Short- But Not Long-Term Effects of Creep Feeding Provided to Suckling Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethic Statement
2.2. Farrowing and Lactation
2.3. Weaning, Growing, and Fattening
2.4. Carcass and Meat Quality
2.5. Rectal Microbiome Extraction
2.6. Statistical Analysis
2.6.1. Farm, Carcass, and Meat Quality Data
2.6.2. Microbiome Data
3. Results
3.1. Mortality
3.2. Weight and Gains
3.3. Carcass and Meat Quality Characteristics
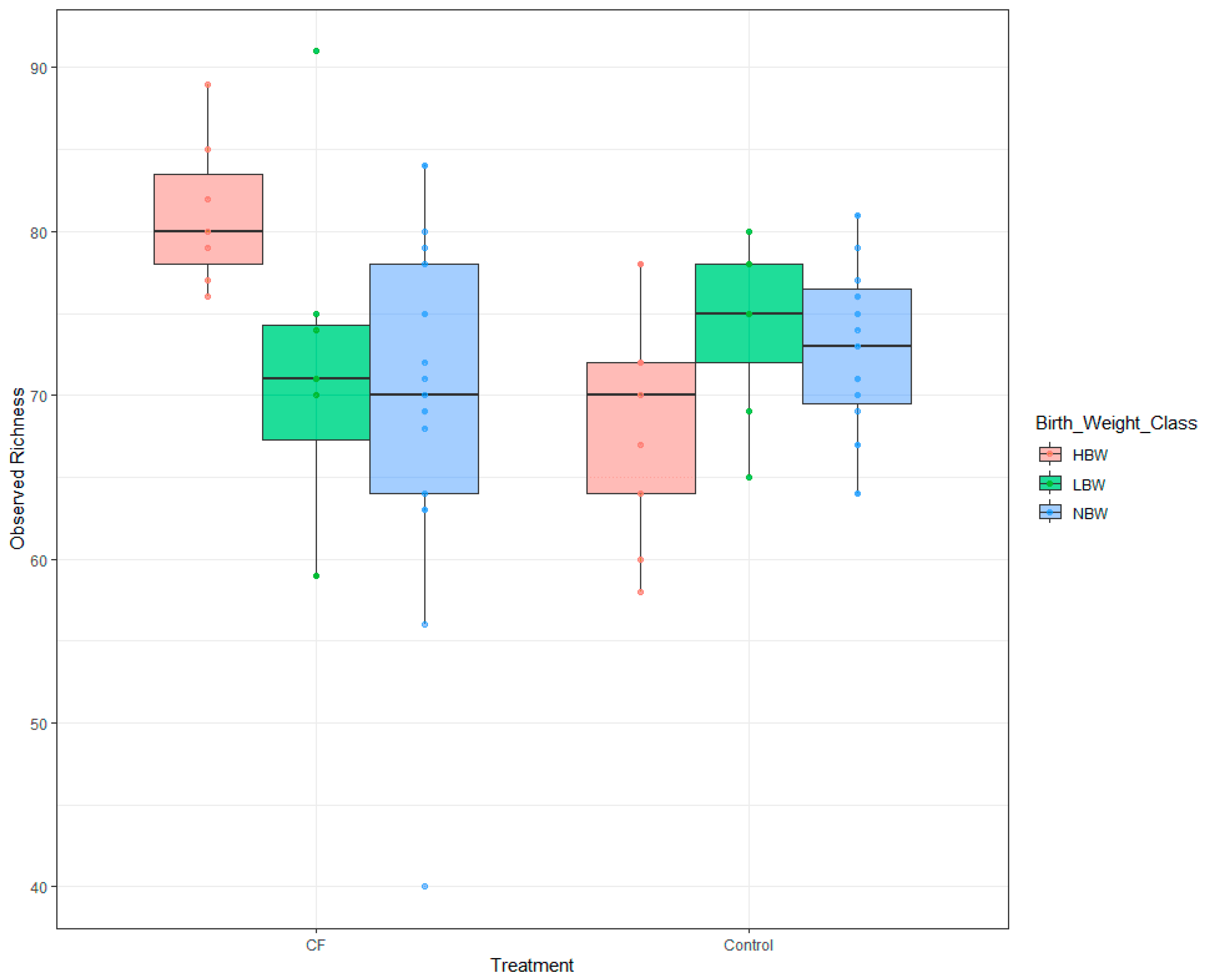
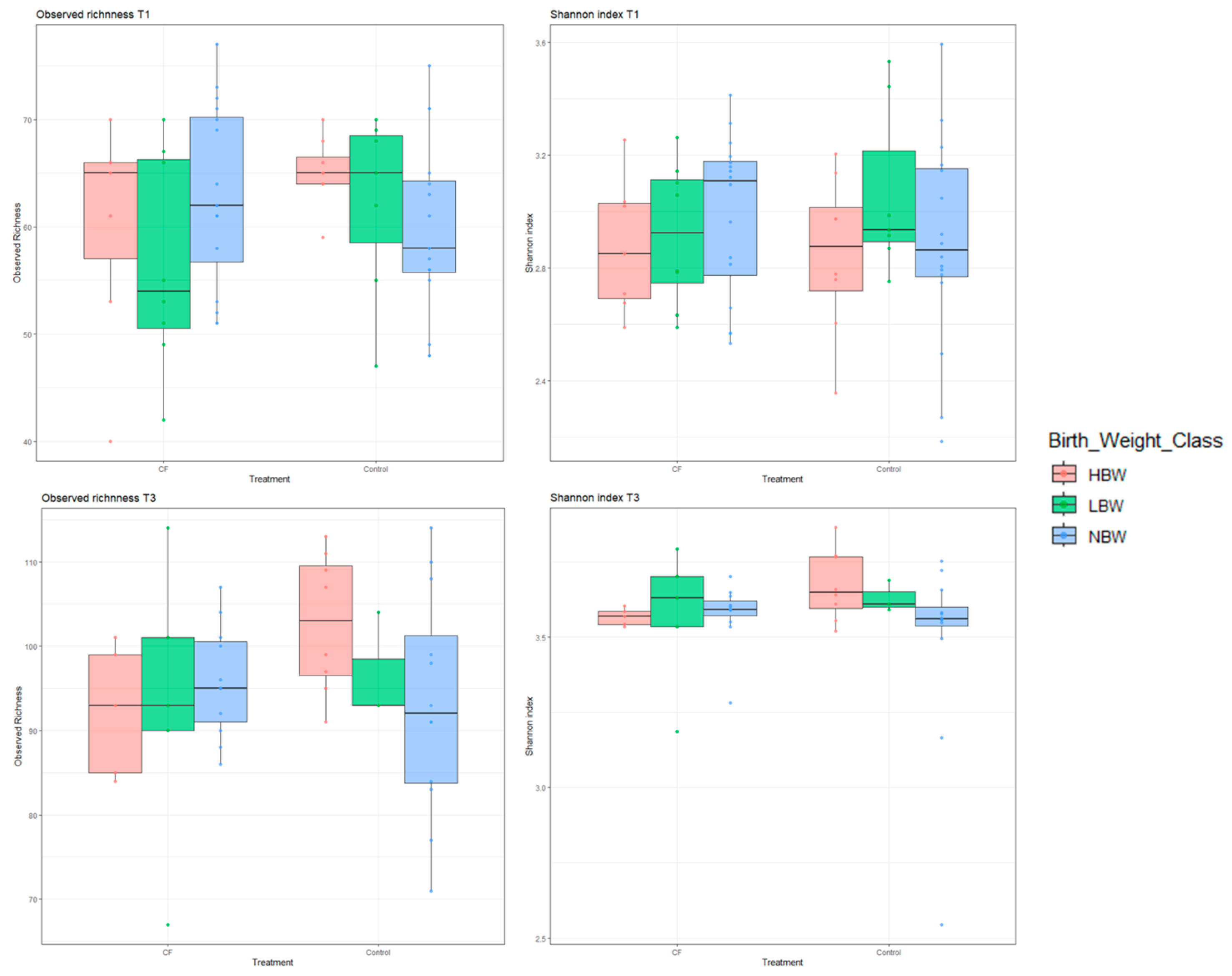
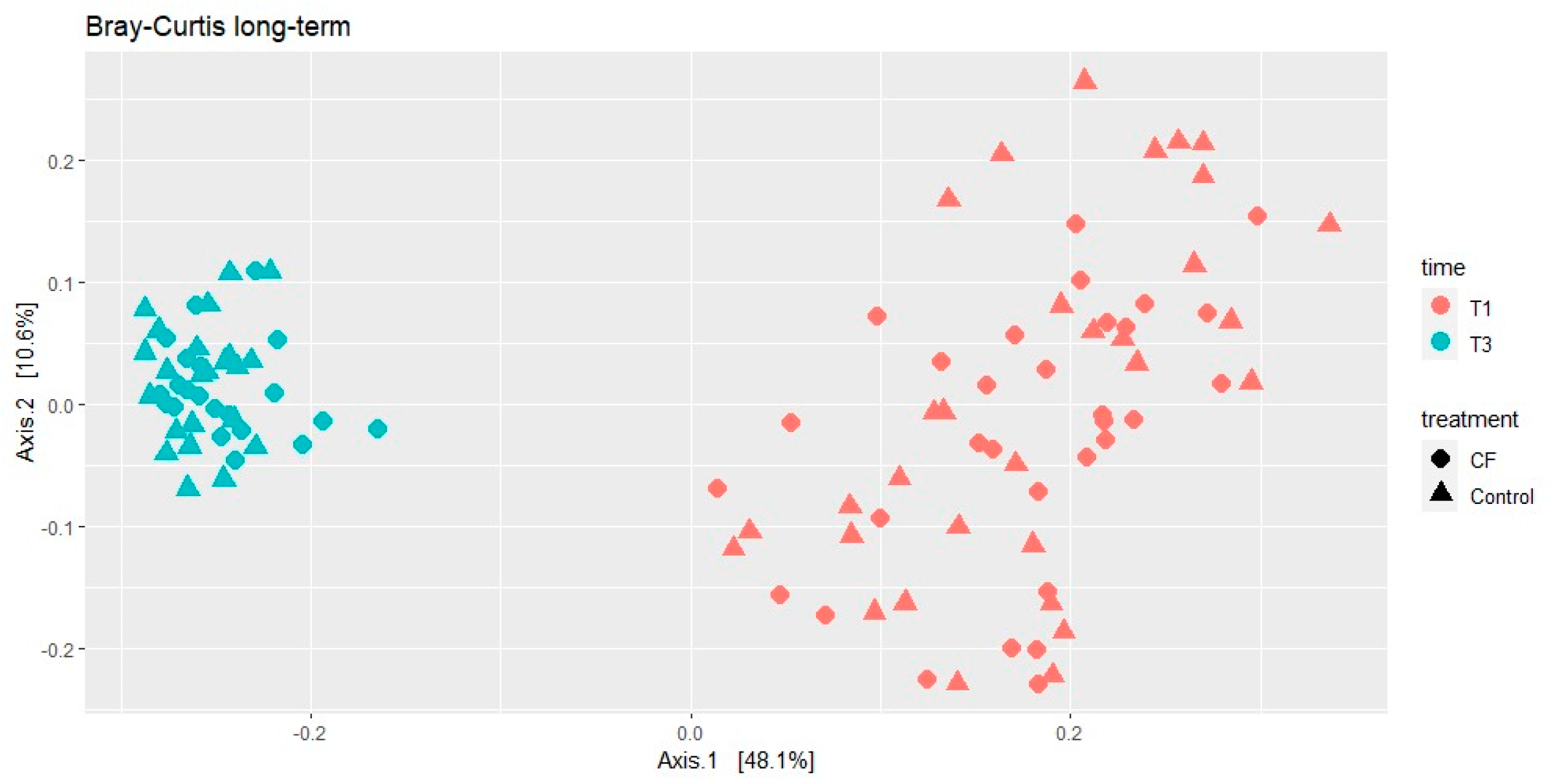
3.4. Microbiome Analyses
3.4.1. Short-Term Effects
3.4.2. Long-Term Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beaulieu, A.D.; Aalhus, J.L.; Williams, N.H.; Patience, J.F. Impact of Piglet Birth Weight, Birth Order, and Litter Size on Subsequent Growth Performance, Carcass Quality, Muscle Composition, and Eating Quality of Pork1. J. Anim. Sci. 2010, 88, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Feldpausch, J.A.; Jourquin, J.; Bergstrom, J.R.; Bargen, J.L.; Bokenkroger, C.D.; Davis, D.L.; Gonzalez, J.M.; Nelssen, J.L.; Puls, C.L.; Trout, W.E.; et al. Birth Weight Threshold for Identifying Piglets at Risk for Preweaning Mortality. Transl. Anim. Sci. 2019, 3, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Morise, A.; Louveau, I.; Le Huërou-Luron, I. Growth and Development of Adipose Tissue and Gut and Related Endocrine Status during Early Growth in the Pig: Impact of Low Birth Weight. Animal 2008, 2, 73–83. [Google Scholar] [CrossRef]
- Michiels, J.; De Vos, M.; Missotten, J.; Ovyn, A.; De Smet, S.; Van Ginneken, C. Maturation of Digestive Function Is Retarded and Plasma Antioxidant Capacity Lowered in Fully Weaned Low Birth Weight Piglets. Br. J. Nutr. 2013, 109, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Declerck, I.; Sarrazin, S.; Dewulf, J.; Maes, D. Sow and Piglet Factors Determining Variation of Colostrum Intake between and within Litters. Anim. Int. J. Anim. Biosci. 2017, 11, 1336–1343. [Google Scholar] [CrossRef]
