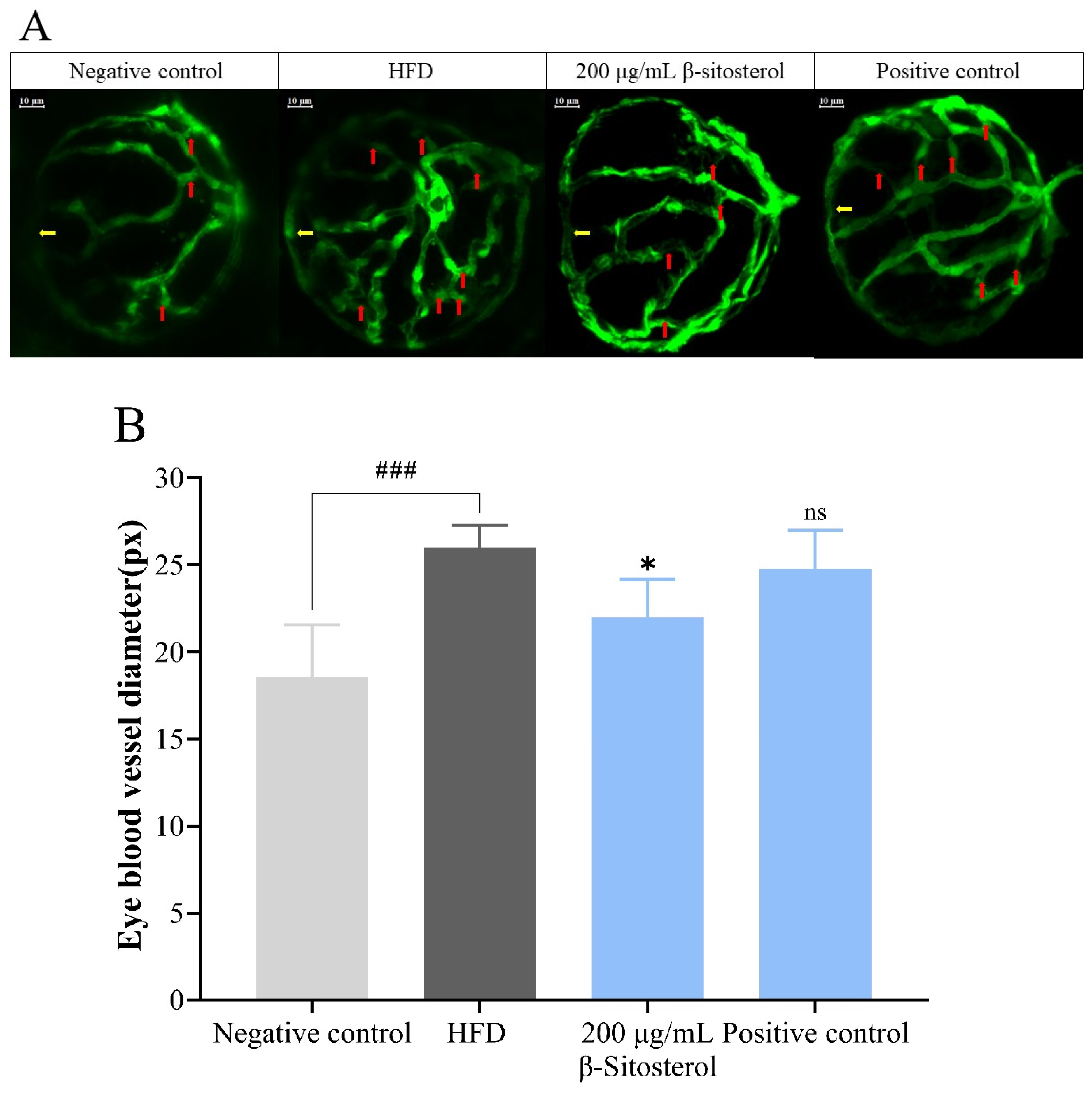
3.2. Effects of β-Sitosterol on the Microvessels of the Zebrafish Vitreous Vascular System
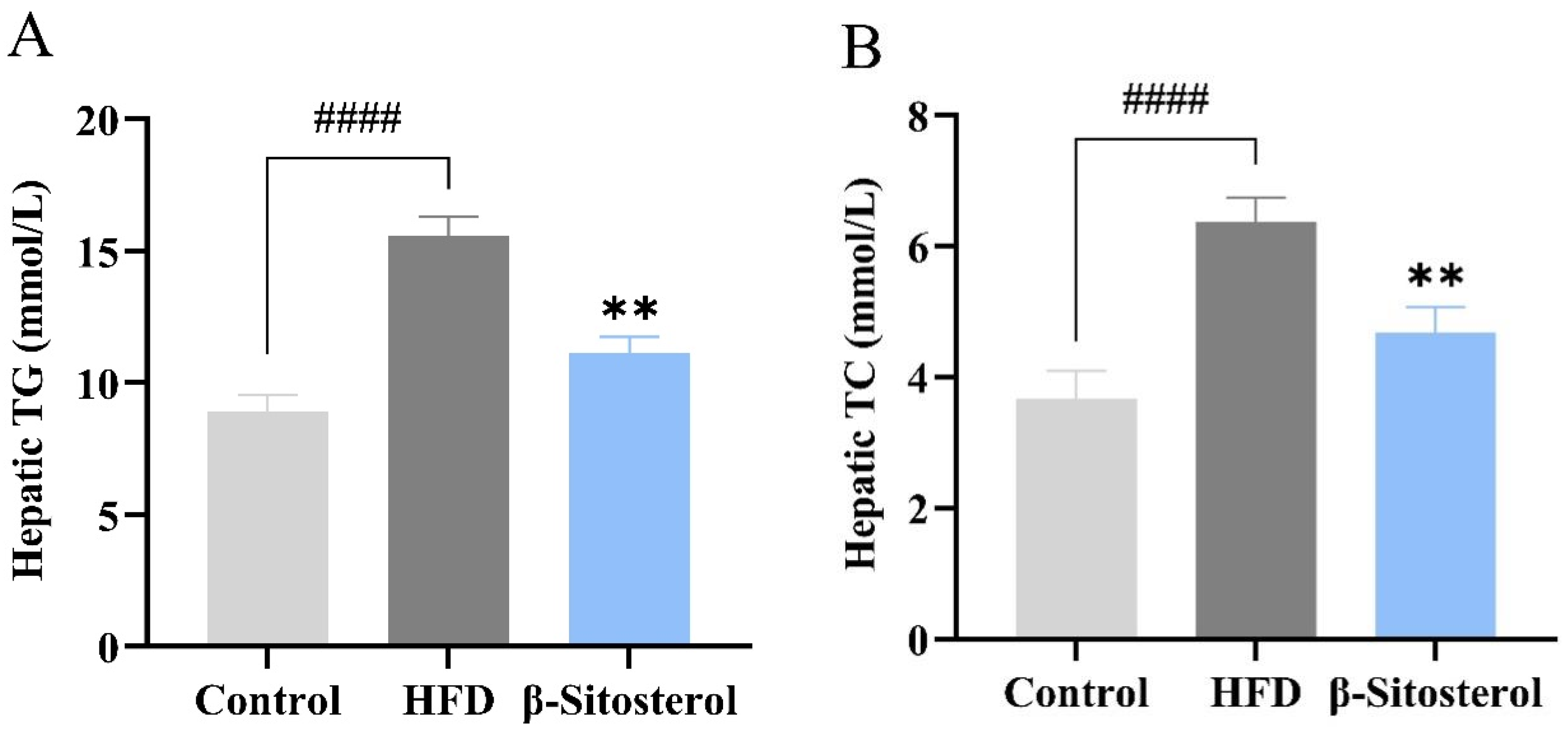
3.3. Effects of β-Sitosterol on Triglyceride and Cholesterol Levels in Adult Zebrafish Liver
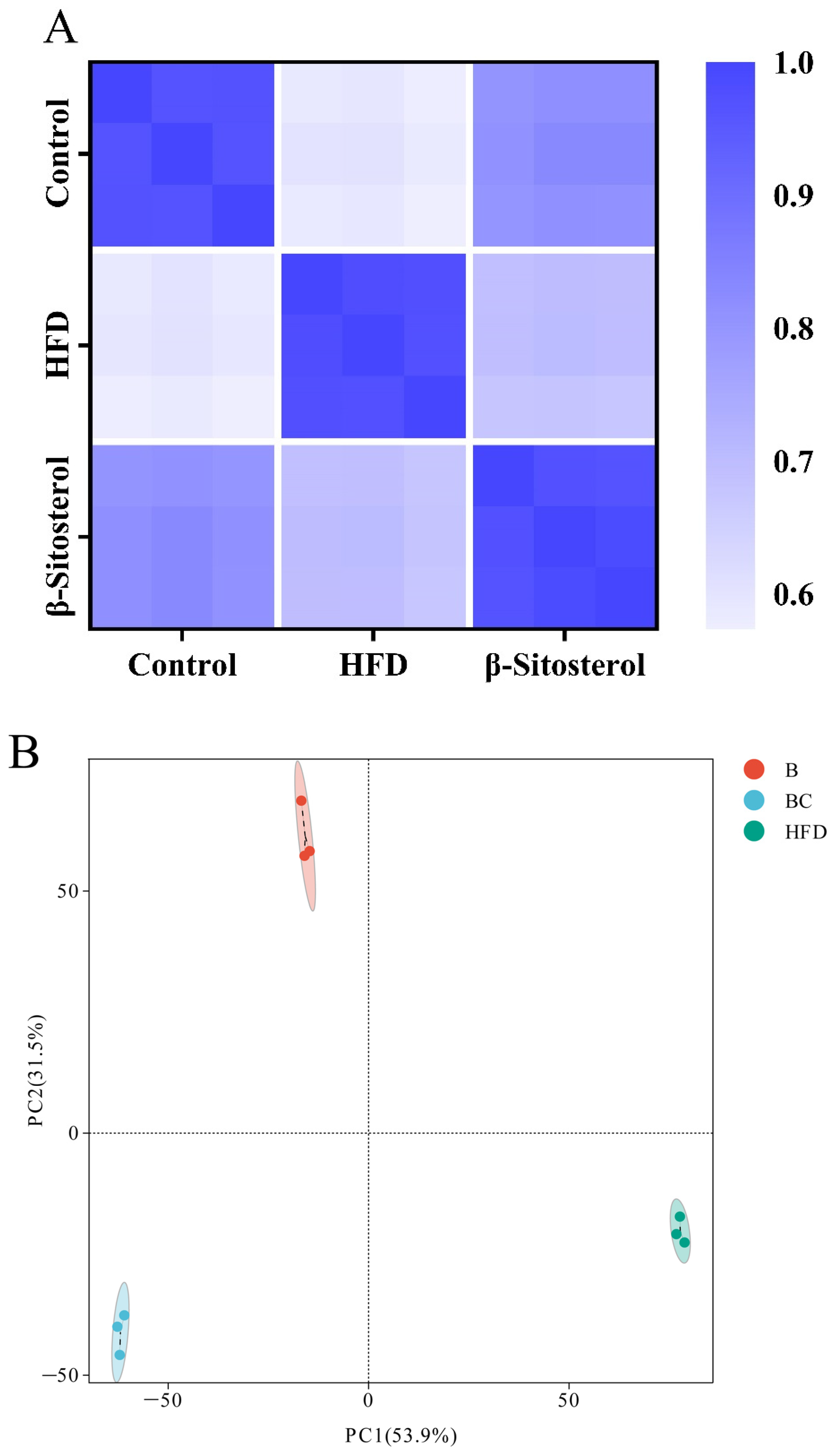
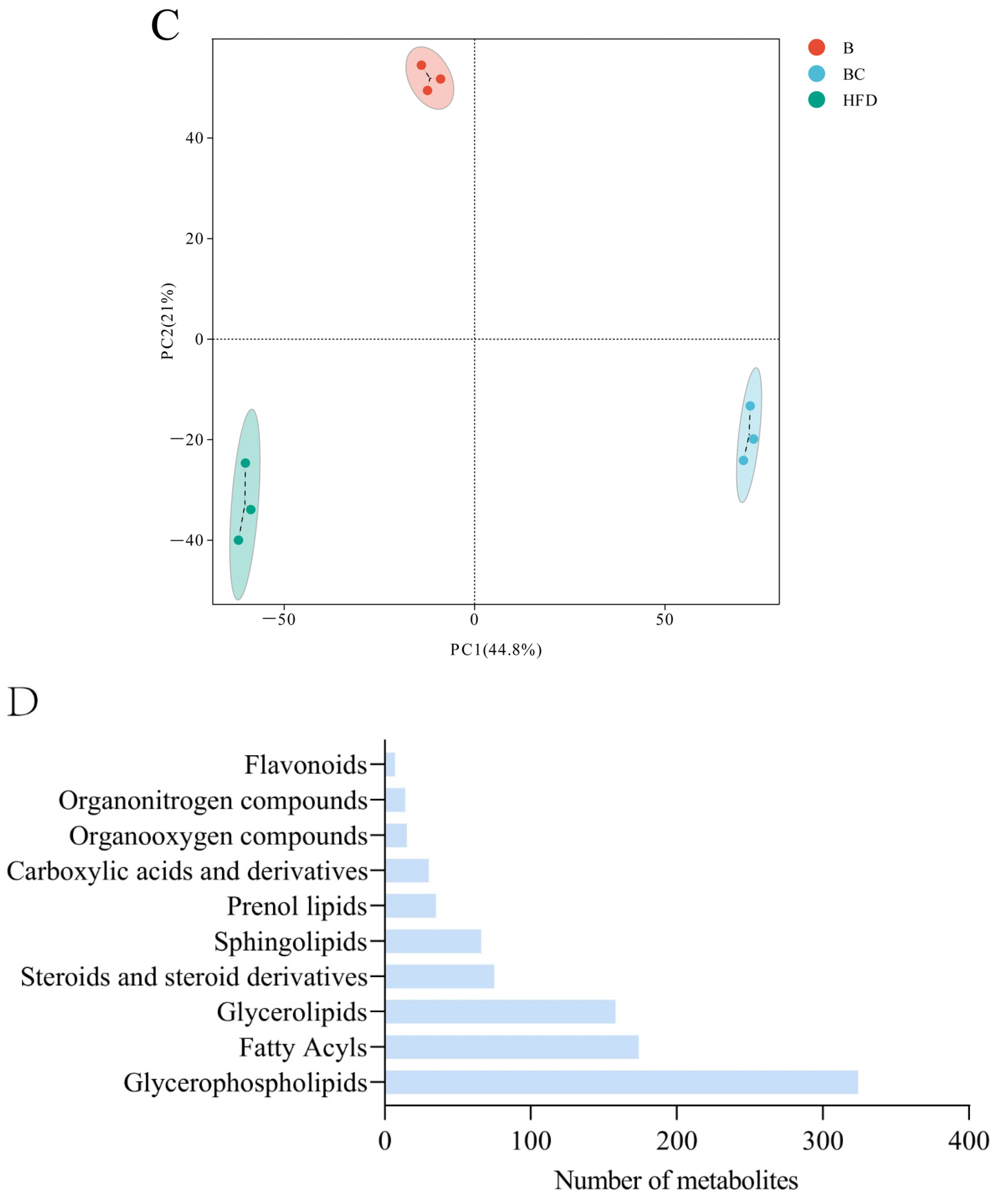
3.4. Lipid Metabolome Analysis of Adult Zebrafish Liver
3.5. Analysis of Differentially Expressed Lipid Metabolites (DELMs) in Zebrafish Liver of HFD and β-Sitosterol Groups
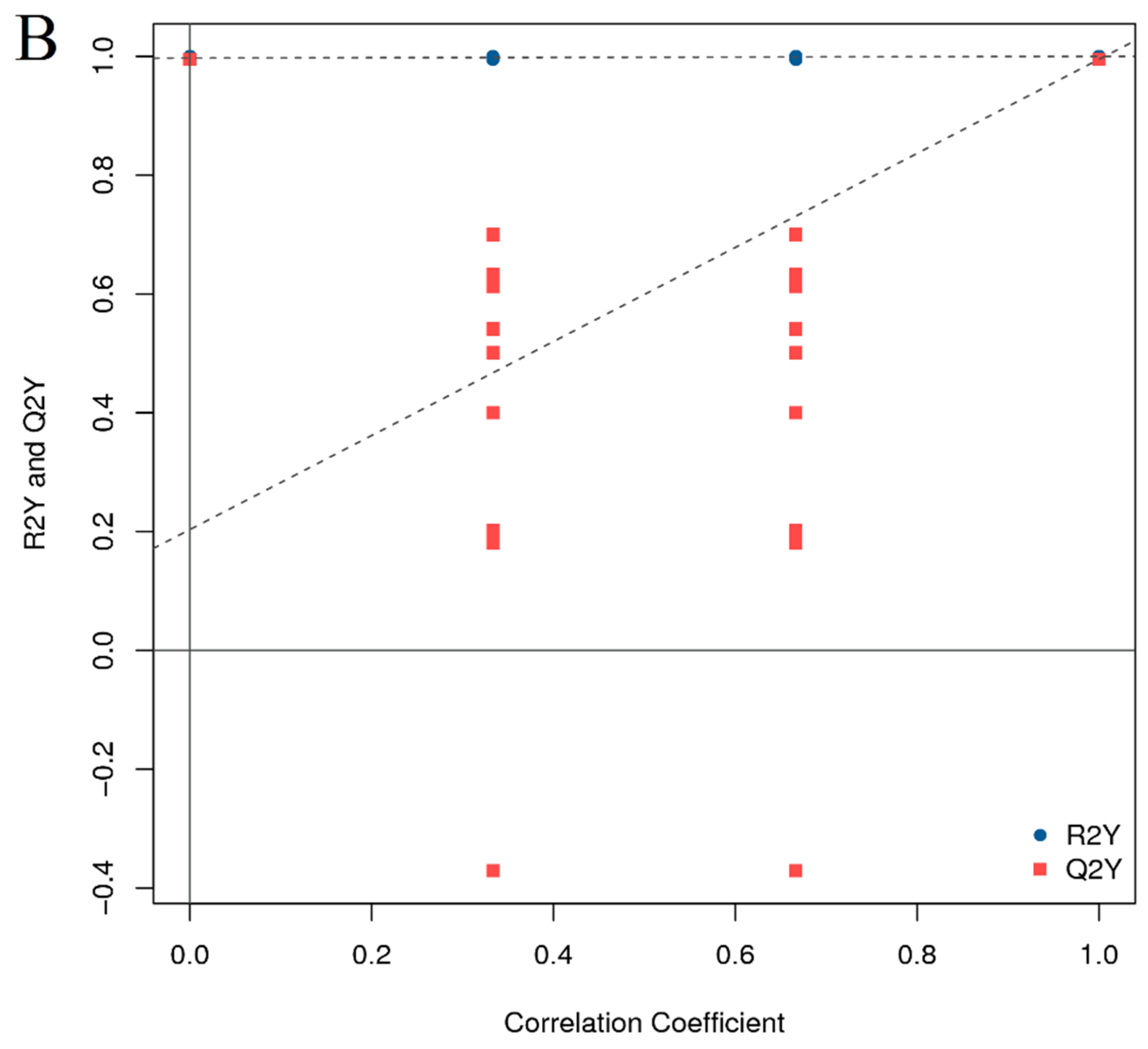
3.6. Analysis of DELMs in Zebrafish Liver of HFD and β-Sitosterol Groups
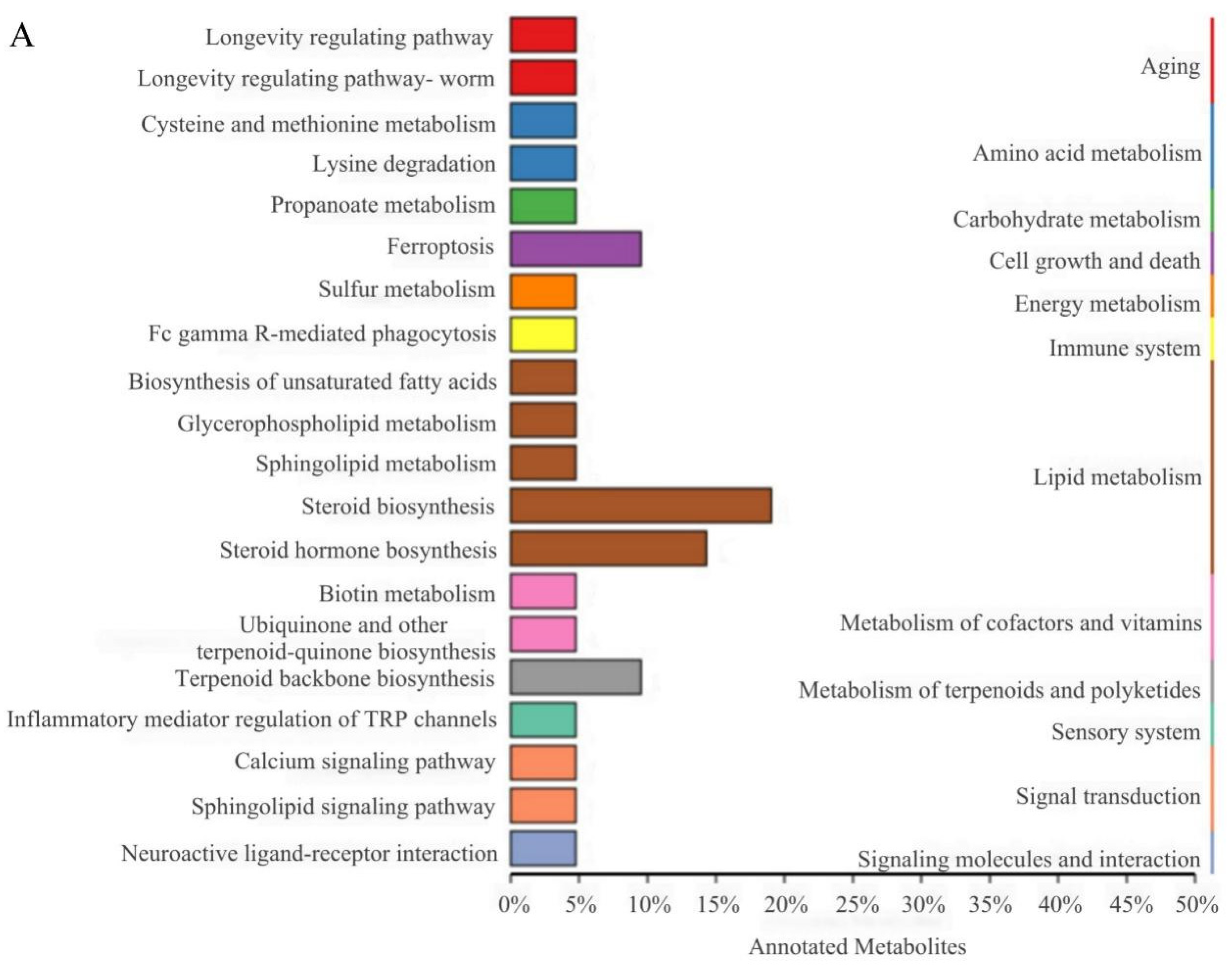
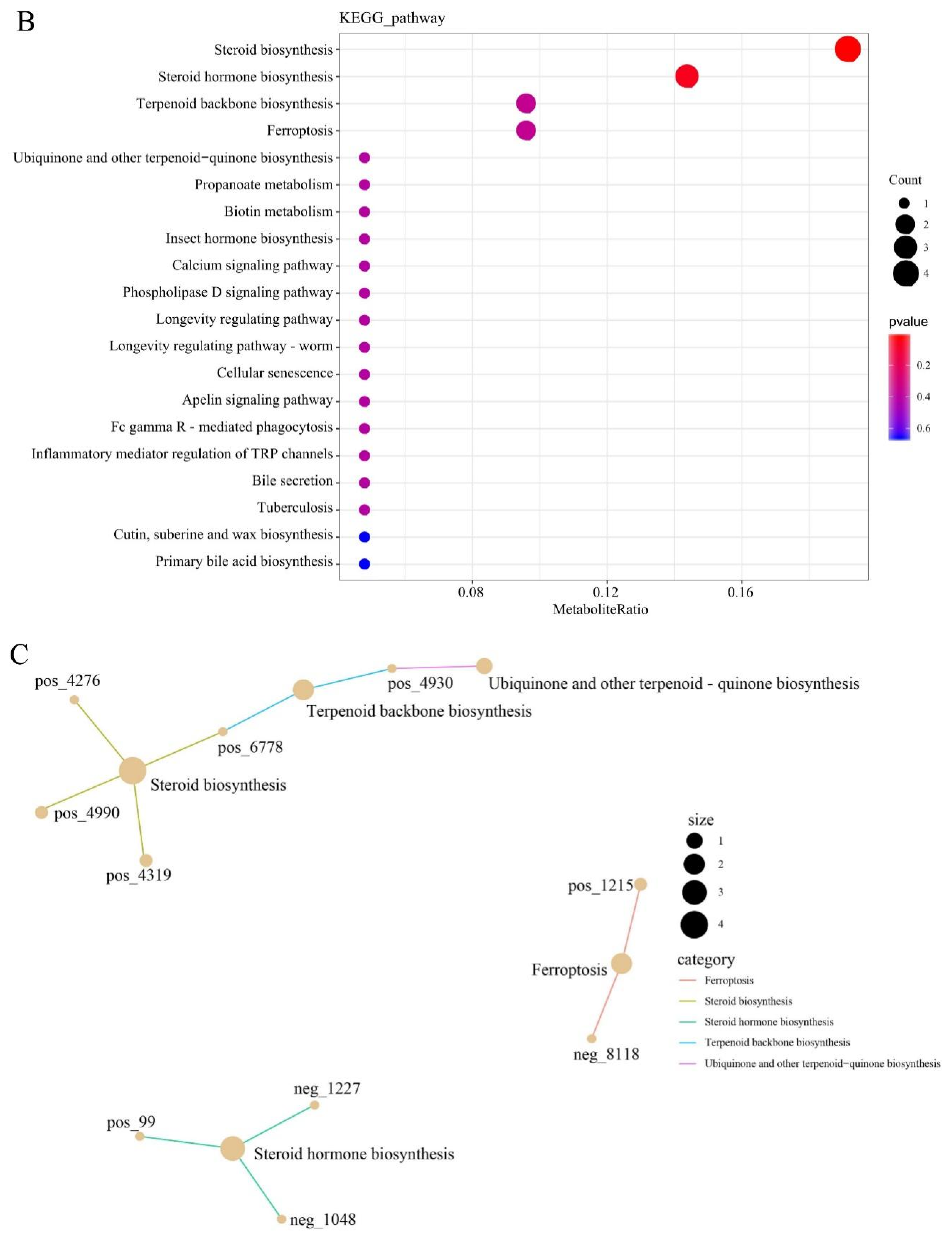
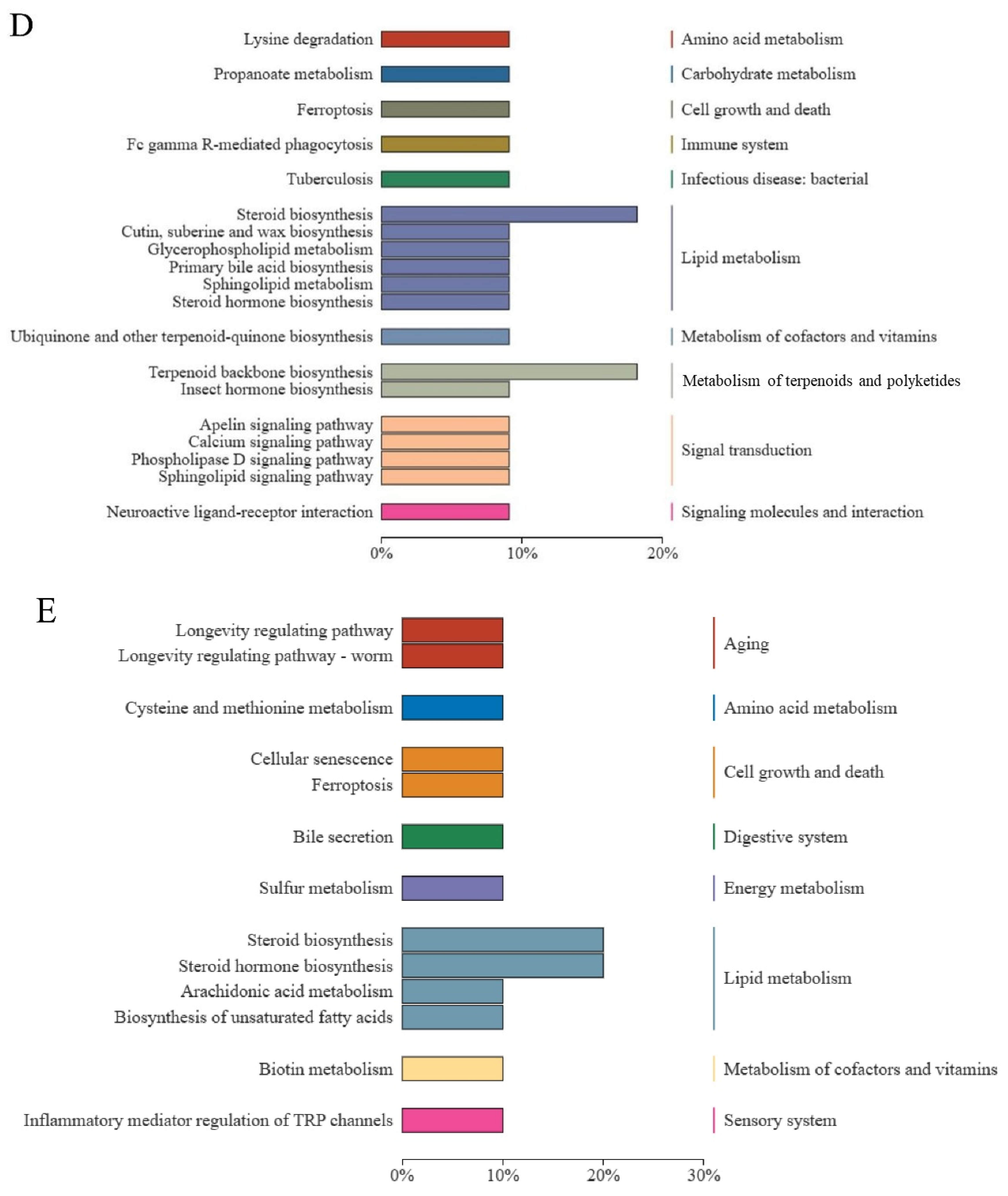
3.7. KEGG Functional Annotation and Enrichment Analysis of DELMs
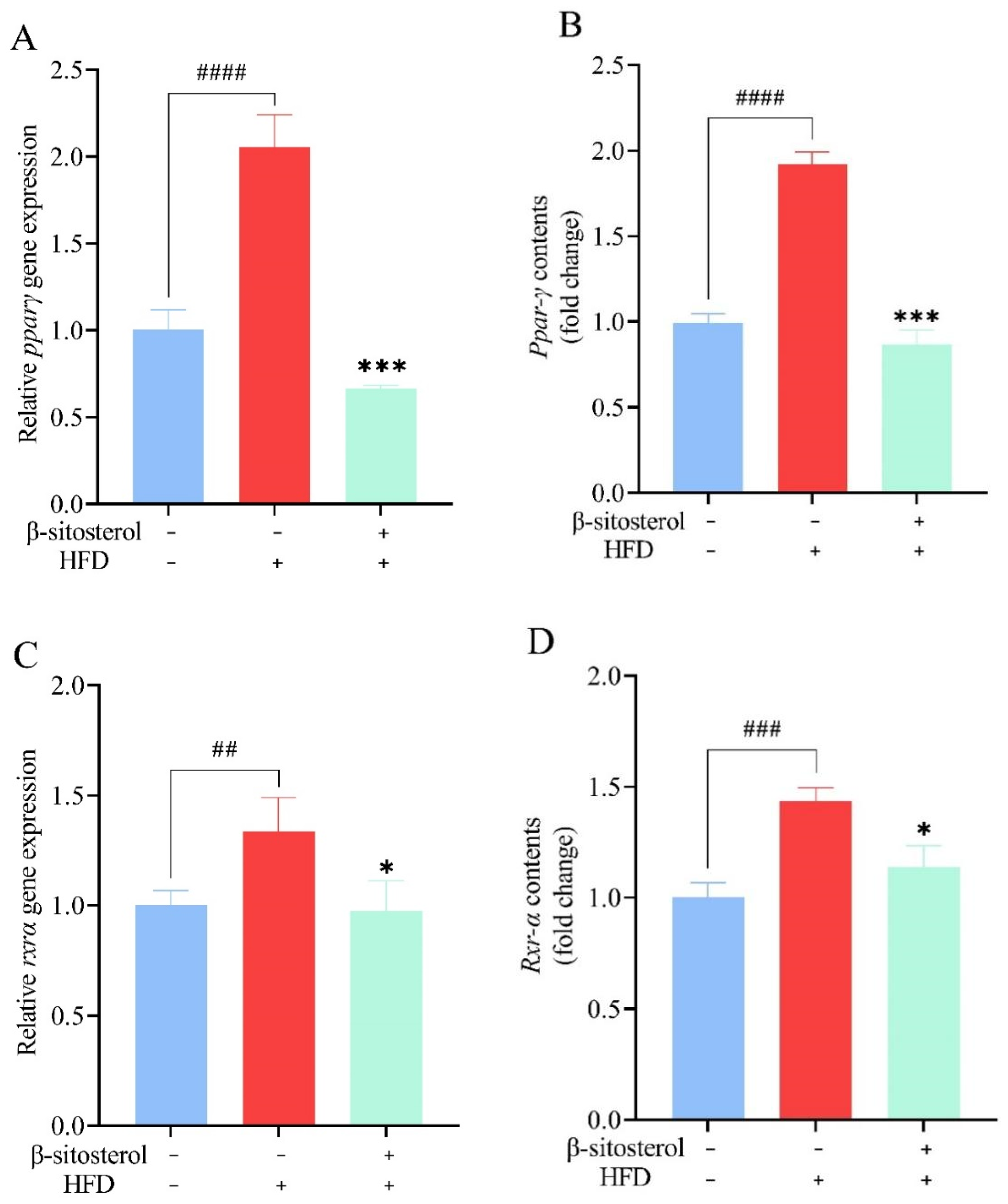
3.8. Effects of β-Sitosterol Treatment on Ppar-γ and Rxr-α in Adult Zebrafish Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slatter, D.A.; Aldrovandi, M.; O’Connor, A.; Allen, S.M.; Brasher, C.J.; Murphy, R.C.; Mecklemann, S.; Ravi, S.; Darley-Usmar, V.; O’Donnell, V.B. Mapping the human platelet lipidome reveals cytosolic phospholipase A2 as a regulator of mitochondrial bioenergetics during activation. Cell Metab. 2016, 23, 930–944. [Google Scholar] [CrossRef]
- Wu, D.; Li, J.; Fan, Z.; Wang, L.; Zheng, X. Resveratrol ameliorates oxidative stress, inflammatory response and lipid metabolism in common carp (Cyprinus carpio) fed with high-fat diet. Front. Immunol. 2022, 13, 965954. [Google Scholar] [CrossRef]
- Huang, W.; Yan, X.; Liu, H.; Tan, B.; Suo, X.; Pan, S.; Li, T.; Yang, Y.; Dong, X. Effects of vitamin E supplementation of a high-lipid diet on the growth and biochemical parameters of hybrid groupers (♀ Epinephelus fuscoguttatus × ♂ E. lanceolatus). Front. Mar. Sci. 2022, 9, 924018. [Google Scholar] [CrossRef]
- Lee, S.-M.; Jeon, I.G.; Lee, J.Y. Effects of digestible protein and lipid levels in practical diets on growth, protein utilization and body composition of juvenile rockfish (Sebastes schlegeli). Aquaculture 2002, 211, 227–239. [Google Scholar] [CrossRef]
- Lu, K.L.; Xu, W.N.; Wang, L.N.; Zhang, D.D.; Zhang, C.N.; Liu, W.B. Hepatic beta-oxidation and regulation of carnitine palmitoyltransferase (CPT) I in blunt snout bream Megalobrama amblycephala fed a high fat diet. PLoS ONE 2014, 9, e93135. [Google Scholar] [CrossRef]
