Morphokinetic Analyses of Fishing Cat–Domestic Cat Interspecies Somatic Cell Nuclear Transfer Embryos Through A Time-Lapse System
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. In Vitro Maturation (IVM) of Oocytes
2.3. Interspecies SCNT
2.3.1. Donor Cells
2.3.2. Nuclear Transfer
2.4. In Vitro Fertilization
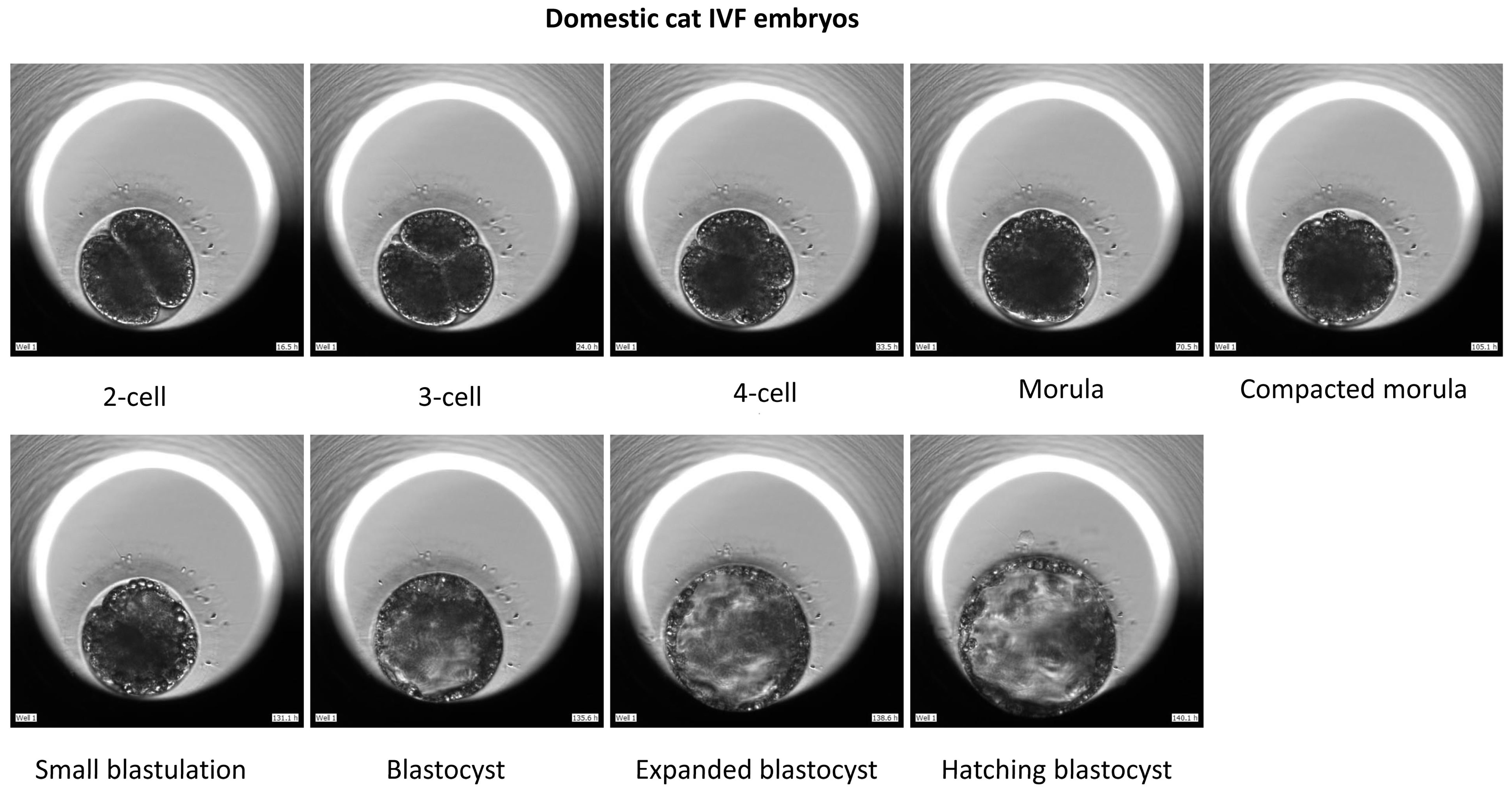
2.5. In Vitro Embryo Culture and Time-Lapse Imaging
2.6. Blastocyst Cell Counting
2.7. Data Analysis
3. Results
3.1. Comparison of In Vitro Developmental Capability of Fishing Cat-Domestic Cat iSCNT or Domestic Cat IVF Embryos
3.2. Comparison of Morphokinetic Parameters of Fishing Cat–Domestic Cat iSCNT Embryos and Domestic Cat IVF Embryos
3.3. Comparison of Morphokinetic Parameters of Normal or Arrested Embryos Derived from Fishing Cat–Domestic Cat iSCNT
3.4. Cleavage Patterns of the First Division in iSCNT and IVF Embryos and Their Relationship to Development to Blastocyst Stage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, C.C.; Loewke, K.E.; Bossert, N.L.; Behr, B.; De Jonge, C.J.; Baer, T.M.; Reijo Pera, R.A. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat. Biotechnol. 2010, 28, 1115–1121. [Google Scholar] [CrossRef]
- Meseguer, M.; Rubio, I.; Cruz, M.; Basile, N.; Marcos, J.; Requena, A. Embryo incubation and selection in a time-lapse monitoring system improves pregnancy outcome compared with a standard incubator: A retrospective cohort study. Fertil. Steril. 2012, 98, 1481–1489.e10. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Ruiz, B.; Basile, N.; Pérez Albalá, S.; Bronet, F.; Remohí, J.; Meseguer, M. Automatic time-lapse instrument is superior to single-point morphology observation for selecting viable embryos: Retrospective study in oocyte donation. Fertil. Steril. 2016, 106, 1379–1385.e10. [Google Scholar] [CrossRef]
