Effects of Dietary Nano-Composite of Copper and Carbon on Antioxidant Capacity, Immunity, and Cecal Microbiota of Weaned Ira White Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals, and Diets
2.2. Sample Collection
2.3. Liver Antioxidant Indicators
2.4. Determination of Jejunal Mucosal Immune Indicators
2.5. qRT-PCR
2.6. Bacterial DNA Extraction and 16S rRNA Gene Sequencing
2.7. Data Analysis and Statistics
3. Results
3.1. Growth Performance
3.2. The Content of Antioxidant Enzymes and the Expression of Related Genes in the Liver
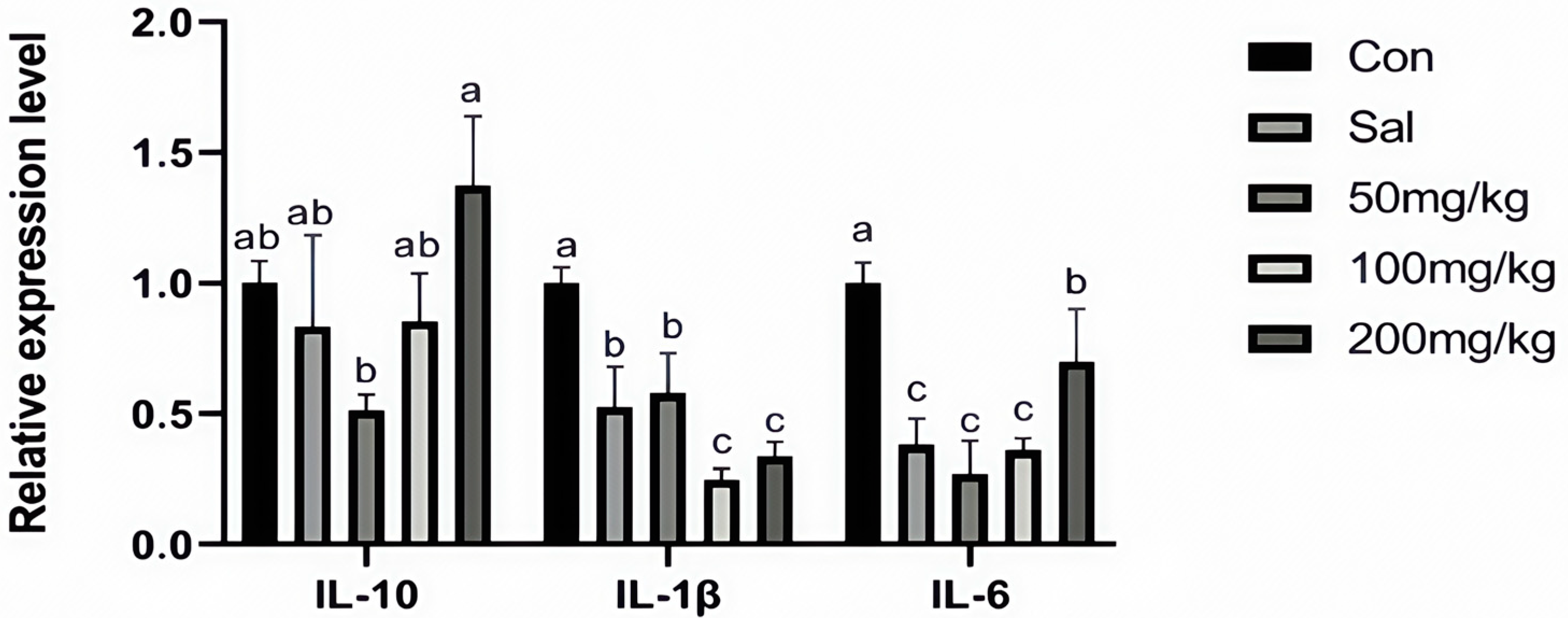
3.3. The Content of Immune Factors in Jejunal Mucosa and the Expression of Related Genes
3.4. Cecal Microbiota
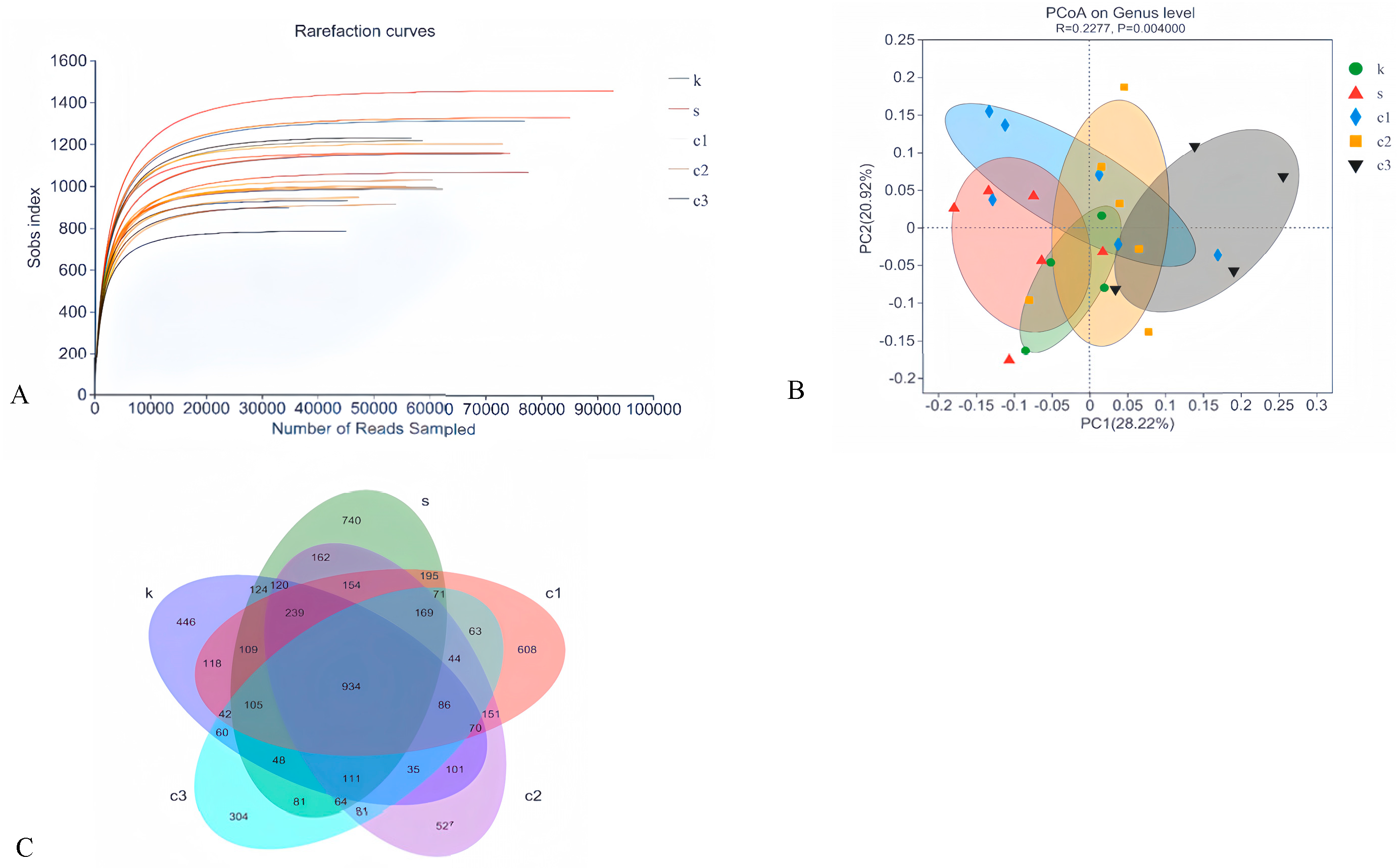