- Du, Z.Y.; Clouet, P.; Huang, L.M.; Degrace, P.; Zheng, W.H.; He, J.G.; Tian, L.X.; Liu, Y.J. Utilization of different dietary lipid sources at high level in herbivorous grass carp (Ctenopharyngodon idella): Mechanism related to hepatic fatty acid oxidation. Aquac. Nutr. 2008, 14, 77–92. [Google Scholar] [CrossRef]
- Lu, K.L.; Xu, W.N.; Liu, W.B.; Wang, L.N.; Zhang, C.N.; Li, X.F. Association of Mitochondrial Dysfunction with Oxidative Stress and Immune Suppression in Blunt Snout Bream Megalobrama amblycephala Fed a High-Fat Diet. J. Aquat. Anim. Health 2014, 26, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-l.; Zhou, Y.-l.; Zhao, H.; Chen, W.-Y.; Chen, Y.-J.; Lin, S.-M. Effect of dietary lipid level on growth, lipid metabolism and oxidative status of largemouth bass, Micropterus salmoides. Aquaculture 2019, 506, 394–400. [Google Scholar] [CrossRef]
- Zhou, Y.L.; Guo, J.L.; Tang, R.J.; Ma, H.J.; Chen, Y.J.; Lin, S.M. High dietary lipid level alters the growth, hepatic metabolism enzyme, and anti-oxidative capacity in juvenile largemouth bass Micropterus salmoides. Fish. Physiol. Biochem. 2020, 46, 125–134. [Google Scholar] [CrossRef]
- Jobling, M.; Larsen, A.V.; Andreassen, B.; Olsen, R.L. Adiposity and growth of post-smolt Atlantic salmon Salmo salar L. Aquac. Res. 2002, 33, 533–541. [Google Scholar] [CrossRef]
- Johansen, S.J.S.; Ekli, M.; Jobling, M. Is there lipostatic regulation of feed intake in Atlantic salmon Salmo salar L. Aquac. Res. 2002, 33, 515–524. [Google Scholar] [CrossRef]
- Beamish, F.; Medland, T. Protein sparing effects in large rainbow trout, Salmo gairdneri. Aquaculture 1986, 55, 35–42. [Google Scholar] [CrossRef]
- Dias, J.; Alvarez, M.; Diez, A.; Arzel, J.; Corraze, G.; Bautista, J.; Kaushik, S. Regulation of hepatic lipogenesis by dietary protein energy in juvenile European seabass (Dicentrarchus labrax). Aquaculture 1998, 161, 169–186. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, N.; Xue, M.; Zhang, M.; Liu, W.; Xu, C.; Fan, Y.; Meng, Y.; Zhang, Q.; Zhou, Y. Anti-Inflammatory and antioxidant properties of beta-sitosterol in copper sulfate-induced inflammation in zebrafish (Danio rerio). Antioxidants 2023, 12, 391. [Google Scholar] [CrossRef]
- Ramalingam, S.; Packirisamy, M.; Karuppiah, M.; Vasu, G.; Gopalakrishnan, R.; Gothandam, K.; Thiruppathi, M. Effect of β-sitosterol on glucose homeostasis by sensitization of insulin resistance via enhanced protein expression of PPRγ and glucose transporter 4 in high fat diet and streptozotocin-induced diabetic rats. Cytotechnology 2020, 72, 357–366. [Google Scholar] [CrossRef]