- Edwards, S.A.; Baxter, E.M. 11. Piglet Mortality: Causes and Prevention. In The Gestating and Lactating Sow; Wageningen Academic Publishers: Wageningen, The Netherlands, 2014; pp. 253–278. ISBN 978-90-8686-253-5. [Google Scholar]
- Christison, G.I.; Wenger, I.I.; Follensbee, M.E. Teat Seeking Success of Newborn Piglets after Drying or Warming. Can. J. Anim. Sci. 1997, 77, 317–319. [Google Scholar] [CrossRef]
- Quesnel, H.; Farmer, C.; Devillers, N. Colostrum Intake: Influence on Piglet Performance and Factors of Variation. Livest. Sci. 2012, 146, 105–114. [Google Scholar] [CrossRef]
- Blavi, L.; Solà-Oriol, D.; Llonch, P.; López-Vergé, S.; Martín-Orúe, S.M.; Pérez, J.F. Management and Feeding Strategies in Early Life to Increase Piglet Performance and Welfare around Weaning: A Review. Animals 2021, 11, 302. [Google Scholar] [CrossRef]
- Romero, M.; Calvo, L.; Morales, J.I.; Rodríguez, A.I.; Escudero, R.M.; Olivares, Á.; López-Bote, C. Short- and Long-Term Effects of Birth Weight and Neonatal Care in Pigs. Anim. Open Access J. 2022, 12, 2936. [Google Scholar] [CrossRef]
- Romero, M.; Calvo, L.; Morales, J.I.; Magro, A.; Rodríguez, A.I.; Segura, J.; Escudero, R.; López-Bote, C.; Olivares, Á. Short- and Long-Term Effects of Split-Suckling in Pigs According to Birth Weight. Animals 2023, 13, 3521. [Google Scholar] [CrossRef] [PubMed]
- Muns, R.; Magowan, E. The Effect of Creep Feed Intake and Starter Diet Allowance on Piglets’ Gut Structure and Growth Performance after Weaning. J. Anim. Sci. 2018, 96, 3815–3823. [Google Scholar] [CrossRef]
- Sulabo, R.C.; Jacela, J.Y.; Tokach, M.D.; Dritz, S.S.; Goodband, R.D.; DeRouchey, J.M.; Nelssen, J.L. Effects of Lactation Feed Intake and Creep Feeding on Sow and Piglet Performance. J. Anim. Sci. 2010, 88, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Sands, J.M.; Rodrigues, L.A.; Wellington, M.O.; Panisson, J.C.; Columbus, D.A. Pre- and Post-Weaning Performance of Piglets Offered Different Types of Creep Feed. Can. J. Anim. Sci. 2022, 102, 189–193. [Google Scholar] [CrossRef]