3.4.1. Amplicon Sequence Variant (ASV) Cluster Analysis and Rarefaction Curve
3.4.2. Beta-Diversity Analysis
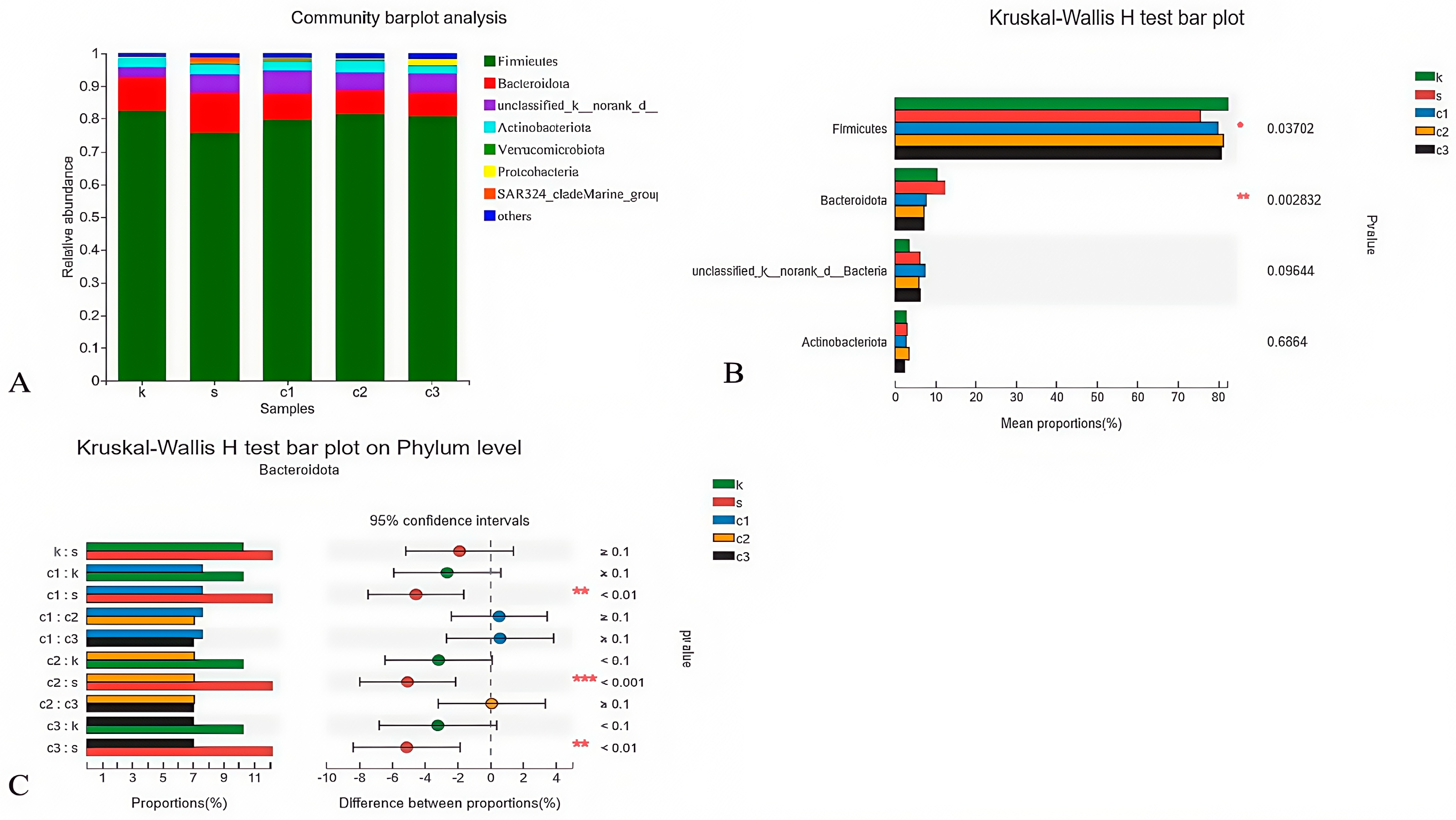
3.4.3. Heat Map Analysis at the Phylum Level
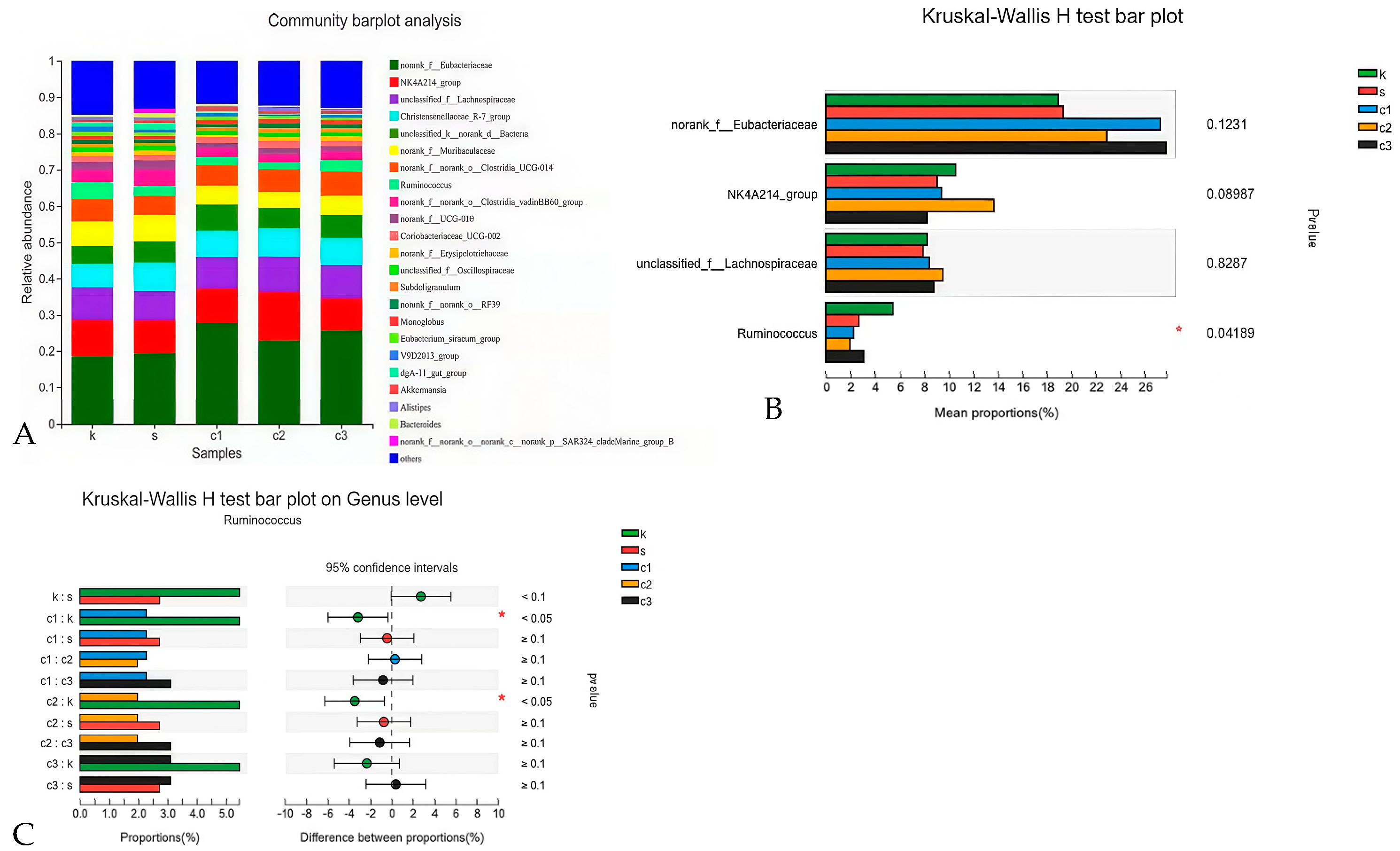
3.4.4. Heat Map Analysis at the Genus Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fels, M.; Benato, L.; Whittaker, A. Editorial: Health and Welfare of Rabbits (on a Farm, in the Laboratory or as a Pet). Front. Vet. Sci. 2024, 11, 1522420. [Google Scholar] [CrossRef] [PubMed]
- Subasinghe, S.K.; Ogbuehi, K.C.; Mitchell, L.; Dias, G.J. Animal Model with Structural Similarity to Human Corneal Collagen Fibrillar Arrangement. Anat. Sci. Int. 2021, 96, 286–293. [Google Scholar] [CrossRef]
- Marco-Jiménez, F.; Viudes-de-Castro, M.P.; Vicente, J.S. Why Choose the Rabbit to Work in Reproductive Technology? Reprod. Domest. Anim. 2024, 59 (Suppl. S3), e14640. [Google Scholar] [CrossRef] [PubMed]
- Agossou, D.J.; Toukourou, Y.; Koluman, N. Sustainable Development of Livestock and Meat Production in Republic of Benin: Strategies and Perspectives. J. Anim. Health Prod. 2018, 6, 35–40. [Google Scholar]
- Kumar, P.; Sharma, N.; Narnoliya, L.K.; Verma, A.K.; Umaraw, P.; Mehta, N.; Ismail-Fitry, M.R.; Kaka, U.; Yong-Meng, G.; Lee, S.-J.; et al. Improving Quality and Consumer Acceptance of Rabbit Meat: Prospects and Challenges. Meat Sci. 2025, 219, 109660. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT Database. Available online: https://www.fao.org/faostat/en/ (accessed on 20 December 2024).
