Comparison of Vaccination Regimens on Immune Responses Using PED Replicon Vaccine: A Field Trial in PED-Negative and PED-Positive Thai Swine Farms
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.1.1. Experiment 1
2.1.2. Experiment 2
2.2. Sample Collection
2.3. Evaluation of PEDV-Specific IgA and IgG in Serum and Colostrum, and Serum Neutralization
2.4. Statistical Analysis
3. Results
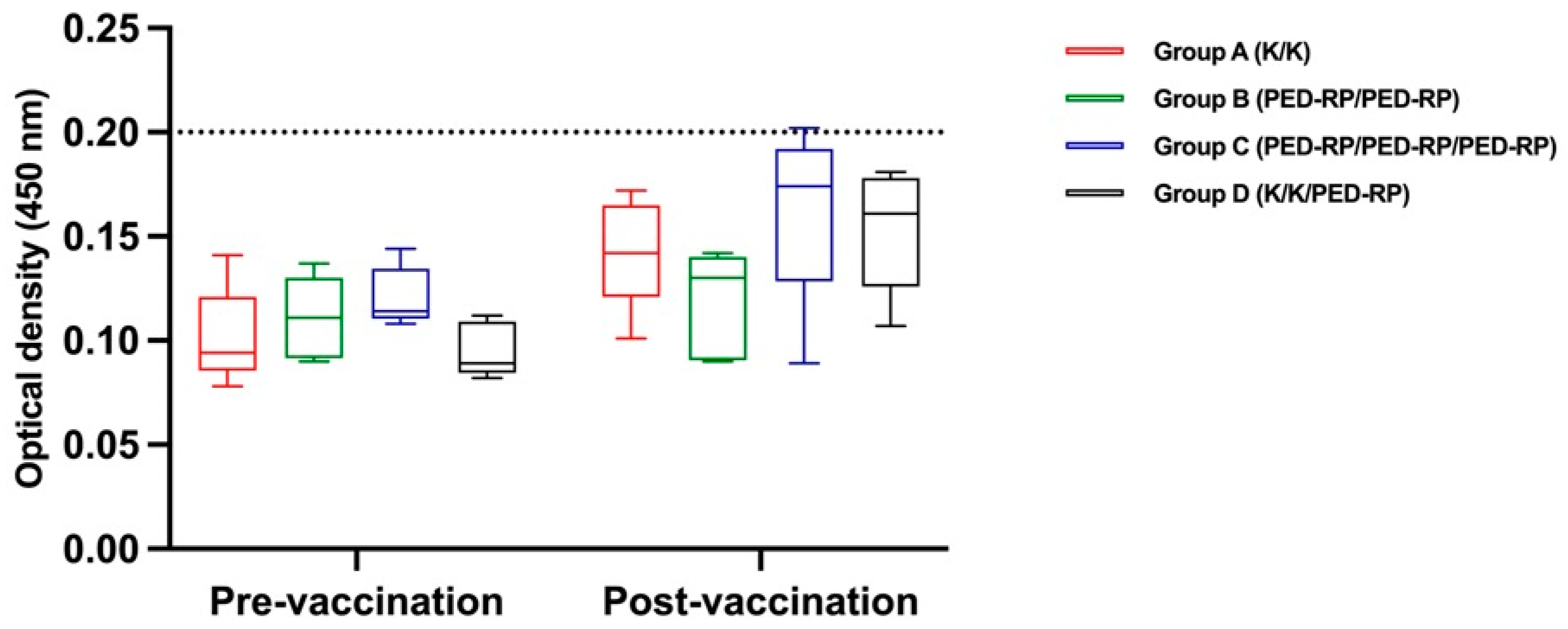
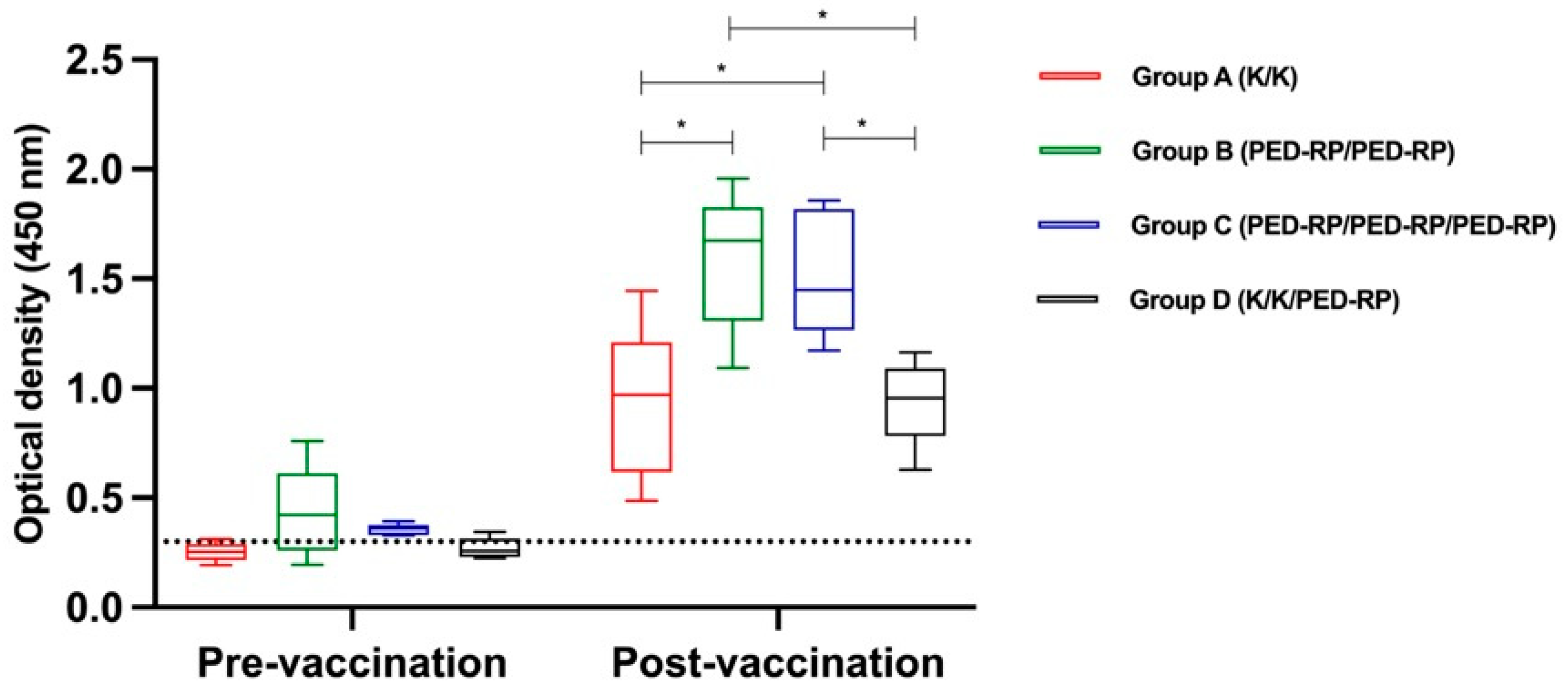
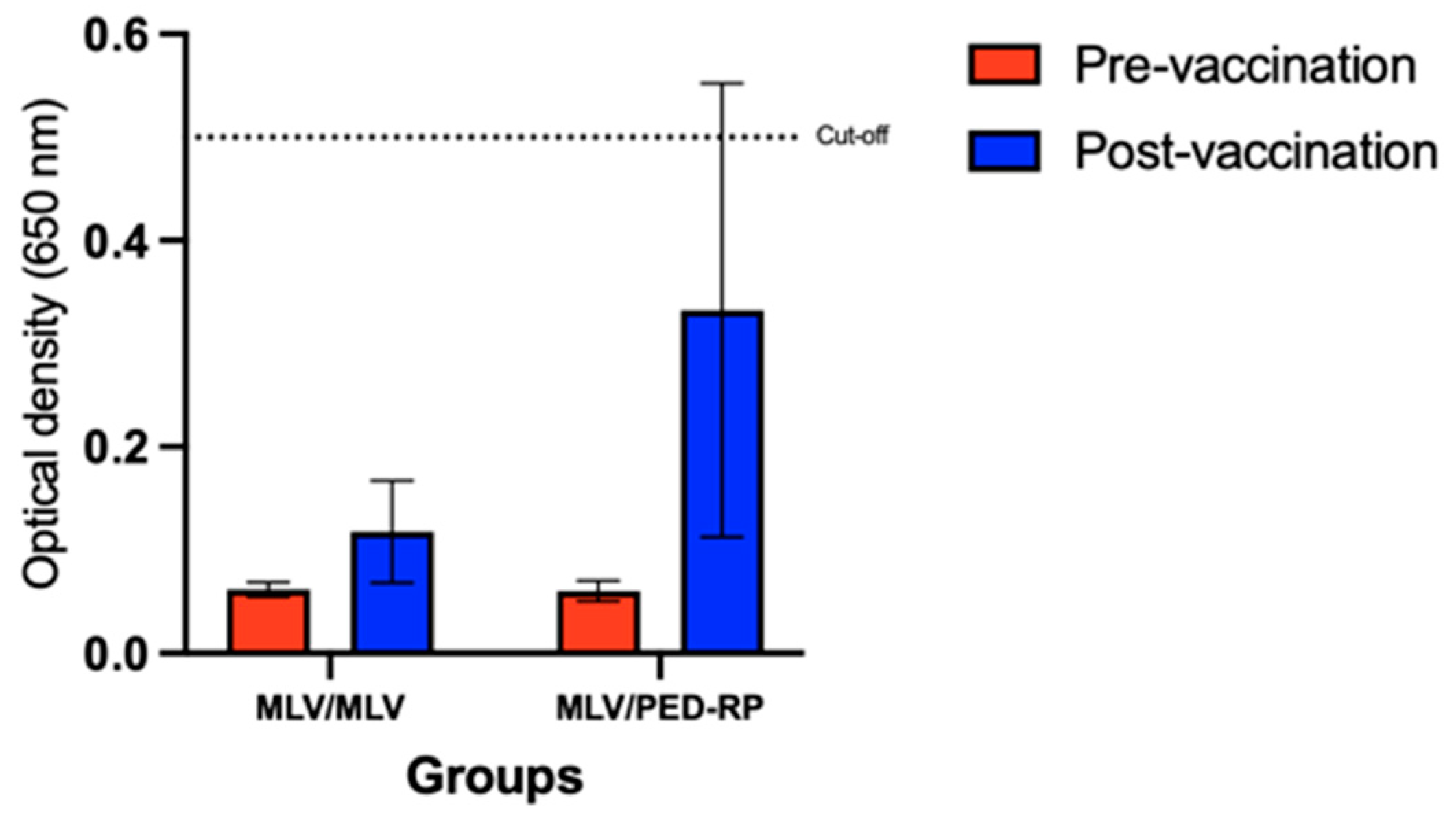
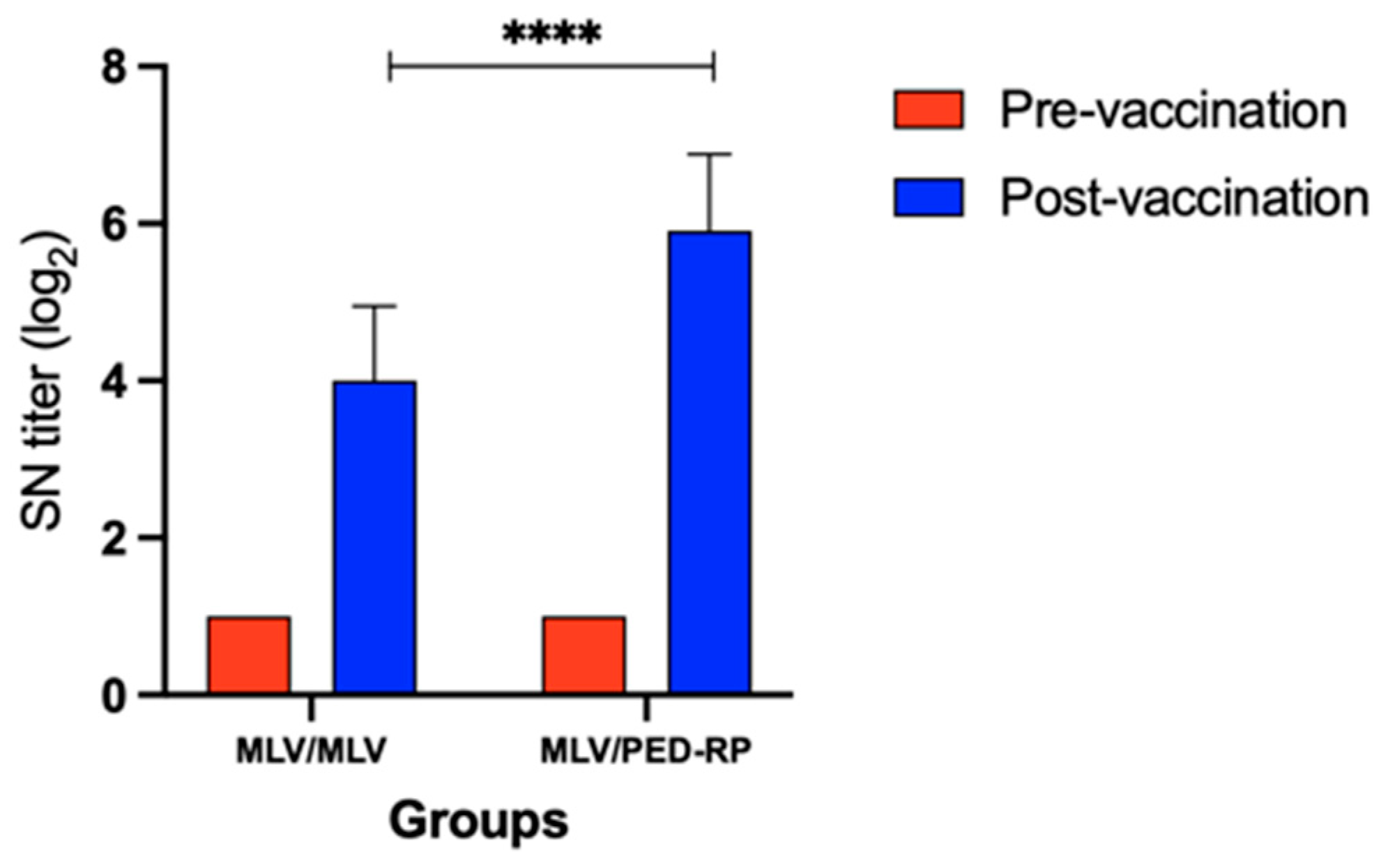
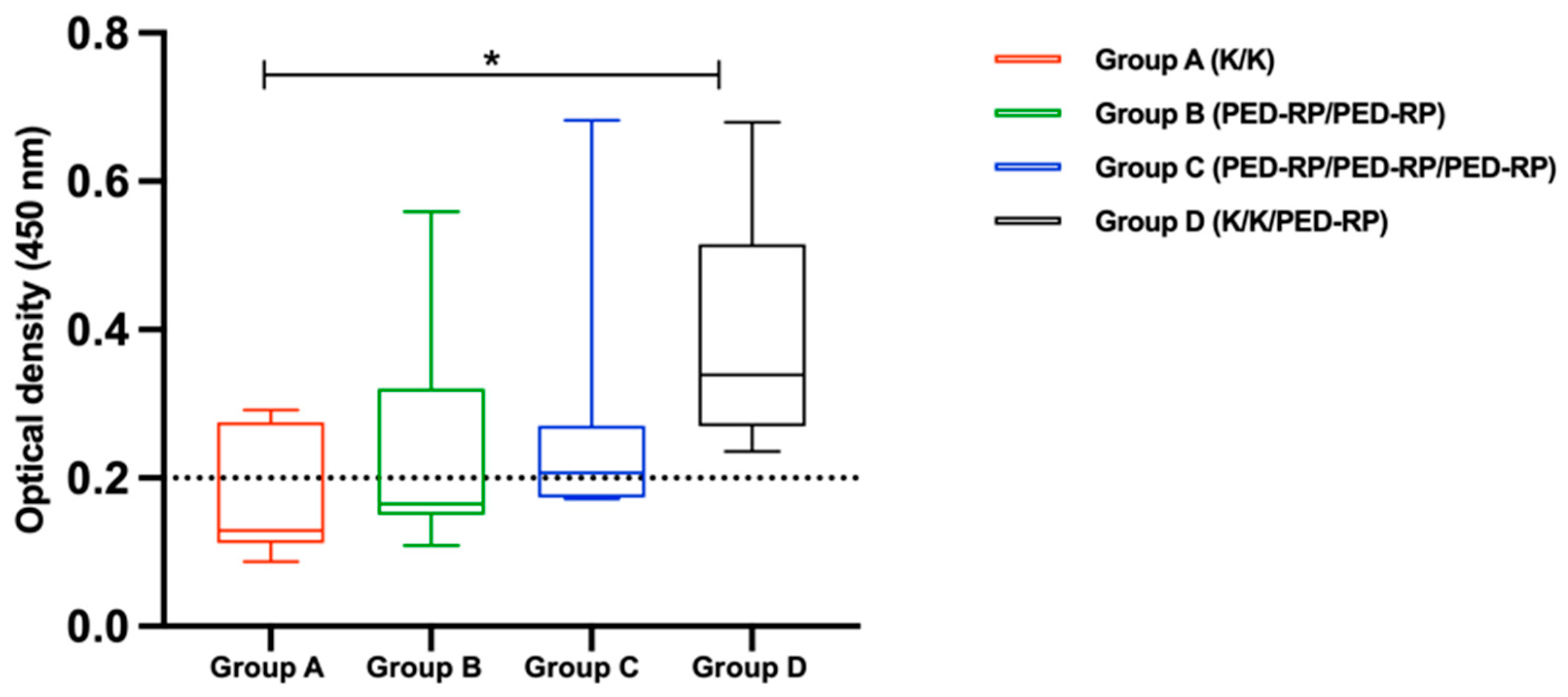
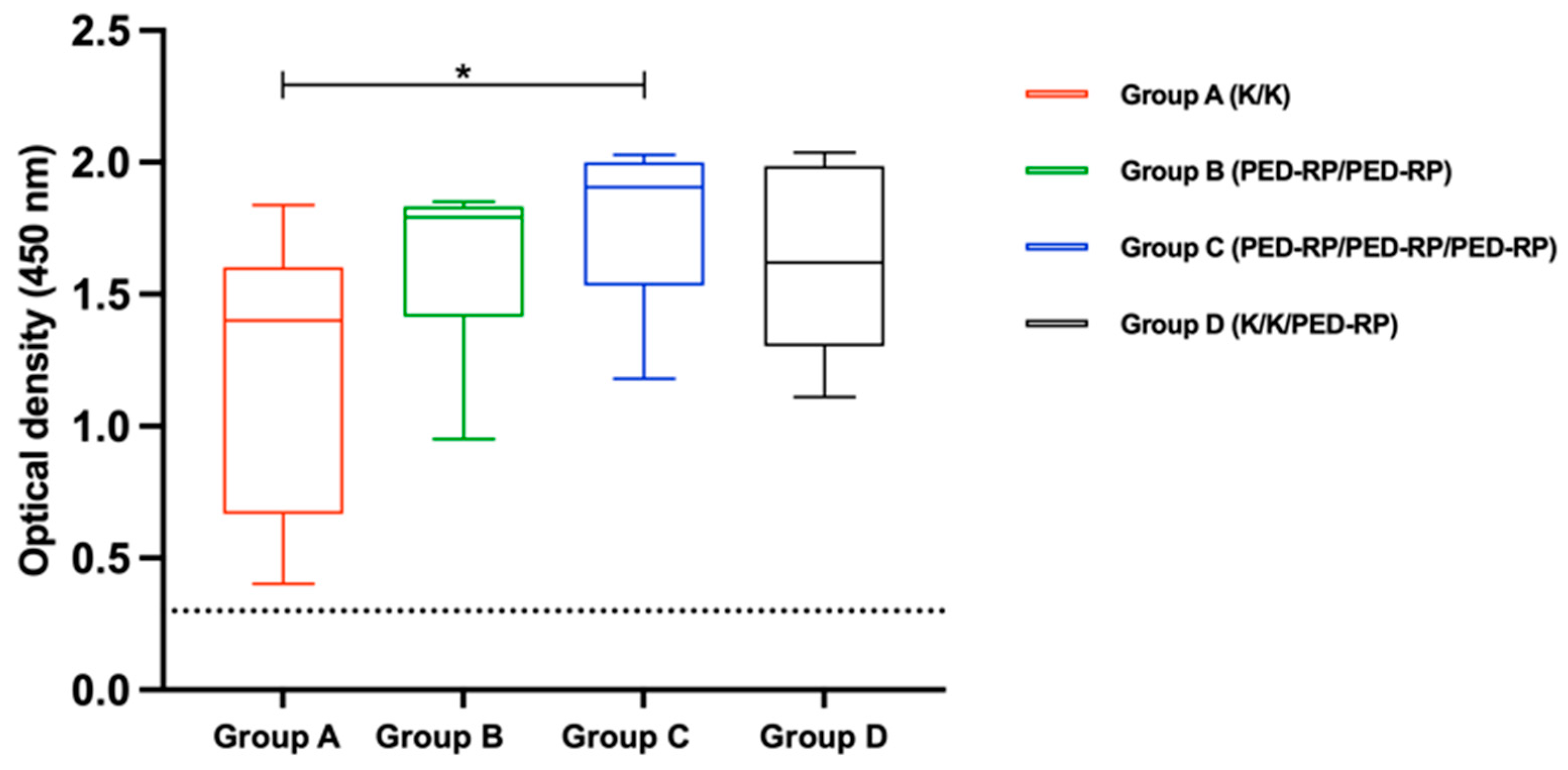
3.1. Evaluation of Immune Responses in Vaccinated Gilts’ Sera During the Acclimatization Period
3.2. Evaluation of Immune Responses in Vaccinated Gilts’ Colostrum at Postpartum Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, J.T.; Chen, Q.; Gauger, P.C.; Gimenez-Lirola, L.G.; Sinha, A.; Harmon, K.M.; Madson, D.M.; Burrough, E.R.; Magstadt, D.R.; Salzbrenner, H.M.; et al. Effect of Porcine Epidemic Diarrhea Virus Infectious Doses on Infection Outcomes in Naive Conventional Neonatal and Weaned Pigs. PLoS ONE 2015, 10, e0139266. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, W.; Zhou, Q.; Li, Q.; Xu, Z.; Shen, H.; Chen, F. Characterization and pathogenicity of Vero cell-attenuated porcine epidemic diarrhea virus CT strain. Virol. J. 2019, 16, 121. [Google Scholar] [CrossRef]