- Craig, J.; Kim, J.; Brewster, C.; Smits, R.; Braden, C.; Pluske, J. Increasing Creep Pellet Size Improves Creep Feed Disappearance of Gilt and Sow Progeny in Lactation and Enhances Pig Production after Weaning. J. Swine Health Prod. 2021, 29, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, P.; Luise, D.; Correa, F.; Bosi, P. Timely Control of Gastrointestinal Eubiosis: A Strategic Pillar of Pig Health. Microorganisms 2021, 9, 313. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, R.; Middelkoop, A.; Boekhorst, J.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E.; Kleerebezem, M. Early Life Feeding Accelerates Gut Microbiome Maturation and Suppresses Acute Post-weaning Stress in Piglets. Environ. Microbiol. 2021, 23, 7201–7213. [Google Scholar] [CrossRef] [PubMed]
- Strathe, A.V.; Bruun, T.S.; Hansen, C.F. Sows with High Milk Production Had Both a High Feed Intake and High Body Mobilization. Animal 2017, 11, 1913–1921. [Google Scholar] [CrossRef]
- Middelkoop, A.; Choudhury, R.; Gerrits, W.J.J.; Kemp, B.; Kleerebezem, M.; Bolhuis, J.E. Dietary Diversity Affects Feeding Behaviour of Suckling Piglets. Appl. Anim. Behav. Sci. 2018, 205, 151–158. [Google Scholar] [CrossRef]
- Klindt, J. Influence of Litter Size and Creep Feeding on Preweaning Gain and Influence of Preweaning Growth on Growth to Slaughter in Barrows1,2. J. Anim. Sci. 2003, 81, 2434–2439. [Google Scholar] [CrossRef] [PubMed]
- Boston, T.E.; Wang, F.; Lin, X.; Leonard, S.; Kim, S.W.; McKilligan, D.; Fellner, V.; Odle, J. Gruel Creep Feeding Accelerates Growth and Alters Intestinal Health of Young Pigs. Animals 2022, 12, 2408. [Google Scholar] [CrossRef] [PubMed]
- Huting, A.M.S.; Almond, K.; Wellock, I.; Kyriazakis, I. What Is Good for Small Piglets Might Not Be Good for Big Piglets: The Consequences of Cross-Fostering and Creep Feed Provision on Performance to Slaughter. J. Anim. Sci. 2017, 95, 4926–4944. [Google Scholar] [CrossRef]
- Branscheid, W.; Dobrowolski, A.; Sack, E. Simplification of the EC Reference Method for the Full Dissection of Pig Carcasses. Fleischwirtschaft 1990, 70, 565–567. [Google Scholar]
- Segura, J.; Lopez-Bote, C.J. A Laboratory Efficient Method for Intramuscular Fat Analysis. Food Chem. 2014, 145, 821–825. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; Stevenes, M.; Wagner, H. Vegan: Community Ecology Package R Package; version 2.0-2; 2012.