- Bivolarski, B.L.; Vachkova, E.G. Morphological and Functional Events Associated to Weaning in Rabbits. J. Anim. Physiol. Anim. Nutr. 2014, 98, 9–18. [Google Scholar] [CrossRef]
- Kovács, M.; Bónai, A.; Szendrő, Z.; Milisits, G.; Lukács, H.; Szabó-Fodor, J.; Tornyos, G.; Matics, Z.; Kovács, F.; Horn, P. Effect of Different Weaning Ages (21, 28 or 35 days) on Production, Growth and Certain Parameters of the Digestive Tract in Rabbits. Anim. Int. J. Anim. Biosci. 2012, 6, 894–901. [Google Scholar] [CrossRef]
- Kelly, D.; Coutts, A.G.P. Development of Digestive and Immunological Function in Neonates: Role of Early Nutrition. Livest. Prod. Sci. 2000, 66, 161–167. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, B.; Wu, Y.; Hu, S.; Mu, L.; Zhu, C.; Pan, Y.; Wu, X. Impacts of Diarrhea on the Immune System, Intestinal Environment, and Expression of PGRPs in New Zealand Rabbits. PeerJ 2017, 5, e4100. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.; Maximiano, M.R.; Franco, O.L. Antimicrobial Peptides Used as Growth Promoters in Livestock Production. Appl. Microbiol. Biotechnol. 2021, 105, 7115–7121. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. Antibiotic Growth Promoters in Agriculture: History and Mode of Action. Poult. Sci. 2005, 84, 634–643. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- Klein, E.Y.; Impalli, I.; Poleon, S.; Denoel, P.; Cipriano, M.; Van Boeckel, T.P.; Pecetta, S.; Bloom, D.E.; Nandi, A. Global Trends in Antibiotic Consumption During 2016–2023 and Future Projections Through 2030. Proc. Natl. Acad. Sci. USA 2024, 121, e2411919121. [Google Scholar] [CrossRef] [PubMed]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Deb Adhikari, M.; Saha, T.; Tiwary, B.K. Quest for Alternatives to Antibiotics: An Urgent Need of the Twenty-First Century. In Alternatives to Antibiotics: Recent Trends and Future Prospects; Saha, T., Deb Adhikari, M., Tiwary, B.K., Eds.; Springer Nature: Singapore, 2022; pp. 3–32. [Google Scholar] [CrossRef]
- Ruiz-Pérez, R.; Newman-Portela, A.M.; Ruiz-Fresneda, M.A. Emerging Global Trends in Next-Generation Alternatives to Classic Antibiotics for Combatting Multidrug-Resistant Bacteria. J. Clean. Prod. 2024, 478, 143895. [Google Scholar] [CrossRef]
- El-Sabrout, K.; Khalifah, A.; Ciani, F. Current Applications and Trends in Rabbit Nutraceuticals. Agriculture 2023, 13, 1424. [Google Scholar] [CrossRef]
- Sadr, S.; Lotfalizadeh, N.; Ghafouri, S.A.; Delrobaei, M.; Komeili, N.; Hajjafari, A. Nanotechnology Innovations for Increasing the Productivity of Poultry and the Prospective of Nanobiosensors. Vet. Med. Sci. 2023, 9, 2118–2131. [Google Scholar] [CrossRef]
- Michalak, I.; Dziergowska, K.; Alagawany, M.; Farag, M.R.; El-Shall, N.A.; Tuli, H.S.; Emran, T.B.; Dhama, K. The Effect of Metal-Containing Nanoparticles on the Health, Performance and Production of Livestock Animals and Poultry. Vet. Q. 2022, 42, 68–94. [Google Scholar] [CrossRef]
- Rana, T. Prospects and Future Perspectives of Selenium Nanoparticles: An Insight of Growth Promoter, Antioxidant and Anti-bacterial Potentials in Productivity of Poultry. J. Trace Elem. Med. Biol. 2021, 68, 126862. [Google Scholar] [CrossRef]
- Marin, M.P.; Nicolae, C.G.; Tapaloaga, D.; Petcu, C.; Tapaloaga, P.R.; Dinita, G. The Metabolic Utilization of Iron and Copper in the Piglet Organism. Curr. Opin. Biotechnol. 2013, 24, S51. [Google Scholar] [CrossRef]
- Tian, Z.; Jiang, S.; Zhou, J.; Zhang, W. Copper Homeostasis and Cuproptosis in Mitochondria. Life Sci. 2023, 334, 122223. [Google Scholar] [CrossRef] [PubMed]
- Olivares, M.; Uauy, R. Copper as an Essential Nutrient. Am. J. Clin. Nutr. 1996, 63, 791s–796s. [Google Scholar] [CrossRef] [PubMed]
- Bost, M.; Houdart, S.; Oberli, M.; Kalonji, E.; Huneau, J.F.; Margaritis, I. Dietary Copper and Human Health: Current Evidence and Unresolved Issues. J. Trace Elem. Med. Biol. 2016, 35, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Fukai, T.; Ushio-Fukai, M.; Kaplan, J.H. Copper Transporters and Copper Chaperones: Roles in Cardiovascular Physiology and Disease. Am. J. Physiol. Cell Physiol 2018, 315, C186–C201. [Google Scholar] [CrossRef]
- Guan, D.F.; Zhao, L.H.; Shi, X.; Ma, X.L.; Chen, Z. Copper in Cancer: From Pathogenesis to Therapy. Biomed. Pharmacother. 2023, 163, 114791. [Google Scholar] [CrossRef]
- Sharif, M.; Rahman, M.A.; Ahmed, B.; Abbas, R.Z.; Hassan, F.U. Copper Nanoparticles as Growth Promoter, Antioxidant and Anti-Bacterial Agents in Poultry Nutrition: Prospects and Future Implications. Biol. Trace Elem. Res. 2021, 199, 3825–3836. [Google Scholar] [CrossRef] [PubMed]
- Gangadoo, S.; Stanley, D.; Hughes, R.J.; Moore, R.J.; Chapman, J. Nanoparticles in Feed: Progress and Prospects in Poultry Research. Trends Food Sci. Technol. 2016, 58, 115–126. [Google Scholar] [CrossRef]
- Patra, A.; Lalhriatpuii, M. Progress and Prospect of Essential Mineral Nanoparticles in Poultry Nutrition and Feeding—A Review. Biol. Trace Elem. Res. 2020, 197, 233–253. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.Q.; Ye, S.S.; Tao, W.J.; Du, Y.J. Effects of Copper-Loaded Chitosan Nanoparticles on Growth and Immunity in Broilers. Poult. Sci. 2011, 90, 2223–2228. [Google Scholar] [CrossRef]
- Refaie, A.; Ghazal, M.; Barakat, S.; Morsy, W.; Meshreky, S.; Younan, G.; Eisa, W. Nano-Copper as a New Growth Promoter in the Diet of Growing New Zealand White Rabbits. Egypt. J. Rabbit. Sci. 2015, 25, 39–57. [Google Scholar] [CrossRef]
- Chand Mali, S.; Dhaka, A.; Sharma, S.; Trivedi, R. Review on Biogenic Synthesis of Copper Nanoparticles and Its Potential Applications. Inorg. Chem. Commun. 2023, 149, 110448. [Google Scholar] [CrossRef]
- Liu, J.; Lin, S.Y.; Wu, S.Q.; Lin, Q.J.; Fan, Z.T.; Wang, C.K.; Ye, D.C.; Guo, P.T. Dietary Supplementation with Nano-Composite of Copper and Carbon on Growth Performance, Immunity, and Antioxidant Ability of Yellow-Feathered Broilers. J. Anim. Sci. 2023, 101, skad362. [Google Scholar] [CrossRef]
- Van Damme, L.G.W.; Ipek, N.; Verwaeren, J.; Delezie, E.; Tuyttens, F.A.M. Cage Enrichment to Minimize Aggression in Part-Time Group-Housed Female Breeding Rabbits. Front. Vet. Sci. 2024, 11, 1401021. [Google Scholar] [CrossRef]
- DB37/T 1835-2011; Meat Rabbits Feeding Standard. Shandong Provincial Bureau of Quality and Technical Supervision: Jinan, China, 2011.