- Babu, A.F.; Koistinen, V.M.; Turunen, S.; Solano-Aguilar, G.; Urban, J.F., Jr.; Zarei, I.; Hanhineva, K. Identification and distribution of sterols, bile acids, and acylcarnitines by LC-MS/MS in humans, mice, and pigs-A qualitative analysis. Metabolites 2022, 12, 49. [Google Scholar] [CrossRef]
- Feng, S.; Gan, L.; Yang, C.S.; Liu, A.B.; Lu, W.; Shao, P.; Dai, Z.; Sun, P.; Luo, Z. Effects of stigmasterol and β-Sitosterol on nonalcoholic fatty liver disease in a mouse model: A lipidomic analysis. J. Agric. Food Chem. 2018, 66, 3417–3425. [Google Scholar] [CrossRef]
- Feng, S.; Dai, Z.; Liu, A.B.; Huang, J.; Narsipur, N.; Guo, G.; Kong, B.; Reuhl, K.; Lu, W.; Luo, Z.; et al. Intake of stigmasterol and β-sitosterol alters lipid metabolism and alleviates NAFLD in mice fed a high-fat western-style diet. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1274–1284. [Google Scholar] [CrossRef]
- Jocken, J.W.; Goossens, G.H.; Boon, H.; Mason, R.R.; Essers, Y.; Havekes, B.; Watt, M.J.; van Loon, L.J.; Blaak, E.E. Insulin-mediated suppression of lipolysis in adipose tissue and skeletal muscle of obese type 2 diabetic men and men with normal glucose tolerance. Diabetologia 2013, 56, 2255–2265. [Google Scholar] [CrossRef]
- Grinberg, L.; Dabbah Assadi, F.; Baum, G.; Zemel, R.; Tur-Kaspa, R.; Shochat, C.; Karasik, D.; Karpuj, M.V. Beneficial effect of Vitamin D on Non-Alcoholic Fatty Liver Disease (NAFLD) progression in the zebrafish model. Nutrients 2023, 15, 1362. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Kim, M.J.; Lim, H.; Kim, K.H.; Kim, S.W.; Lee, E.S.; Kim, H.H.; Kim, S.J.; Hur, K.Y.; et al. An autophagy enhancer ameliorates diabetes of human IAPP-transgenic mice through clearance of amyloidogenic oligomer. Nat. Commun. 2021, 12, 183. [Google Scholar] [CrossRef]
- Yang, W.Y.; Rao, P.S.; Luo, Y.C.; Lin, H.K.; Huang, S.H.; Yang, J.M.; Yuh, C.H. Omics-based investigation of diet-induced obesity synergized with HBx, Src, and p53 mutation accelerating hepatocarcinogenesis in zebrafish model. Cancers 2019, 11, 1899. [Google Scholar] [CrossRef]
- Harvie, E.A.; Huttenlocher, A. Non-invasive Imaging of the Innate Immune Response in a Zebrafish Larval Model of Streptococcus iniae Infection. J. Vis. Exp. 2015, 98, 52788. [Google Scholar] [CrossRef]
- Zang, L.; Shimada, Y.; Nishimura, N. Development of a novel zebrafish model for type 2 diabetes mellitus. Sci. Rep. 2017, 7, 1461. [Google Scholar] [CrossRef]
- Oka, T.; Nishimura, Y.; Zang, L.; Hirano, M.; Shimada, Y.; Wang, Z.; Umemoto, N.; Kuroyanagi, J.; Nishimura, N.; Tanaka, T. Diet-induced obesity in zebrafish shares common pathophysiological pathways with mammalian obesity. BMC Physiol. 2010, 10, 21. [Google Scholar] [CrossRef]