- Wickham, H. Ggplot2; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-24275-0. [Google Scholar]
- Zhou, H.; He, K.; Chen, J.; Zhang, X. LinDA: Linear Models for Differential Abundance Analysis of Microbiome Compositional Data. Genome Biol. 2022, 23, 95. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-Infectious Causes of Pre-Weaning Mortality in Piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Rekiel, A.; Więcek, J.; Batorska, M.; Kulisiewicz, J. Effect of Piglet Birth Weight on Carcass Muscle and Fat Content and Pork Quality—A Review. Ann. Anim. Sci. 2015, 15, 271–287. [Google Scholar] [CrossRef]
- Vanden Hole, C.; Ayuso, M.; Aerts, P.; Prims, S.; Van Cruchten, S.; Van Ginneken, C. Glucose and Glycogen Levels in Piglets That Differ in Birth Weight and Vitality. Heliyon 2019, 5, e02510. [Google Scholar] [CrossRef] [PubMed]
- Gondret, F.; Lefaucheur, L.; Juin, H.; Louveau, I.; Lebret, B. Low Birth Weight Is Associated with Enlarged Muscle Fiber Area and Impaired Meat Tenderness of the Longissimus Muscle in Pigs. J. Anim. Sci. 2006, 84, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Wolter, B.F.; Ellis, M.; Corrigan, B.P.; DeDecker, J.M. The Effect of Birth Weight and Feeding of Supplemental Milk Replacer to Piglets during Lactation on Preweaning and Postweaning Growth Performance and Carcass Characteristics. J. Anim. Sci. 2002, 80, 301–308. [Google Scholar] [CrossRef]
- Van Den Brand, H.; Wamsteeker, D.; Oostindjer, M.; Van Enckevort, L.C.M.; Van Der Poel, A.F.B.; Kemp, B.; Bolhuis, J.E. Effects of Pellet Diameter during and after Lactation on Feed Intake of Piglets Pre- and Postweaning1. J. Anim. Sci. 2014, 92, 4145–4153. [Google Scholar] [CrossRef]
- Pluske, J.R.; Kerton, D.K.; Cranwell, P.D.; Campbell, R.G.; Mullan, B.P.; King, R.H.; Power, G.N.; Pierzynowski, S.G.; Westrom, B.; Rippe, C.; et al. Age, Sex, and Weight at Weaning Influence Organ Weight and Gastrointestinal Development of Weanling Pigs. Aust. J. Agric. Res. 2003, 54, 515–527. [Google Scholar] [CrossRef]