- Di, B.; Yang, G.-F.; Liao, L.-W.; He, J.-Y.; Zhong, X.-Y.; Guo, P.-T.; Gao, Y.-Y.; Jin, L. Effects of Nano-Composites of Copper and Carbon on Growth Performance and Jejunal Mechanical Barrier of Weaned Ira Rabbits. Chin. J. Anim. Nutr. 2023, 35, 4575–4586. (In Chinese) [Google Scholar]
- Gallois, M.; Le Huërou-Luron, I.; Fortun-Lamothe, L.; Lallès, J.P.; Gidenne, T. Adaptability of the Digestive Function According to Age at Weaning in the Rabbit: I. Effect on Feed Intake and Digestive Functionality. Anim. Int. J. Anim. Biosci. 2008, 2, 525–535. [Google Scholar] [CrossRef]
- Gharib, H.S.A.; Abdel-Fattah, A.F.; Mohammed, H.A.; Abdel-Fattah, D.M. Weaning Induces Changes in Behavior and Stress Indicators in Young New Zealand Rabbits. J. Adv. Vet. Anim. Res. 2018, 5, 166–172. [Google Scholar]
- Yin, D.; Zhang, Z.; Zhu, Y.; Xu, Z.; Liu, W.; Liang, K.; Li, F. Assessment of the Impact of Dietary Supplementation with Epigallocatechin Gallate (EGCG) on Antioxidant Status, Immune Response, and Intestinal Microbiota in Post-Weaning Rabbits. Animals 2024, 14, 3011. [Google Scholar] [CrossRef]
- Festa, R.A.; Thiele, D.J. Copper: An Essential Metal in Biology. Curr. Biol. 2011, 21, R877–R883. [Google Scholar] [CrossRef]
- Wang, L.; Wu, N.; Zhang, Y.; Wang, G.-L.; Pu, S.; Guan, T.; Zhu, C.; Wang, H.; Li, J. Effects of Copper on Non-Specific Immunity and Antioxidant in the Oriental River Prawn (Macrobrachium nipponense). Ecotoxicol. Environ. Saf. 2022, 236, 113465. [Google Scholar]
- Liu, Z.; Wu, X.; Zhang, T.; Cui, H.; Guo, J.; Guo, Q.; Gao, X.; Yang, F. Influence of Dietary Copper Concentrations on Growth Performance, Serum Lipid Profiles, Antioxidant Defenses, and Fur Quality in Growing–Furring Male Blue Foxes (Vulpes lagopus). J. Anim. Sci. 2016, 94, 1095–1104. [Google Scholar] [CrossRef]
- Campbell, J. Malondialdehyde (MDA): Structure, Biochemistry and Role in Disease; Nova Science Pub. Inc.: Huntington, NY, USA, 2015. [Google Scholar]
- Ghosh, N.; Das, A.; Chaffee, S.; Roy, S.; Sen, C.K. Chapter 4—Reactive Oxygen Species, Oxidative Damage and Cell Death. In Immunity and Inflammation in Health and Disease; Chatterjee, S., Jungraithmayr, W., Bagchi, D., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 45–55. [Google Scholar] [CrossRef]
- Levy, M.A.; Tsai, Y.-H.; Reaume, A.G.; Bray, T.M. Cellular Response of Antioxidant Metalloproteins in Cu/Zn SOD Transgenic Mice Exposed to Hyperoxia. J. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 281, L172–L182. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide Dismutases: Dual Roles in Controlling ROS Damage and Regulating ROS Signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Min, J.X.; Wang, F.D. Copper Homeostasis and Cuproptosis in Health and Disease. Signal Transduct. Target. Ther. 2022, 7, 378. [Google Scholar] [CrossRef]
- Nassiri, M.; Ahmadi, F. Effects of Copper Oxide Nanoparticles on the Growth Performance, Antioxidant Enzymes Activity and Gut Morphology of Broiler Chickens. J. World Acad. Sci. Eng. Technol. Int. J. Agric. Biosyst. Eng. 2015, 9, 1–11. Available online: https://api.semanticscholar.org/CorpusID:86925100 (accessed on 5 November 2024).