- Weng, L.; Weersink, A.; Poljak, Z.; de Lange, K.; von Massow, M. An economic evaluation of intervention strategies for Porcine Epidemic Diarrhea (PED). Prev. Vet. Med. 2016, 134, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Puranaveja, S.; Poolperm, P.; Lertwatcharasarakul, P.; Kesdaengsakonwut, S.; Boonsoongnern, A.; Urairong, K.; Kitikoon, P.; Choojai, P.; Kedkovid, R.; Teankum, K.; et al. Chinese-like strain of porcine epidemic diarrhea virus, Thailand. Emerg. Infect. Dis. 2009, 15, 1112–1115. [Google Scholar] [CrossRef]
- Temeeyasen, G.; Srijangwad, A.; Tripipat, T.; Tipsombatboon, P.; Piriyapongsa, J.; Phoolcharoen, W.; Chuanasa, T.; Tantituvanont, A.; Nilubol, D. Genetic diversity of ORF3 and spike genes of porcine epidemic diarrhea virus in Thailand. Infect. Genet. Evol. 2014, 21, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Yang, M.; Goyal, S.M.; Cheeran, M.C.; Torremorell, M. Evaluation of biosecurity measures to prevent indirect transmission of porcine epidemic diarrhea virus. BMC Vet. Res. 2017, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Li, B.; Shi, Y.; Feng, S.; Yin, Y.; Long, F.; Pan, Y.; Wei, Y. Phylogenetic and Evolutionary Analysis of Porcine Epidemic Diarrhea Virus in Guangxi Province, China, during 2020 and 2024. Viruses 2024, 16, 1126. [Google Scholar] [CrossRef]