- Ma, B.X.; Zhang, H.; Jin, L.; Huang, B. Neonatal Outcomes of Embryos Cultured in a Time-Lapse Incubation System: An Analysis of More Than 15,000 Fresh Transfer Cycles. Reprod. Sci. 2022, 29, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Kij, B.; Kochan, J.; Nowak, A.; Niżański, W.; Prochowska, S.; Fryc, K.; Bugno-Poniewierska, M. Using Time Lapse Monitoring for Determination of Morphological Defect Frequency in Feline Embryos after In Vitro Fertilization (IVF). Animals 2020, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Kochan, J.; Nowak, A.; Kij, B.; Prochowska, S.; Niżański, W. Analysis of Morphokinetic Parameters of Feline Embryos Using a Time-Lapse System. Animals 2021, 9, 748. [Google Scholar] [CrossRef] [PubMed]
- Magata, F.; Ideta, A.; Okubo, H.; Matsuda, F.; Urakawa, M.; Oono, Y. Growth potential of bovine embryos presenting abnormal cleavage observed through time lapse cinematography. Theriogenology 2019, 133, 119–124. [Google Scholar] [CrossRef]
- Fryc, K.; Nowak, A.; Kij, B.; Kochan, J.; Bartlewski, P.M.; Murawski, M. Timing of cleavage divisions determined with time-lapse imaging is linked to blastocyst formation rates and quality of in vitro-produced ovine embryos. Theriogenology 2021, 159, 147–152. [Google Scholar] [CrossRef]
- Park, M.J.; Kim, E.Y.; Kang, M.J.; Lee, J.B.; Jeong, C.J.; Park, S.P. Investigation of the Developmental Potential and Developmental Kinetics of Bovine Parthenogenetic and Somatic Cell Nuclear Transfer Embryos Using a Time-Lapse Monitoring System. Cell. Reprogram. 2017, 19, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Son, Y.J.; Lee, S.E.; Park, Y.G.; Jeong, S.G.; Shin, M.Y.; Kim, E.Y.; Park, S.P. Fibroblast Growth Factor 10 Enhances the Developmental Efficiency of Somatic Cell Nuclear Transfer Embryos by Accelerating the Kinetics of Cleavage During In Vitro Maturation. Cell Reprogram. 2018, 20, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Mallol, A.; Piqué, L.; Santaló, J.; Ibáñez, E. Morphokinetics of cloned mouse embryos treated with epigenetic drugs and blastocyst prediction. Reproduction 2016, 151, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Swegen, A.; Appeltant, R.; Williams, S.A. Cloning in action: Can embryo splitting, induced pluripotency and somatic cell nuclear transfer contribute to endangered species conservation? Biol. Rev. Camb. Philos. Soc. 2023, 98, 1225–1249. [Google Scholar] [CrossRef] [PubMed]