- El-Kazaz, S.E.; Hafez, M.H. Evaluation of Copper Nanoparticles and Copper Sulfate Effect on Immune Status, Behavior, and Productive Performance of Broilers. J. Adv. Vet. Anim. Res. 2020, 7, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Atanasiu, R.; Stéa, D.; Mateescu, M.A.; Vergely, C.; Dalloz, F.; Briot, F.; Maupoil, V.; Nadeau, R.; Rochette, L. Direct Evidence of Caeruloplasmin Antioxidant Properties. J. Mol. Cell. Biochem. 2004, 189, 127–135. [Google Scholar] [CrossRef]
- Pu, Z.; Xu, W.; Lin, Y.; He, J.; Huang, M. Oxidative Stress Markers and Metal Ions Are Correlated with Cognitive Function in Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dementiasr 2017, 32, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ognik, K.; Krauze, M. The Potential for Using Enzymatic Assays to Assess the Health of Turkeys. World’s Poult. Sci. J. 2016, 72, 535–550. [Google Scholar] [CrossRef]
- Angelova, M.; Asenova, S.; Nedkova, V.N.; Koleva-Kolarova, R. Copper in the Human Organism. Trakia J. Sci. 2011, 9, 88–98. [Google Scholar]
- Chen, J.; Jiang, Y.H.; Shi, H.; Peng, Y.G.; Fan, X.Y.; Li, C.H. The Molecular Mechanisms of Copper Metabolism and Its Roles in Human Diseases. Pflug. Arch.-Eur. J. Physiol. 2020, 472, 1415–1429. [Google Scholar] [CrossRef]
- Mandarano, A.H.; McGargill, M.A. The Critical Role of Copper Homeostasis During the Immune Response. J. Immunol. 2023, 210 (Suppl. S1), 148.13. [Google Scholar] [CrossRef]
- Deo, C.; Biswas, A.; Sharma, D.; Tiwari, A.K. Effects of Different Concentration of Copper on Performance, Immunity and Carcass Traits in Broiler Japanese Quails. J. Biol. Trace Elem. Res. 2022, 201, 4530–4537. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Peng, G.; Lu, Y.; Wang, K.; Ju, Q.N.; Ju, Y.L.; Ouyang, M.Z. Relationship Between Copper and Immunity: The Potential Role of Copper in Tumor Immunity. Front. Oncol. 2022, 12, 1019153. [Google Scholar] [CrossRef]
- Shurson, G.C.; Ku, P.K.; Waxler, G.L.; Yokoyama, M.T.; Miller, E.R. Physiological Relationships Between Microbiological Status and Dietary Copper Levels in the Pig. J. Anim. Sci. 1990, 68, 1061–1071. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The Pro- and Anti-Inflammatory Properties of the Cytokine Interleukin-6. Biochim. Et Biophys. Acta-Mol. Cell Res. 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Hunter, C.A.; Jones, S.A. IL-6 as a Keystone Cytokine in Health and Disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Interleukin (IL-6) Immunotherapy. Cold Spring Harb. Perspect. Biol. 2018, 10, a028456. [Google Scholar] [CrossRef]
- Luan, J.; Chen, W.; Fan, J.; Wang, S.; Zhang, X.; Zai, W.; Jin, X.; Wang, Y.; Feng, Z.; Zhang, J.; et al. GSDMD Membrane Pore Is Critical for IL-1β Release and Antagonizing IL-1β by Hepatocyte-Specific Nanobiologics Is a Promising Therapeutics for Murine Alcoholic Steatohepatitis. Biomaterials 2020, 227, 119570. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.F.; Zhang, Q.H.; Wu, H.; Wang, C.C.; Cao, S.T.; Feng, J.; Hu, C.H. Influences of Copper/Zinc-Loaded Montmorillonite on Growth Performance, Mineral Retention, Intestinal Morphology, Mucosa Antioxidant Capacity, and Cytokine Contents in Weaned Piglets. Biol. Trace Elem. Res. 2018, 185, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhu, M.; Jiang, Q.; Wang, L. Effects of Copper Sources and Levels on Lipid Profiles, Immune Parameters, Antioxidant Defenses, and Trace Element Residues in Broilers. Biol. Trace Elem. Res. 2020, 194, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Aliyu, M.; Zohora, F.T.; Anka, A.U.; Ali, K.; Maleknia, S.; Saffarioun, M.; Azizi, G. Interleukin-6 Cytokine: An Overview of the Immune Regulation, Immune Dysregulation, and Therapeutic Approach. Int. Immunopharmacol. 2022, 111, 109130. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- de Almada, C.N.; Nunes de Almada, C.; Martinez, R.C.R.; Sant’Ana, A.d.S. Characterization of the Intestinal Microbiota and Its Interaction with Probiotics and Health Impacts. Appl. Microbiol. Biotechnol. 2015, 99, 4175–4199. [Google Scholar] [CrossRef] [PubMed]