- Yu, L.; Gong, L.; Wang, C.; Hu, N.; Tang, Y.; Zheng, L.; Dai, X.; Li, Y. Radix polygoni multiflori and Its main component emodin attenuate non-alcoholic fatty liver disease in zebrafish by regulation of AMPK signaling pathway. Drug Des. Dev. Ther. 2020, 14, 1493–1506. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, Y.; Liu, J.; Zuo, J.; Yan, L.; Thring, R.W.; Ba, X.; Qi, D.; Wu, M.; Gao, Y.; et al. Tauroursodeoxycholic acid functions as a critical effector mediating insulin sensitization of metformin in obese mice. Redox Biol. 2022, 57, 102481. [Google Scholar] [CrossRef]
- Wiggenhauser, L.M.; Qi, H.; Stoll, S.J.; Metzger, L.; Bennewitz, K.; Poschet, G.; Krenning, G.; Hillebrands, J.L.; Hammes, H.P.; Kroll, J. Activation of retinal angiogenesis in hyperglycemic pdx1−/− zebrafish mutants. Diabetes 2020, 69, 1020–1031. [Google Scholar] [CrossRef]
- Chen, B.; Zheng, Y.M.; Zhang, J.P. Comparative study of different diets-induced NAFLD models of zebrafish. Front. Endocrinol. 2018, 9, 366. [Google Scholar] [CrossRef]
- Chen, Z.; Zang, L.; Wu, Y.; Nakayama, H.; Shimada, Y.; Shrestha, R.; Zhao, Y.; Miura, Y.; Chiba, H.; Hui, S.-P.; et al. Lipidomic profiling on oxidized phospholipids in type 2 diabetes mellitus model zebrafish. Anal. Sci. 2018, 34, 1201–1208. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Cheng, X.L.; Lin, R.C. Lipidomics applications for discovering biomarkers of diseases in clinical chemistry. Int. Rev. Cell Mol. Biol. 2014, 313, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Huang, Y.; Li, J.; Yang, K.; Yang, W.; Shen, G.; Li, X.; Lei, Y.; Pang, S.; Wang, C.; et al. New insights into the mechanism of phthalate-induced developmental effects. Environ. Pollut. 2018, 241, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.J.; Lin, C.H.; Li, M.; Nishtala, K.; Alaei, S.; Rossello, F.; Sonntag, C.; Hersey, L.; Miles, L.B.; Krisp, C.; et al. FKRP-dependent glycosylation of fibronectin regulates muscle pathology in muscular dystrophy. Nat. Commun. 2021, 12, 2951. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.J.; Kim, K.J.; Choi, J.; Jeon, H.J.; Seo, M.J.; Lee, B.Y. Ginsenoside Rg1 suppresses early stage of adipocyte development via activation of C/EBP homologous protein-10 in 3T3-L1 and attenuates fat accumulation in high fat diet-induced obese zebrafish. J. Ginseng Res. 2017, 41, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Zhou, C.; Zeng, T.; Li, Y.; Lai, Y.; Mo, C.; Chen, Y.; Huang, S.; Lv, Z.; Gao, L. P-Hydroxyacetophenone ameliorates alcohol-Induced steatosis and oxidative stress via the NF-kappaB signaling pathway in zebrafish and hepatocytes. Front. Pharmacol. 2019, 10, 1594. [Google Scholar] [CrossRef]