- Gong, D.; Gong, X.; Wang, L.; Yu, X.; Dong, Q. Involvement of Reduced Microbial Diversity in Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2016, 2016, e6951091. [Google Scholar] [CrossRef] [PubMed]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet Shapes the Gut Microbiome of Pigs during Nursing and Weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef]
- De Rodas, B.; Youmans, B.P.; Danzeisen, J.L.; Tran, H.; Johnson, T.J. Microbiome Profiling of Commercial Pigs from Farrow to Finish. J. Anim. Sci. 2018, 96, 1778–1794. [Google Scholar] [CrossRef]
- Holman, D.B.; Chénier, M.R. Temporal Changes and the Effect of Subtherapeutic Concentrations of Antibiotics in the Gut Microbiota of Swine. FEMS Microbiol. Ecol. 2014, 90, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Mach, N.; Berri, M.; Estellé, J.; Levenez, F.; Lemonnier, G.; Denis, C.; Leplat, J.-J.; Chevaleyre, C.; Billon, Y.; Doré, J.; et al. Early-Life Establishment of the Swine Gut Microbiome and Impact on Host Phenotypes. Environ. Microbiol. Rep. 2015, 7, 554–569. [Google Scholar] [CrossRef] [PubMed]
- Lerch, F.; Yosi, F.; Vötterl, J.C.; Koger, S.; Ehmig, J.; Sharma, S.; Verhovsek, D.; Metzler-Zebeli, B.U. An Insight into the Temporal Dynamics in the Gut Microbiome, Metabolite Signaling, Immune Response, and Barrier Function in Suckling and Weaned Piglets under Production Conditions. Front. Vet. Sci. 2023, 10, 1184277. [Google Scholar] [CrossRef] [PubMed]
- Muro, B.B.D.; Carnevale, R.F.; Monteiro, M.S.; Yao, R.; Ferreira, F.N.A.; Neta, C.S.S.; Pereira, F.A.; Maes, D.; Janssens, G.P.J.; Almond, G.W.; et al. A Systematic Review and Meta-Analysis of Creep Feeding Effects on Piglet Pre- and Post-Weaning Performance. Animals 2023, 13, 2156. [Google Scholar] [CrossRef] [PubMed]
- Bruininx, E.M.A.M.; Schellingerhout, A.B.; Binnendijk, G.P.; Van der Peet-Schwering, C.M.C.; Schrama, J.W.; Den Hartog, L.A.; Everts, H.; Beynen, A.C. Individually Assessed Creep Food Consumption by Suckled Piglets: Influence on Post-Weaning Food Intake Characteristics and Indicators of Gut Structure and Hind-Gut Fermentation. Anim. Sci. 2004, 78, 67–75. [Google Scholar] [CrossRef]