- Tian, L.; Luo, Y.; Wen, T.; Yang, W.; Zhao, Y.; Huang, P.; He, H.; Wu, J.; Li, Z.; Pan, C. A quadruple protection procedure for resuming pig production in small-scale ASFV-positive farms in China. Curr. Res. Microb. Sci. 2021, 2, 100014. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Lu, H.; Geng, C.; Yang, K.; Liu, W.; Liu, Z.; Yuan, F.; Gao, T.; Wang, S.; Wen, P.; et al. Epidemic and Evolutionary Characteristics of Swine Enteric Viruses in South-Central China from 2018 to 2021. Viruses 2022, 14, 1420. [Google Scholar] [CrossRef] [PubMed]
- Suwan, P.; Boonsoongnern, A.; Phuttapatimok, S.; Sukmak, M.; Jirawattanapong, P.; Chumsing, W.; Boodde, O.; Woramahatthanon, K.; Woonwong, Y. Effectiveness of gilt acclimatization—Improvement procedures in a farm with recurrent outbreaks of porcine epidemic diarrhea. Vet. World 2023, 16, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Jermsutjarit, P.; Mebumroong, S.; Watcharavongtip, P.; Lin, H.; Tantituvanont, A.; Kaeoket, K.; Pineyro, P.; Nilubol, D. Evolution and virulence of porcine epidemic diarrhea virus following in vitro and in vivo propagation. Sci. Rep. 2024, 14, 12279. [Google Scholar] [CrossRef] [PubMed]
- Langel, S.N.; Paim, F.C.; Alhamo, M.A.; Buckley, A.; Van Geelen, A.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Stage of Gestation at Porcine Epidemic Diarrhea Virus Infection of Pregnant Swine Impacts Maternal Immunity and Lactogenic Immune Protection of Neonatal Suckling Piglets. Front. Immunol. 2019, 10, 727. [Google Scholar] [CrossRef]