- Cowl, V.B.; Comizzoli, P.; Appeltant, R.; Bolton, R.L.; Browne, R.K.; Holt, W.V.; Penfold, L.M.; Swegen, A.; Walker, S.L.; Williams, S.A. Cloning for the Twenty-First Century and Its Place in Endangered Species Conservation. Annu. Rev. Anim. Biosci. 2024, 12, 91–112. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.C.; Pope, C.E.; Giraldo, A.; Lyons, L.A.; Harris, R.F.; King, A.L.; Cole, A.; Godke, R.A.; Dresser, B.L. Nuclear transfer of sand cat cells into enucleated domestic cat oocytes is affected by cryopreservation of donor cells. Cloning Stem Cells 2004, 6, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.C.; Pope, C.E.; Kutner, R.H.; Ricks, D.M.; Lyons, L.A.; Ruhe, M.; Dumas, C.; Lyons, J.; López, M.; Dresser, B.L.; et al. Birth of African Wildcat cloned kittens born from domestic cats. Cloning Stem Cells 2008, 10, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.J.; Lee, Y.; Lee, H.; Kim, N.; Kim, L.; Shin, H.; Kong, I. In vitro production and initiation of pregnancies in inter-genus nuclear transfer embryos derived from leopard cat (Prionailurus bengalensis) nuclei fused with domestic cat (Felis silverstris catus) enucleated oocytes. Theriogenology 2006, 66, 275–282. [Google Scholar] [CrossRef]
- Thongphakdee, A.; Siriaroonrat, B.; Manee-in, S.; Klincumhom, N.; Kamolnorranath, S.; Chatdarong, K.; Techakumphu, M. Intergeneric somatic cell nucleus transfer in marbled cat and flat-headed cat. Theriogenology 2010, 73, 120–128. [Google Scholar] [CrossRef]
- Veraguas, D.; Aguilera, C.; Echeverry, D.; Saez-Ruiz, D.; Castro, F.O.; Rodriguez-Alvarez, L. Embryo aggregation allows the production of kodkod (Leopardus guigna) blastocysts after interspecific SCNT. Theriogenology 2020, 158, 148–157. [Google Scholar] [CrossRef]
- Mukherjee, S.; Appel, A.; Duckworth, J.W.; Sanderson, J.; Dahal, S.; Willcox, D.H.A.; Herranz Muñoz, V.; Malla, G.; Ratnayaka, A.; Kantimahanti, M.; et al. The IUCN Red List of Threatened Species. 2016. Prionailurus viverrinus, e.T18150A50662615. Available online: https://www.iucnredlist.org/species/18150/221434864 (accessed on 3 June 2022).