- Kuller, W.I.; Soede, N.M.; van Beers-Schreurs, H.M.G.; Langendijk, P.; Taverne, M.A.M.; Kemp, B.; Verheijden, J.H.M. Effects of Intermittent Suckling and Creep Feed Intake on Pig Performance from Birth to Slaughter. J. Anim. Sci. 2007, 85, 1295–1301. [Google Scholar] [CrossRef] [PubMed]
- Arnaud, E.A.; Gardiner, G.E.; Halpin, K.M.; Ribas, C.; O’Doherty, J.V.; Sweeney, T.; Lawlor, P.G. Postpartum Meloxicam Administration to Sows but Not Split-Suckling Increases Piglet Growth and Reduces Clinical Incidence of Disease in Suckling Piglets. J. Anim. Sci. 2023, 101, skad275. [Google Scholar] [CrossRef]
- George, A.D.; Burugupalli, S.; Paul, S.; Mansell, T.; Burgner, D.; Meikle, P.J. The Role of Human Milk Lipids and Lipid Metabolites in Protecting the Infant against Non-Communicable Disease. Int. J. Mol. Sci. 2022, 23, 7490. [Google Scholar] [CrossRef] [PubMed]


| Birth Weight (BW) | Treatment (T) | Parity (P) | Sex (S) | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LBW | NBW | HBW | Ctr | CF | PP | MP | F | CM | BW | T | P | S | |
| Birth to 7 days | 36.4 | 9.6 | 5.6 | 14.0 | 12.0 | 10.9 | 14.1 | 11.1 | 14.5 | 0.001 | 0.51 | 0.34 | 0.28 |
| CF to weaning | 4.1 | 2.8 | 0.0 | 1.6 | 3.7 | 3.3 | 2.1 | 2.5 | 2.3 | 0.062 | 0.11 | 0.47 | 0.86 |
| Weaning + 7 d | 0.0 | 2.2 | 1.2 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 1.7 | 0.453 | 0.97 | 0.96 | 0.98 |
| Cumulative mortality from birth | |||||||||||||
| Weaning | 39.0 | 12.5 | 5.6 | 15.2 | 15.9 | 14.5 | 15.9 | 13.1 | 17.0 | 0.001 | 0.95 | 0.45 | 0.33 |
| Weaning + 7 d | 39.0 | 14.1 | 6.7 | 16.7 | 16.8 | 15.2 | 17.4 | 14.6 | 18.1 | 0.001 | 0.96 | 0.57 | 0.32 |
| Treatment (T) | Parity (P) | Sex (S) | SD | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Ctr | CF | PP | MP | F | CM | T | P | S | ||
| Birth weight (kg) | 1.37 | 1.30 | 1.21 | 1.38 | 1.33 | 1.34 | 0.36 | 0.269 | 0.010 | 0.224 |
| Weaning weight (kg) | 5.72 | 5.69 | 5.18 | 5.90 | 5.72 | 5.70 | 1.48 | 0.836 | 0.021 | 0.377 |
| Nursery end weight (kg) | 29.20 | 28.14 | 26.92 | 29.49 | 23.18 | 29.27 | 6.77 | 0.706 | 0.073 | 0.003 |