- Salmon, H.; Berri, M.; Gerdts, V.; Meurens, F. Humoral and cellular factors of maternal immunity in swine. Dev. Comp. Immunol. 2009, 33, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Srijangwad, A.; Stott, C.J.; Temeeyasen, G.; Senasuthum, R.; Chongcharoen, W.; Tantituvanont, A.; Nilubol, D. Immune response of gilts to single and double infection with porcine epidemic diarrhea virus. Arch. Virol. 2017, 162, 2029–2034. [Google Scholar] [CrossRef]
- Niederwerder, M.C.; Hesse, R.A. Swine enteric coronavirus disease: A review of 4 years with porcine epidemic diarrhoea virus and porcine deltacoronavirus in the United States and Canada. Transbound. Emerg. Dis. 2018, 65, 660–675. [Google Scholar] [CrossRef]
- Jang, G.; Park, J.; Lee, C. Successful Eradication of Porcine Epidemic Diarrhea in an Enzootically Infected Farm: A Two-Year Follow-Up Study. Pathogens 2021, 10, 830. [Google Scholar] [CrossRef]
- Lauring, A.S.; Jones, J.O.; Andino, R. Rationalizing the development of live attenuated virus vaccines. Nat. Biotechnol. 2010, 28, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Yong, C.Y.; Ong, H.K.; Yeap, S.K.; Ho, K.L.; Tan, W.S. Recent Advances in the Vaccine Development Against Middle East Respiratory Syndrome-Coronavirus. Front. Microbiol. 2019, 10, 1781. [Google Scholar] [CrossRef]