- Gallois, M.; Fortun-Lamothe, L.; Michelan, A.; Gidenne, T. Adaptability of the Digestive Function According to Age at Weaning in the Rabbit: II. Effect on Nutrient Digestion in the Small Intestine and in the Whole Digestive Tract. Anim. Int. J. Anim. Biosci. 2008, 2, 536–547. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, J.; Fan, H.M.; Xia, S.Q.; Shao, J.H.; Tang, T.; Chen, L.; Bai, X.; Sun, W.Q.; Jia, X.B.; Chen, S.Y.; et al. Microbiome, Transcriptome, and Metabolomic Analyses Revealed the Mechanism of Immune Response to Diarrhea in Rabbits Fed Antibiotic-Free Diets. Front. Microbiol. 2022, 13, 888984. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Mussard, E.; Barilly, C.; Lencina, C.; Gress, L.; Painteaux, L.; Gabinaud, B.; Cauquil, L.; Aymard, P.; Canlet, C.; et al. Developmental Stage, Solid Food Introduction, and Suckling Cessation Differentially Influence the Comaturation of the Gut Microbiota and Intestinal Epithelium in Rabbits. J. Nutr. 2022, 152, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Zhou, J.; Dong, Z.L.; Li, G.Y.; Wang, J.J.; Li, Y.K.; Wan, D.; Yang, H.S.; Yin, Y.L. Effect of Dietary Copper on Intestinal Microbiota and Antimicrobial Resistance Profiles of in Weaned Piglets. Front. Microbiol. 2019, 10, 2808. [Google Scholar] [CrossRef] [PubMed]
- Di Giancamillo, A.; Rossi, R.; Martino, P.A.; Aidos, L.; Maghin, F.; Domeneghini, C.; Corino, C. Copper Sulphate Forms in Piglet Diets: Microbiota, Intestinal Morphology and Enteric Nervous System Glial Cells. Anim. Sci. J. Nihon Chikusan Gakkaiho 2018, 89, 616–624. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, M.; Paës, C.; Mussard, E.; Knudsen, C.; Cauquil, L.; Aymard, P.; Barilly, C.; Gabinaud, B.; Zemb, O.; Fourre, S.; et al. Gut Microbiota Derived Metabolites Contribute to Intestinal Barrier Maturation at the Suckling-to-Weaning Transition. Gut Microbes 2020, 11, 1268–1286. [Google Scholar] [CrossRef] [PubMed]
- Agradi, S.; Cremonesi, P.; Menchetti, L.; Balzaretti, C.; Severgnini, M.; Riva, F.; Castiglioni, B.; Draghi, S.; Di Giancamillo, A.; Castrica, M.; et al. Bovine Colostrum Supplementation Modulates the Intestinal Microbial Community in Rabbits. Animals 2023, 13, 976. [Google Scholar] [CrossRef]
- Paganin, A.C.L.; Monzani, P.S.; Carazzolle, M.F.; Araújo, R.B.; Gonzalez-Esquerra, R.; Haese, D.; Kill, J.L.; Rezende, G.S.; de Lima, C.G.; Malavazi, I.; et al. Assessment of Cecal Microbiota Modulation from Piglet Dietary Supplementation with Copper. BMC Microbiol. 2023, 23, 92. [Google Scholar] [CrossRef] [PubMed]
- Forouzandeh, A.; Blavi, L.; Abdelli, N.; Melo-Duran, D.; Vidal, A.; Rodríguez, M.; Monteiro, A.N.T.R.; Pérez, J.F.; Darwich, L.; Solà-Oriol, D. Effects of Dicopper Oxide and Copper Sulfate on Growth Performance and Gut Microbiota in Broilers. Poult. Sci. 2021, 100, 101224. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wen, J.S.; Jiao, L.F.; Wang, C.C.; Hong, Q.H.; Feng, J.; Hu, C.H. Dietary Copper/Zinc-Loaded Montmorillonite Improved Growth Performance and Intestinal Barrier and Changed Gut Microbiota in Weaned Piglets. J. Anim. Physiol. Anim. Nutr. 2021, 105, 678–686. [Google Scholar] [CrossRef]
- Nguyen, H.T.T.; Kheravii, S.K.; Wu, S.-B.; Roberts, J.R.; Swick, R.A.; Toghyani, M. Sources and Levels of Copper Affect Liver Copper Profile, Intestinal Morphology and Cecal Microbiota Population of Broiler Chickens Fed Wheat-Soybean Meal Diets. Sci. Rep. 2022, 12, 2249. [Google Scholar] [CrossRef]
- Du, Y.A.; Tu, Y.; Zhou, Z.Y.; Hong, R.; Yan, J.Y.; Zhang, G.W. Effects of Organic and Inorganic Copper on Cecal Microbiota and Short-Chain Fatty Acids in Growing Rabbits. Front. Vet. Sci. 2023, 10, 1179374. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Ge, F.; Yao, X.X.; Guo, X.; Bao, P.J.; Ma, X.M.; Wu, X.Y.; Chu, M.; Yan, P.; Liang, C.N. Microbiome and Metabolomics Reveal the Effects of Different Feeding Systems on the Growth and Ruminal Development of Yaks. Front. Microbiol. 2021, 12, 682989. [Google Scholar] [CrossRef]
- Li, L.; Xiong, L.S.; Yao, J.H.; Zhuang, X.J.; Zhang, S.H.; Yu, Q.; Xiao, Y.L.; Cui, Y.; Chen, M.H. Increased Small Intestinal Permeability and RNA Expression Profiles of Mucosa from Terminal Ileum in Patients with Diarrhoea-Predominant Irritable Bowel Syndrome. Dig. Liver Dis. 2016, 48, 880–887. [Google Scholar] [CrossRef]
- Fukumoto, S.; Tatewaki, M.; Yamada, T.; Fujimiya, M.; Mantyh, C.; Voss, M.; Eubanks, S.; Harris, M.; Pappas, T.N.; Takahashi, T. Short-Chain Fatty Acids Stimulate Colonic Transit Via Intraluminal 5-HT Release in Rats. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2003, 284, R1269–R1276. [Google Scholar] [CrossRef]
- Abdelli, N.; Solà-Oriol, D.; Pérez, J.F. Phytogenic Feed Additives in Poultry: Achievements, Prospective and Challenges. Animals 2021, 11, 3471. [Google Scholar] [CrossRef]