- Lockwood, N.; Parker, J.; Wilson, C.; Frankel, P. Optimal anesthetic regime for motionless three-dimensional image acquisition during longitudinal studies of adult nonpigmented zebrafish. Zebrafish 2017, 14, 133–139. [Google Scholar] [CrossRef] [PubMed]
- van den Boomen, D.J.H.; Sienkiewicz, A.; Berlin, I.; Jongsma, M.L.M.; van Elsland, D.M.; Luzio, J.P.; Neefjes, J.J.C.; Lehner, P.J. A trimeric Rab7 GEF controls NPC1-dependent lysosomal cholesterol export. Nat. Commun. 2020, 11, 5559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, X.; Xue, M.; Jiang, N.; Li, Y.; Fan, Y.; Zhang, P.; Liu, N.; Xiao, Z.; Zhang, Q.; et al. Oral vaccination of largemouth bass (Micropterus salmoides) against Largemouth Bass Ranavirus (LMBV) using yeast surface display technology. Animals 2023, 13, 1183. [Google Scholar] [CrossRef]
- Xue, S.; Ly, T.T.N.; Vijayakar, R.S.; Chen, J.; Ng, J.; Mathuru, A.S.; Magdinier, F.; Reversade, B. HOX epimutations driven by maternal SMCHD1/LRIF1 haploinsufficiency trigger homeotic transformations in genetically wildtype offspring. Nat. Commun. 2022, 13, 3583. [Google Scholar] [CrossRef]
- Li, F.; Hao, X.; Chen, Y.; Bai, L.; Gao, X.; Lian, Z.; Wei, H.; Sun, R.; Tian, Z. The microbiota maintain homeostasis of liver-resident γδT-17 cells in a lipid antigen/CD1d-dependent manner. Nat. Commun. 2017, 7, 13839. [Google Scholar] [CrossRef]
- Yan, Z.; Luo, H.; Xie, B.; Tian, T.; Li, S.; Chen, Z.; Liu, J.; Zhao, X.; Zhang, L.; Deng, Y.; et al. Targeting adaptor protein SLP76 of RAGE as a therapeutic approach for lethal sepsis. Nat. Commun. 2021, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Ye, M.; Wen, C.; Cheng, X.; Zou, L.; Li, M.; Liu, X.; Liu, Z.; Wen, L.; Wang, J. Compound dark tea ameliorates obesity and hepatic steatosis and modulates the gut microbiota in mice. Front. Nutr. 2023, 10, 1082250. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, Y.; Yi, P.; Cao, H.; Wang, Q.; Zhao, X. Dietary interventions of salmon and silver carp phospholipids on mice with metabolic syndrome based on lipidomics. Cells 2022, 11, 3199. [Google Scholar] [CrossRef] [PubMed]
- Limbu, S.M.; Ma, Q.; Zhang, M.L.; Du, Z.Y. High fat diet worsens the adverse effects of antibiotic on intestinal health in juvenile Nile tilapia (Oreochromis niloticus). Sci. Total Environ. 2019, 680, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Liao, K.; Wang, T.; Mai, K.; Xu, W.; Ai, Q. Dietary lipid levels influence lipid deposition in the liver of large yellow croaker (Larimichthys crocea) by regulating lipoprotein receptors, fatty acid uptake and triacylglycerol synthesis and catabolism at the transcriptional level. PLoS ONE 2015, 10, e0129937. [Google Scholar] [CrossRef]