- Langel, S.N.; Paim, F.C.; Lager, K.M.; Vlasova, A.N.; Saif, L.J. Lactogenic immunity and vaccines for porcine epidemic diarrhea virus (PEDV): Historical and current concepts. Virus Res. 2016, 226, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E. Porcine Epidemic Diarrhea: Insights and Progress on Vaccines. Vaccines 2024, 12, 212. [Google Scholar] [CrossRef]
- Schwartz, T.J.; Rademacher, C.J. Evaluation of the effects of PEDv vaccine on PEDv naive and previously PEDv-exposed sows in a challenge model comparing immune response and preweaning mortality. In Proceedings of the 2016 AASV Annual Meeting: Standing on the Shoulders of Giants: Collaboration and Teamwork, New Orleans, LA, USA, 27 February–1 March 2016. [Google Scholar]
- Perri, S.; Greer, C.E.; Thudium, K.; Doe, B.; Legg, H.; Liu, H.; Romero, R.E.; Tang, Z.; Bin, Q.; Dubensky, T.W., Jr.; et al. An alphavirus replicon particle chimera derived from venezuelan equine encephalitis and sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 2003, 77, 10394–10403. [Google Scholar] [CrossRef] [PubMed]
- Pushko, P.; Parker, M.; Ludwig, G.V.; Davis, N.L.; Johnston, R.E.; Smith, J.F. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: Expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 1997, 239, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Kamrud, K.I.; Alterson, K.; Custer, M.; Dudek, J.; Goodman, C.; Owens, G.; Smith, J.F. Development and characterization of promoterless helper RNAs for the production of alphavirus replicon particle. J. Gen. Virol. 2010, 91, 1723–1727. [Google Scholar] [CrossRef] [PubMed]