- Yang, S.T.; Shi, J.X.; Gong, F.; Zhang, S.P.; Lu, C.F.; Tan, K.; Leng, L.Z.; Hao, M.; He, H.; Gu, Y.F.; et al. Cleavage pattern predicts developmental potential of day 3 human embryos produced by IVF. Reprod. Biomed. Online 2015, 30, 625–634. [Google Scholar] [CrossRef]
- Zhan, Q.; Ye, Z.; Clarke, R.; Rosenwaks, Z.; Zaninovic, N. Direct Unequal Cleavages: Embryo Developmental Competence, Genetic Constitution and Clinical Outcome. PLoS ONE 2016, 11, e0166398. [Google Scholar] [CrossRef]
- Barrie, A.; Homburg, R.; McDowell, G.; Brown, J.; Kingsland, C.; Troup, S. Preliminary investigation of the prevalence and implantation potential of abnormal embryonic phenotypes assessed using time-lapse imaging. Reprod. Biomed. Online 2017, 34, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Goldberg, J.M.; Austin, C.; Falcone, T. Are cleavage anomalies, multinucleation, or specific cell cycle kinetics observed with time-lapse imaging predictive of embryo developmental capacity or ploidy? Fertil. Steril. 2018, 109, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Wood, T.C.; Wildt, D.E. Effect of the quality of the cumulus-oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J. Reprod. Fertil. 1997, 110, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.J.; Liu, R.M. Dynamic changes in chromatin and microtubules at the first cell cycle in SCNT or IVF goat embryos. Cell Biol. Int. 2018, 42, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.W.; Zeng, Y.; Tay, E.; Teo, J.H.J.; Cipta, N.O.; Hamashima, K.; Yi, Y.; Liu, H.; Warrier, T.; Le, M.T.N.; et al. Nuclear receptor-SINE B1 network modulates expanded pluripotency in blastoids and blastocysts. Nat. Commun. 2024, 15, 10011. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wilmut, I.; Schnieke, A.E.; McWhir, J.; Kind, A.J.; Campbell, K.H. Viable offspring derived from fetal and adult mammalian cells. Nature 1997, 385, 810–813. [Google Scholar] [CrossRef]
- Klinger, B.; Schnieke, A. 25th ANNIVERSARY OF CLONING BY SOMATIC-CELL NUCLEAR TRANSFER Twenty-five years after Dolly: How far have we come? Reproduction 2021, 162, F1–F10. [Google Scholar] [CrossRef] [PubMed]
- Matoba, S.; Zhang, Y. Somatic Cell Nuclear Transfer Reprogramming: Mechanisms and Applications. Cell Stem Cell. 2018, 4, 471–485. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Warrier, T.; El Farran, C.; Zeng, Y.; Ho, B.S.Q.; Bao, Q.; Zheng, Z.H.; Bi, X.; Ng, H.H.; Ong, D.S.T.; Chu, J.J.H.; et al. SETDB1 acts as a topological accessory to Cohesin via an H3K9me3-independent, genomic shunt for regulating cell fates. Nucleic Acids Res. 2022, 50, 7326–7349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hamashima, K.; Wong, K.W.; Sam, T.W.; Teo, J.H.J.; Taneja, R.; Le, M.T.N.; Li, Q.J.; Hanna, J.H.; Li, H.; Loh, Y.H. Single-nucleus multiomic mapping of m6A methylomes and transcriptomes in native populations of cells with sn-m6A-CT. Mol. Cell. 2023, 83, 3205–3216. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Loi, P.; Palazzese, L.; Scapolo, P.A.; Fulka, J.; Fulka, H.; Czernik, M. 25th ANNIVERSARY OF CLONING BY SOMATIC-CELL NUCLEAR TRANSFER: Scientific and technological approaches to improve SCNT efficiency in farm animals and pets. Reproduction 2021, 162, F33–F43. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, L.; Liu, W.; Qiao, Z.; Shen, S.; Zhu, Q.; Gao, R.; Wang, M.; Wang, M.; Li, C.; et al. Dux-Mediated Corrections of Aberrant H3K9ac during 2-Cell Genome Activation Optimize Efficiency of Somatic Cell Nuclear Transfer. Cell Stem Cell 2021, 28, 150–163.e5. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zeng, Y.; Sam, T.W.; Hamashima, K.; Tan, R.J.R.; Warrier, T.; Phua, J.X.; Taneja, R.; Liou, Y.C.; Li, H.; et al. Ribosomal proteins regulate 2-cell-stage transcriptome in mouse embryonic stem cells. Stem Cell Rep. 2023, 18, 463–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mrowiec, P.; Bugno-Poniewierska, M.; Młodawska, W. The perspective of the incompatible of nucleus and mitochondria in interspecies somatic cell nuclear transfer for endangered species. Reprod. Domest. Anim. 2021, 56, 199–207. [Google Scholar] [CrossRef]
- Long, C.; Li, H.; Li, X.; Yang, W.; Zuo, Y. Nuclear transfer arrest embryos show massive dysregulation of genes involved in transcription pathways. Int. J. Mol. Sci. 2021, 22, 8187. [Google Scholar] [CrossRef]
- Ochota, M.; Niżański, W. Time of early cleavage affects the developmental potential of feline preimplantation embryos in vitro. Theriogenology 2017, 89, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Ochota, M.; Wojtasik, B.; Niżański, W. Total Cell Number and its Allocation to Trophectoderm and Inner Cell Mass in In Vitro Obtained Cats’ Blastocysts. Reprod. Domest. Anim. 2016, 51, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Kochan, J.; Nowak, A.; Kij, B.; Fryc, K.; Prochowska, S.; Niżański, W. A comparison of in vitro culture systems for cat embryos. Theriogenology 2022, 179, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Rubio, I.; Kuhlmann, R.; Agerholm, I.; Kirk, J.; Herrero, J.; Escribá, M.J.; Bellver, J.; Meseguer, M. Limited implantation success of direct-cleaved human zygotes: A time-lapse study. Fertil. Steril. 2012, 98, 1458–1463. [Google Scholar] [CrossRef]
- Zheng, W.; Chen, L.; Dai, J.; Dai, C.; Guo, J.; Lu, C.; Gong, F.; Lu, G.; Lin, G. New biallelic mutations in PADI6 cause recurrent preimplantation embryonic arrest characterized by direct cleavage. J. Assist. Reprod. Genet. 2020, 37, 205–212. [Google Scholar] [CrossRef]



| Group | No. of Reconstructed Couplets | No. of Fused Couplets (%) | No. of Inseminated Oocytes | No. of Cultured Embryos | No. of Cleavage (%) |
|---|---|---|---|---|---|
| iSCNT | 72 | 49 (68.1) | --- | 44 | 32 (72.7) |
| IVF | --- | --- | 16 | 16 | 14 (87.5) |