- Du, Z.-Y.; Liu, Y.-J.; Tian, L.-X.; Wang, J.-T.; Wang, Y.; Liang, G.-Y. Effect of dietary lipid level on growth, feed utilization and body composition by juvenile grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2005, 11, 139–146. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Pergolizzi, S.; Capillo, G.; Kuciel, M.; Alesci, A.; Faggio, C. Immunohistochemical characterization of Toll-like receptor 2 in gut epithelial cells and macrophages of goldfish Carassius auratus fed with a high-cholesterol diet. Fish. Shellfish. Immunol. 2016, 59, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lai, W.; Chen, Q.; Fang, W.; Cui, K.; He, Y.; Li, X.; Mai, K.; Ai, Q. Calcium pyruvate attenuates fat deposition by augmenting fatty acid oxidation and inhibiting glucose oxidation in juvenile large yellow croaker (Larimichthys crocea) consuming a high-fat diet. Aquaculture 2023, 562, 738778. [Google Scholar] [CrossRef]
- Gao, S.; Chen, W.; Zhang, Y.; Zhao, X.; Chang, K.; Huang, Y. Guar gum improves growth performance, intestinal microbiota homeostasis, and hepatic lipid metabolism in juvenile largemouth bass (Micropterus salmoides) fed high-fat diets. Int. J. Biol. Macromol. 2023, 235, 123807. [Google Scholar] [CrossRef]
- Kong, Y.; Li, Y.; Dai, Z.R.; Qin, M.; Fan, H.L.; Hao, J.G.; Zhang, C.X.; Zhong, Q.P.; Qi, C.; Wang, P. Glycosaminoglycan from Ostrea rivularis attenuates hyperlipidemia and regulates gut microbiota in high-cholesterol diet-fed zebrafish. Food Sci. Nutr. 2021, 9, 5198–5210. [Google Scholar] [CrossRef]
- Lei, L.; Zhu, H.; Zhang, C.; Wang, X.; Ma, K.Y.; Wang, L.; Zhao, Y.; Chen, Z.Y. Dietary β-sitosterol is more potent in reducing plasma cholesterol than sesamin in hypercholesterolemia hamsters. Eur. J. Lipid Sci. Technol. 2016, 119, 1600349. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, X.; Long, X.; Jin, J.; Jin, R. Effect of beta-sitosterol self-microemulsion and beta-sitosterol ester with linoleic acid on lipid-lowering in hyperlipidemic mice. Lipids Health Dis. 2019, 18, 157. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.L.; Drolet, M.; MacLatchy, D.L. Investigation of de novo cholesterol synthetic capacity in the gonads of goldfish (Carassius auratus) exposed to the phytosterol beta-sitosterol. Reprod. Biol. Endocrinol. 2006, 4, 60. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.B.; Zhang, L.Y.; Chen, C.; Zhang, T.T.; Xue, C.H.; Yanagita, T.; Li, Z.J.; Wang, Y.M. Sea cucumber sterol alleviates the lipid accumulation in high-Fat-fructose diet fed mice. J. Agric. Food Chem. 2020, 68, 9707–9717. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.; Wei, Y.; Wang, M.; Tao, J.; Ouyang, H.; Du, Z.; Li, S.; Jiang, H. Network pharmacology combined with metabolomics and lipidomics to reveal the hypolipidemic mechanism of Alismatis rhizoma in hyperlipidemic mice. Food Funct. 2022, 13, 4714–4733. [Google Scholar] [CrossRef]
- Couvillion, S.P.; Danczak, R.E.; Naylor, D.; Smith, M.L.; Stratton, K.G.; Paurus, V.L.; Bloodsworth, K.J.; Farris, Y.; Schmidt, D.J.; Richardson, R.E.; et al. Rapid remodeling of the soil lipidome in response to a drying-rewetting event. Microbiome 2023, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding Is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Fukunaga, T.; Zou, W.; Rohatgi, N.; Colca, J.R.; Teitelbaum, S.L. An insulin-sensitizing thiazolidinedione, which minimally activates PPARgamma, does not cause bone loss. J. Bone Miner. Res. 2015, 30, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.C.; Zhuo, M.Q.; Zhang, D.G.; Cui, H.Y.; Luo, Z. Nano-Zn increased Zn accumulation and triglyceride content by up-regulating lipogenesis in freshwater teleost, yellow catfish Pelteobagrus fulvidraco. Int. J. Mol. Sci. 2020, 21, 1615. [Google Scholar] [CrossRef]
- Korpal, M.; Puyang, X.; Jeremy Wu, Z.; Seiler, R.; Furman, C.; Oo, H.Z.; Seiler, M.; Irwin, S.; Subramanian, V.; Julie Joshi, J.; et al. Evasion of immunosurveillance by genomic alterations of PPARgamma/RXRalpha in bladder cancer. Nat. Commun. 2017, 8, 103. [Google Scholar] [CrossRef]
- Kai, M.; Miyoshi, M.; Fujiwara, M.; Nishiyama, Y.; Inoue, T.; Maeshige, N.; Hamada, Y.; Usami, M. A lard-rich high-fat diet increases hepatic peroxisome proliferator-activated receptors in endotoxemic rats. J. Surg. Res. 2017, 212, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Garay-Lugo, N.; Dominguez-Lopez, A.; Miliar Garcia, A.; Aguilar Barrera, E.; Gomez Lopez, M.; Gomez Alcala, A.; Martinez Godinez Mde, L.; Lara-Padilla, E. n-3 Fatty acids modulate the mRNA expression of the Nlrp3 inflammasome and Mtor in the liver of rats fed with high-fat or high-fat/fructose diets. Immunopharmacol. Immunotoxicol. 2016, 38, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Song, J.; Park, J.-S.; Lee, S.; Lee, J.; Park, H.-J.; Kim, W.-K.; Yoon, S.; Chun, H.-S. 3,4-Dichloroaniline promotes fatty liver in zebrafish larvae. Mol. Cell. Toxicol. 