- Langereis, M.A.; Albulescu, I.C.; Stammen-Vogelzangs, J.; Lambregts, M.; Stachura, K.; Miller, S.; Bosco-Lauth, A.M.; Hartwig, A.E.; Porter, S.M.; Allen, M.; et al. An alphavirus replicon-based vaccine expressing a stabilized Spike antigen induces protective immunity and prevents transmission of SARS-CoV-2 between cats. NPJ Vaccines 2021, 6, 122. [Google Scholar] [CrossRef] [PubMed]
- Vander Veen, R.L.; Loynachan, A.T.; Mogler, M.A.; Russell, B.J.; Harris, D.L.; Kamrud, K.I. Safety, immunogenicity, and efficacy of an alphavirus replicon-based swine influenza virus hemagglutinin vaccine. Vaccine 2012, 30, 1944–1950. [Google Scholar] [CrossRef]
- Sawattrakool, K.; Stott, C.J.; Bandalaria-Marca, R.D.; Srijangwad, A.; Palabrica, D.J.; Nilubol, D. Field trials evaluating the efficacy of porcine epidemic diarrhea vaccine, RNA (Harrisvaccine) in the Philippines. Trop. Anim. Health Prod. 2020, 52, 2743–2747. [Google Scholar] [CrossRef] [PubMed]
- Paudel, S.; Park, J.E.; Jang, H.; Shin, H.J. Comparison of serum neutralization and enzyme-linked immunosorbent assay on sera from porcine epidemic diarrhea virus vaccinated pigs. Vet. Q. 2014, 34, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Jang, G.; Lee, D.; Shin, S.; Lim, J.; Won, H.; Eo, Y.; Kim, C.H.; Lee, C. Porcine epidemic diarrhea virus: An update overview of virus epidemiology, vaccines, and control strategies in South Korea. J. Vet. Sci. 2023, 24, e58. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, Y.; Zhang, B.; Zhao, Y.; Guan, R.; Zhou, Y.; Du, J.; Zhang, Z.; Li, X. Serum IgA antibody level against porcine epidemic diarrhea virus is a potential pre-evaluation indicator of immunization effects in sows during parturition under field conditions. Porc. Health Manag. 2024, 10, 32. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Briones, M.C.; Silva-Pilipich, N.; Herrador-Canete, G.; Vanrell, L.; Smerdou, C. A new generation of vaccines based on alphavirus self-amplifying RNA. Curr. Opin. Virol. 2020, 44, 145–153. [Google Scholar] [CrossRef]
- Ljungberg, K.; Liljestrom, P. Self-replicating alphavirus RNA vaccines. Expert Rev. Vaccines 2015, 14, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Maruggi, G.; Zhang, C.; Li, J.; Ulmer, J.B.; Yu, D. mRNA as a Transformative Technology for Vaccine Development to Control Infectious Diseases. Mol. Ther. 2019, 27, 757–772. [Google Scholar] [CrossRef] [PubMed]
| Experimental Groups (n = 120) | Vaccination Protocols 1 | Doses of Vaccination | |
|---|---|---|---|
| Acclimatization Phase | Gestation Phase 2 | ||
| Group A (n = 30) | K/K | K, 2 shots | - |
| Group B (n = 30) | PED-RP/PED-RP | PED-RP, 2 shots | - |
| Group C (n = 30) | PED-RP/PED-RP/PED-RP | PED-RP, 2 shots | PED-RP, 1 shot |