| No. of Morula (cleavage %) | No. of Compacted Morula (cleavage %) | No. of Blastocyst (cleavage %) | No. Hatching Blastocyst (blastocyst %) | Cell Number of Blastocyst | |
| iSCNT | 13 (40.6) a | 12 (37.5) | 8 (25) a | 4 (50) | 88.3 + 9.4 |
| IVF | 14 (100) b | 12 (85.7) | 11 (78.6) b | 10 (90.9) | 121.5 ± 17.5 |
| Variables | iSCNT | No. of Embryos * | IVF | No. of Embryos ** |
|---|---|---|---|---|
| t2 | 22.5 ± 3.53 a | 7 | 18.75 ± 3.42 b | 8 |
| t3 | 30.61 ± 3.24 a | 7 | 23.3 ± 3.84 b | 8 |
| t4 | 39.4 ± 7.6 a | 7 | 28.84 ± 3.98 b | 8 |
| cc2 | 8.11 ± 3.6 | 7 | 4.55 ± 2.53 | 8 |
| s2 | 8.79 ± 5.59 | 7 | 5.54 ± 2.93 | 8 |
| tM | 81.45 ± 6.03 a | 8 | 59.05 ± 6.64 b | 11 |
| tCM | 99 ± 6.42 a | 8 | 84.26 ± 7.94 b | 11 |
| tSB | 126.93 ± 9.32 | 8 | 125.4 ± 12.71 | 11 |
| tB | 130.3 ± 5.54 | 7 | 135.16 ± 10.17 | 11 |
| tEB | 140.87 ± 10.33 | 6 | 141.5 ± 12.46 | 11 |
| tHB | 148.2 ± 15.11 | 4 | 143.26 ± 6.61 | 10 |
| Variables | Normal Emb | No. of Embryos * | Arrested Emb | No. of Embryos ** |
|---|---|---|---|---|
| t2 | 22.5 ± 3.53 | 7 | 22.36 ± 6.15 | 7 *** |
| t3 | 30.61 ± 3.24 | 7 | 33.53 ± 7.81 | 7 |
| t4 | 39.4 ± 7.6 | 7 | 39.5 ± 11.41 | 5 |
| cc2 | 8.11 ± 3.6 | 7 | 11.17 ± 3.4 | 7 |
| s2 | 9.5 ± 5.74 | 7 | 7.58 ± 7.23 | 5 |
| tM | 81.45 ± 6.03 a | 8 | 67.32 ± 2.61 b | 5 |
| tCM | 99 ± 6.42 a | 8 | 75.9 ± 1.86 b | 4 |
| Group | iSCNT | IVF | |||
|---|---|---|---|---|---|
| Cleavage Pattern | Cleaved | Blastocyst (Cleavage %) | Cleaved | Blastocyst (Cleavage %) | |
| Normal cleavage | 16 | 7 (43.8) a | 9 | 8 (88.9) | |
| Direct cleavage | 14 | 1 (7.7) b,c | 5 | 3 (60) d | |
| Uneven cleavage | 2 | 0 | |||
| Total | 32 | 8 (25.8) | 14 | 11 (78.6) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-J.; Oh, S.J.W.Y.; Tay, N.L.; Lim, C.Y.; Hsu, C.-D.; Chua, D.H.H.; Teo, W.K.L.; Loh, Y.-H.; Ng, S.C. Morphokinetic Analyses of Fishing Cat–Domestic Cat Interspecies Somatic Cell Nuclear Transfer Embryos Through A Time-Lapse System. Animals 2025, 15, 148. https://doi.org/10.3390/ani15020148
Liu H-J, Oh SJWY, Tay NL, Lim CY, Hsu C-D, Chua DHH, Teo WKL, Loh Y-H, Ng SC. Morphokinetic Analyses of Fishing Cat–Domestic Cat Interspecies Somatic Cell Nuclear Transfer Embryos Through A Time-Lapse System. Animals. 2025; 15(2):148. https://doi.org/10.3390/ani15020148
Chicago/Turabian StyleLiu, Hai-Jun, Serena Jocelyn Wai Yin Oh, Nicole Liling Tay, Christina Yingyan Lim, Chia-Da Hsu, Delia Hwee Hoon Chua, Winnie Koon Lay Teo, Yuin-Han Loh, and Soon Chye Ng. 2025. "Morphokinetic Analyses of Fishing Cat–Domestic Cat Interspecies Somatic Cell Nuclear Transfer Embryos Through A Time-Lapse System" Animals 15, no. 2: 148. https://doi.org/10.3390/ani15020148
APA StyleLiu, H.-J., Oh, S. J. W. Y., Tay, N. L., Lim, C. Y., Hsu, C.-D., Chua, D. H. H., Teo, W. K. L., Loh, Y.-H., & Ng, S. C. (2025). Morphokinetic Analyses of Fishing Cat–Domestic Cat Interspecies Somatic Cell Nuclear Transfer Embryos Through A Time-Lapse System. Animals, 15(2), 148. https://doi.org/10.3390/ani15020148