- Gao, Y.-Y.; Zhou, Y.-H.; Liu, X.-P.; Di, B.; He, J.-Y.; Wang, Y.-T.; Guo, P.-T.; Zhang, J.; Wang, C.-K.; Jin, L. Ganoderma Lucidum Polysaccharide Promotes Broiler Health by Regulating Lipid Metabolism, Antioxidants, and Intestinal Microflora. Int. J. Biol. Macromol. 2024, 280, 135918. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Qin, L.; Zhang, F.; Fan, X.; Jin, H.; Du, Z.; Guo, Y.; Liu, W.; Liu, Q. Effects of JUNCAO Ganoderma lucidum Polysaccharide Peptide on Slaughter Performance and Intestinal Health of Minxinan Black Rabbits. Anim. Biotechnol. 2024, 35, 2259436. [Google Scholar] [CrossRef]
- Lebeloane, M.M.; Famuyide, I.M.; Kgosana, K.G.; Elgorashi, E.; Ndivhuwo, K.K.; Maharaj, V.; McGaw, L.J. Anti-Inflammatory Activity of Seven Plant Species with Potential Use as Livestock Feed Additives. S. Afr. J. Bot. 2024, 167, 322–332. [Google Scholar] [CrossRef]

| Items | Content |
|---|---|
| Ingredients | |
| Alfalfa meal | 38.00 |
| Corn | 9.00 |
| Wheat bran | 16.90 |
| Wheat DDGS | 6.00 |
| Rice husk powder | 9.00 |
| Soybean meal | 5.00 |
| Rice bran meal | 10.00 |
| Wheat middling | 3.00 |
| Limestone | 1.20 |
| Methionine | 0.10 |
| Lysine | 0.20 |
| NaCl | 0.60 |
| Premix 1 | 1.00 |
| Total | 100.00 |
| Nutrient levels 2 | |
| Digestible energy/(MJ/kg) | 9.96 |
| Crude protein | 15.48 |
| Crude fiber | 17.35 |
| Neutral detergent fiber | 33.56 |
| Acid detergent fiber | 20.51 |
| Acid detergent lignin | 5.90 |
| Calcium | 0.90 |
| Total phosphorus | 0.51 |
| Lysine | 0.74 |
| Methionine + Cystine | 0.51 |
| Gene | Primer Sequence (5′→3′) | Gene Bank |
|---|---|---|
| GADPH | F:5′-GGTAGTGAAGGCTGCTGCTGATG-3′ | NC_013676.1 |
| R:5′-GTCTCGCACTCCAATCTCTGTTCC-3′ | ||
| SOD1 | F:5′-AGTTCGACACCGACCTGAAG-3′ | NC_013682.1 |
| R:5′-TCCTGGTATTGAGGGCTGTC-3′ | ||
| CER | F:5′-TATGGCAACAGAGTGGCTCG-3′ | NC_013682.1 |
| R:5′-GCTGGGTGGGTAGGATGTTT-3′ | ||
| IL-1β | F:5′-TCTGCAACACCTGGGATGAC-3′ | NC_013670.1 |
| R:5′-TCAGCTCATACGTGCCAGAC-3′ | ||
| IL-6 | F:5′-ACGATCCACTTCATCCTGCG-3′ | NC_013678.1 |
| R:5′-GGATGGTGTGTTCTGACCGT-3′ | ||
| IL-10 | F:5′-TCACCGATTTTCTCCCCTGTG-3′ | NC_013684.1 |
| R:5′-ATGTCAAACTCATGGCTT-3′ |
| Items 2 | Groups 1 | p-Value | ||||
|---|---|---|---|---|---|---|
| CON | SAL | NCCC I | NCCC II | NCCC III | ||
| MDA (nmol/mg) | 1.979 ± 0.115 a | 1.850 ± 0.310 ab | 1.594 ± 0.162 b | 1.627 ± 0.232 b | 1.726 ± 0.173 ab | 0.000 |
| CER/(U/L) | 17.39 ± 1.41 a | 17.09 ± 2.56 a | 14.72 ± 0.77 b | 14.98 ± 1.41 b | 16.38 ± 1.24 ab | 0.004 |
| GSH-Px (pg/mL) | 29.37 ± 1.56 | 28.09 ± 3.41 | 27.23 ± 1.50 | 27.13 ± 3.07 | 27.37 ± 1.72 | 0.134 |
| Cu/Zn-SOD (pg/mL) | 41.44 ± 0.81 a | 40.32 ± 6.13 ab | 35.64 ± 3.83 b | 37.29 ± 4.26 b | 39.29 ± 4.17 ab | 0.006 |
| Items 2 | Groups 1 | p-Value | ||||
|---|---|---|---|---|---|---|
| CON | SAL | NCCC I | NCCC II | NCCC III | ||
| CER/(U/L) | 1.00 ± 0.25 a | 0.51 ± 0.13 b | 0.46 ± 0.11 b | 0.43 ± 0.19 b | 0.60 ± 0.17 ab | 0.015 |
| Cu/Zn-SOD/(pg/mL) | 1.00 ± 0.46 b | 1.99 ± 0.57 a | 1.10 ± 0.32 b | 1.81 ± 0.27 b | 1.98 ± 0.27 a | 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.-H.; Liu, X.-P.; Gu, X.-M.; Lv, H.-X.; Yang, Y.; Cai, Z.-X.; Di, B.; Wang, C.-K.; Gao, Y.-Y.; Jin, L. Effects of Dietary Nano-Composite of Copper and Carbon on Antioxidant Capacity, Immunity, and Cecal Microbiota of Weaned Ira White Rabbits. Animals 2025, 15, 184. https://doi.org/10.3390/ani15020184
Zhou Y-H, Liu X-P, Gu X-M, Lv H-X, Yang Y, Cai Z-X, Di B, Wang C-K, Gao Y-Y, Jin L. Effects of Dietary Nano-Composite of Copper and Carbon on Antioxidant Capacity, Immunity, and Cecal Microbiota of Weaned Ira White Rabbits. Animals. 2025; 15(2):184. https://doi.org/10.3390/ani15020184
Chicago/Turabian StyleZhou, Ying-Huan, Xiao-Ping Liu, Xiao-Ming Gu, Hai-Xuan Lv, Yun Yang, Zai-Xing Cai, Bin Di, Chang-Kang Wang, Yu-Yun Gao, and Ling Jin. 2025. "Effects of Dietary Nano-Composite of Copper and Carbon on Antioxidant Capacity, Immunity, and Cecal Microbiota of Weaned Ira White Rabbits" Animals 15, no. 2: 184. https://doi.org/10.3390/ani15020184
APA StyleZhou, Y.-H., Liu, X.-P., Gu, X.-M., Lv, H.-X., Yang, Y., Cai, Z.-X., Di, B., Wang, C.-K., Gao, Y.-Y., & Jin, L. (2025). Effects of Dietary Nano-Composite of Copper and Carbon on Antioxidant Capacity, Immunity, and Cecal Microbiota of Weaned Ira White Rabbits. Animals, 15(2), 184. https://doi.org/10.3390/ani15020184