| Group D (n = 30) | K/K/PED-RP | K, 2 shots | PED-RP, 1 shot |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirisereewan, C.; Nguyen, T.C.; Iampraphat, N.; Lin, H.; Ellerma, L.; Sirithanyakul, P.; Kedkovid, R.; Thanawongnuwech, R. Comparison of Vaccination Regimens on Immune Responses Using PED Replicon Vaccine: A Field Trial in PED-Negative and PED-Positive Thai Swine Farms. Animals 2025, 15, 273. https://doi.org/10.3390/ani15020273
Sirisereewan C, Nguyen TC, Iampraphat N, Lin H, Ellerma L, Sirithanyakul P, Kedkovid R, Thanawongnuwech R. Comparison of Vaccination Regimens on Immune Responses Using PED Replicon Vaccine: A Field Trial in PED-Negative and PED-Positive Thai Swine Farms. Animals. 2025; 15(2):273. https://doi.org/10.3390/ani15020273
Chicago/Turabian StyleSirisereewan, Chaitawat, Thanh Che Nguyen, Nanthiya Iampraphat, Hongyao Lin, Leonardo Ellerma, Pisit Sirithanyakul, Roongtham Kedkovid, and Roongroje Thanawongnuwech. 2025. "Comparison of Vaccination Regimens on Immune Responses Using PED Replicon Vaccine: A Field Trial in PED-Negative and PED-Positive Thai Swine Farms" Animals 15, no. 2: 273. https://doi.org/10.3390/ani15020273
APA StyleSirisereewan, C., Nguyen, T. C., Iampraphat, N., Lin, H., Ellerma, L., Sirithanyakul, P., Kedkovid, R., & Thanawongnuwech, R. (2025). Comparison of Vaccination Regimens on Immune Responses Using PED Replicon Vaccine: A Field Trial in PED-Negative and PED-Positive Thai Swine Farms. Animals, 15(2), 273. https://doi.org/10.3390/ani15020273