| ADG lactation (g/d) | 164.73 | 165.30 | 152.60 | 169.59 | 164.98 | 165.07 | 49.5 | 0.868 | 0.091 | 0.421 |
| ADG nursery (g/d) | 674.10 | 666.17 | 619.79 | 697.94 | 521.86 | 685.93 | 164.6 | 0.869 | 0.066 | 0.004 |
| Birth Weight (BW) | Treatment (T) | Parity (P) | SD | p-Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LBW | NBW | HBW | Ctr | CF | PP | MP | BW | T | P | Cov-CW | ||
| Slaughter weight (kg) | 118.3 a | 131.1 b | 140.9 c | 130.5 | 130 | 123.8 | 132.8 | 12.3 | 0.001 | 0.304 | 0.058 | -- |
| Carcass weight (kg) | 92.7 a | 100.5 b | 107.7 c | 100.4 | 99.7 | 97.6 | 101.1 | 11.5 | 0.001 | 0.255 | 0.495 | -- |
| Carcass yield (%) | 77.2 | 77.1 | 76.7 | 77.4 | 76.7 | 77.8 | 76.7 | 7.7 | 0.859 | 0.875 | 0.713 | -- |
| Subcutaneous fat | ||||||||||||
| P2 (mm) | 21.45 | 20.67 | 20.18 | 20.78 | 20.76 | 20.25 | 21.28 | 3.43 | 0.662 | 0.978 | 0.28 | 0.001 |
| Ham (mm) | 15.61 | 14.99 | 14.25 | 15.03 | 14.87 | 14.89 | 15.01 | 2.39 | 0.386 | 0.819 | 0.861 | 0.001 |
| Lean (%) | 55.85 | 56.97 | 57.19 | 56.71 | 56.63 | 57.1 | 56.24 | 2.89 | 0.432 | 0.914 | 0.285 | 0.001 |
| Birth Weight (BW) | Treatment (T) | Parity (P) | SD | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LBW | NBW | HBW | Ctr | CF | PP | MP | BW | T | P | Cov-CW | |||
| IMF | 2.18 a | 1.83 b | 1.63 b | 1.80 | 1.96 | 1.74 | 2.02 | 0.47 | 0.009 | 0.245 | 0.044 | 0.01 | |
| Moisture | 74.13 b | 74.29 ab | 74.67 a | 74.43 | 74.30 | 74.51 | 74.22 | 0.50 | 0.038 | 0.388 | 0.049 | 0.00 | |
| Protein | 23.03 | 23.24 | 23.12 | 23.18 | 23.08 | 23.15 | 23.11 | 0.37 | 0.163 | 0.397 | 0.751 | 0.14 | |
| Ash | 1.74 | 1.76 | 1.72 | 1.74 | 1.74 | 1.75 | 1.74 | 0.05 | 0.050 | 0.973 | 0.620 | 0.24 | |
| pHu | 5.69 | 5.67 | 5.66 | 5.68 | 5.67 | 5.67 | 5.68 | 0.11 | 0.827 | 0.606 | 0.668 | 0.00 | |
| L value | 58.61 | 58.41 | 59.34 | 58.67 | 58.90 | 58.84 | 58.74 | 2.31 | 0.556 | 0.729 | 0.883 | 0.00 | |
| a value | 2.57 | 2.59 | 2.30 | 2.41 | 2.57 | 2.49 | 2.49 | 0.52 | 0.324 | 0.310 | 0.998 | 0.37 | |
| b value | 13.80 | 13.75 | 13.69 | 13.64 | 13.86 | 13.76 | 13.73 | 0.83 | 0.954 | 0.350 | 0.896 | 0.03 | |
| Fatty acids in subcutaneous fat | |||||||||||||