2020, 16, 159–165. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Wang, Z.; Qin, J.; Wang, W.; Tian, H.; Ru, S. Bisphenol S induces obesogenic effects through deregulating lipid metabolism in zebrafish (Danio rerio) larvae. Chemosphere 2018, 199, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, Y.R.; Bang, E.; Ha, S.; Noh, S.G.; Kim, B.M.; Jeong, S.H.; Jung, H.J.; Lee, J.Y.; Chung, H.Y. Mechanism of lipid accumulation through PAR2 signaling in diabetic male mice. Endocrinol. Metab. 2021, 36, 171–184. [Google Scholar] [CrossRef]
- Nagatomo, A.; Nishida, N.; Matsuura, Y.; Shibata, N. Rosehip extract inhibits lipid accumulation in white adipose tissue by suppressing the expression of peroxisome proliferator-activated receptor gamma. Prev. Nutr. Food Sci. 2013, 18, 85–91. [Google Scholar] [CrossRef]



| Genes | GenBank Accession NO. | Amplicon Length (bp) | Primer Sequences (5′-3′) | |
|---|---|---|---|---|
| pparγ | NM_131467.1 | 152 | F | CTGCCGCATACACAAGAAGA |
| R | TCACGTCACTGGAGAACTCG | |||
| rxra | U29940 | 128 | F | CTGCCAGATAGACAAACGCCA |
| R | CATTATCACTCCTCTCCCGACC | |||
| β-actin | NM_181601.4 | 131 | F | AGGTCATCACCATTGGCAAT |
| R | GATGTCGACGTCACACTTCAT | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Liu, N.; Xue, M.; Zhang, M.; Xiao, Z.; Xu, C.; Fan, Y.; Qiu, J.; Zhang, Q.; Zhou, Y. β-Sitosterol Reduces the Content of Triglyceride and Cholesterol in a High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease Zebrafish (Danio rerio) Model. Animals 2024, 14, 1289. https://doi.org/10.3390/ani14091289
Zhang P, Liu N, Xue M, Zhang M, Xiao Z, Xu C, Fan Y, Qiu J, Zhang Q, Zhou Y. β-Sitosterol Reduces the Content of Triglyceride and Cholesterol in a High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease Zebrafish (Danio rerio) Model. Animals. 2024; 14(9):1289. https://doi.org/10.3390/ani14091289
Chicago/Turabian StyleZhang, Peng, Naicheng Liu, Mingyang Xue, Mengjie Zhang, Zidong Xiao, Chen Xu, Yuding Fan, Junqiang Qiu, Qinghua Zhang, and Yong Zhou. 2024. "β-Sitosterol Reduces the Content of Triglyceride and Cholesterol in a High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease Zebrafish (Danio rerio) Model" Animals 14, no. 9: 1289. https://doi.org/10.3390/ani14091289
APA StyleZhang, P., Liu, N., Xue, M., Zhang, M., Xiao, Z., Xu, C., Fan, Y., Qiu, J., Zhang, Q., & Zhou, Y. (2024). β-Sitosterol Reduces the Content of Triglyceride and Cholesterol in a High-Fat Diet-Induced Non-Alcoholic Fatty Liver Disease Zebrafish (Danio rerio) Model. Animals, 14(9), 1289. https://doi.org/10.3390/ani14091289