| C16:0 | 23.59 | 23.20 | 23.10 | 23.36 | 23.23 | 23.05 | 23.54 | 0.90 | 0.284 | 0.596 | 0.062 | 0.03 | |
| C18:0 | 12.59 | 12.56 | 12.68 | 12.76 | 12.46 | 12.56 | 12.66 | 0.64 | 0.861 | 0.115 | 0.599 | 0.12 | |
| C18:1n-9 | 38.72 | 38.57 | 37.63 | 38.06 | 38.55 | 37.78 | 38.83 | 1.99 | 0.371 | 0.398 | 0.070 | 0.61 | |
| C18:2n-6 | 10.90 | 11.36 | 12.20 | 11.55 | 11.42 | 12.01 | 10.96 | 1.88 | 0.242 | 0.817 | 0.057 | 0.08 | |
| Fatty acids in intramuscular fat | |||||||||||||
| C16:0 | 23.58 | 23.12 | 22.95 | 23.29 | 23.14 | 23.28 | 23.15 | 1.07 | 0.259 | 0.628 | 0.662 | 0.01 | |
| C18:0 | 12.37 | 12.42 | 12.85 | 12.65 | 12.44 | 12.71 | 12.38 | 1.28 | 0.611 | 0.566 | 0.366 | 0.11 | |
| C18:1n-9 | 41.28 | 40.53 | 41.00 | 41.14 | 40.73 | 41.19 | 40.69 | 4.87 | 0.867 | 0.768 | 0.722 | 0.63 | |
| C18:2n-6 | 12.63 | 13.24 | 13.50 | 13.03 | 13.22 | 12.92 | 13.33 | 1.48 | 0.266 | 0.658 | 0.335 | 0.04 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero, M.; Heras-Molina, A.; Muñoz, M.; Calvo, L.; Morales, J.I.; Rodríguez, A.I.; Escudero, R.; López-Bote, C.; Óvilo, C.; Olivares, Á. Short- But Not Long-Term Effects of Creep Feeding Provided to Suckling Piglets. Animals 2025, 15, 253. https://doi.org/10.3390/ani15020253
Romero M, Heras-Molina A, Muñoz M, Calvo L, Morales JI, Rodríguez AI, Escudero R, López-Bote C, Óvilo C, Olivares Á. Short- But Not Long-Term Effects of Creep Feeding Provided to Suckling Piglets. Animals. 2025; 15(2):253. https://doi.org/10.3390/ani15020253
Chicago/Turabian StyleRomero, María, Ana Heras-Molina, María Muñoz, Luis Calvo, José Ignacio Morales, Ana Isabel Rodríguez, Rosa Escudero, Clemente López-Bote, Cristina Óvilo, and Álvaro Olivares. 2025. "Short- But Not Long-Term Effects of Creep Feeding Provided to Suckling Piglets" Animals 15, no. 2: 253. https://doi.org/10.3390/ani15020253
APA StyleRomero, M., Heras-Molina, A., Muñoz, M., Calvo, L., Morales, J. I., Rodríguez, A. I., Escudero, R., López-Bote, C., Óvilo, C., & Olivares, Á. (2025). Short- But Not Long-Term Effects of Creep Feeding Provided to Suckling Piglets. Animals, 15(2), 253. https://doi.org/10.3390/ani15020253






