Diosmetin Delays In Vitro Aging of Porcine Oocytes by Improving Mitochondrial Function and Reducing Oxidative Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Chemicals
2.2. Ethical Statement
2.3. Oocyte Collection and Maturation In Vitro
2.4. Parthenogenetic Activation (PA) and Embryo In Vitro Culture (IVC)
2.5. Determination of ROS and GSH Levels
2.6. Determination of Mitochondrial Distribution
2.7. Mitochondrial Membrane Potential Assay
2.8. ATP Staining
2.9. Intracellular Senescence-Associated β-Galactosidase (SA-β-Gal) Activity Assay
2.10. Annexin-V Staining
2.11. Immunofluorescent Staining
2.12. Total Cell Count of Blastocysts
2.13. RNA Extraction and qRT-PCR Assay
2.14. Statistical Analysis
3. Results
3.1. DIOS Supplementation Improves Cleavage Rate, Embryonic Developmental Competence, and Cell Number After Orphan Activation in Senescent Oocytes
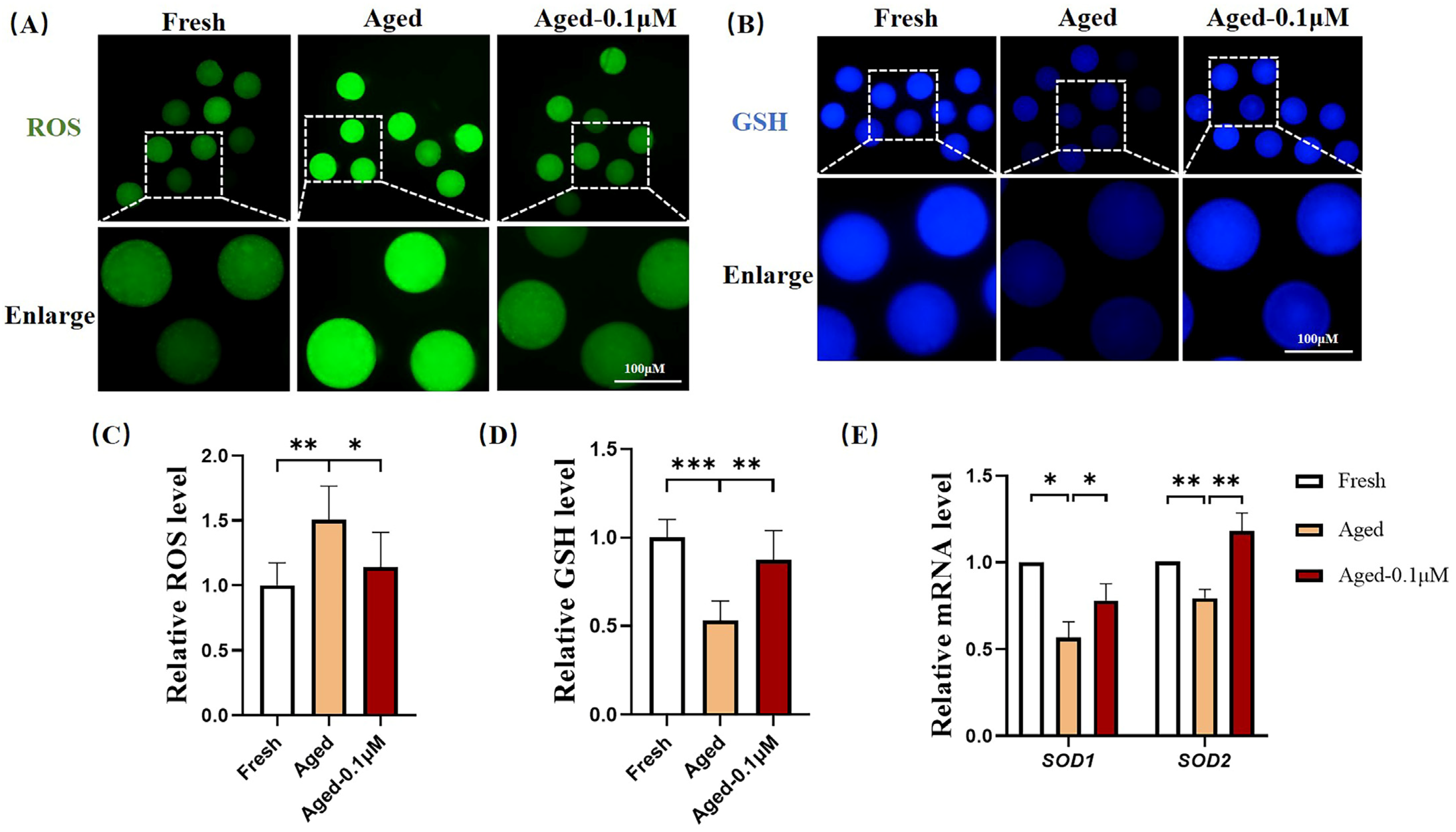
3.2. DIOS Supplementation Enhances the Antioxidant Capacity of Senescent Oocytes
3.3. DIOS Supplementation Improves Mitochondrial Function in Senescent Oocytes
3.4. DIOS Reduces Apoptosis Levels by Mitigating Cellular Senescence

3.5. DIOS Supplementation Alleviates Endoplasmic Reticulum Stress Levels in Senescent Oocytes
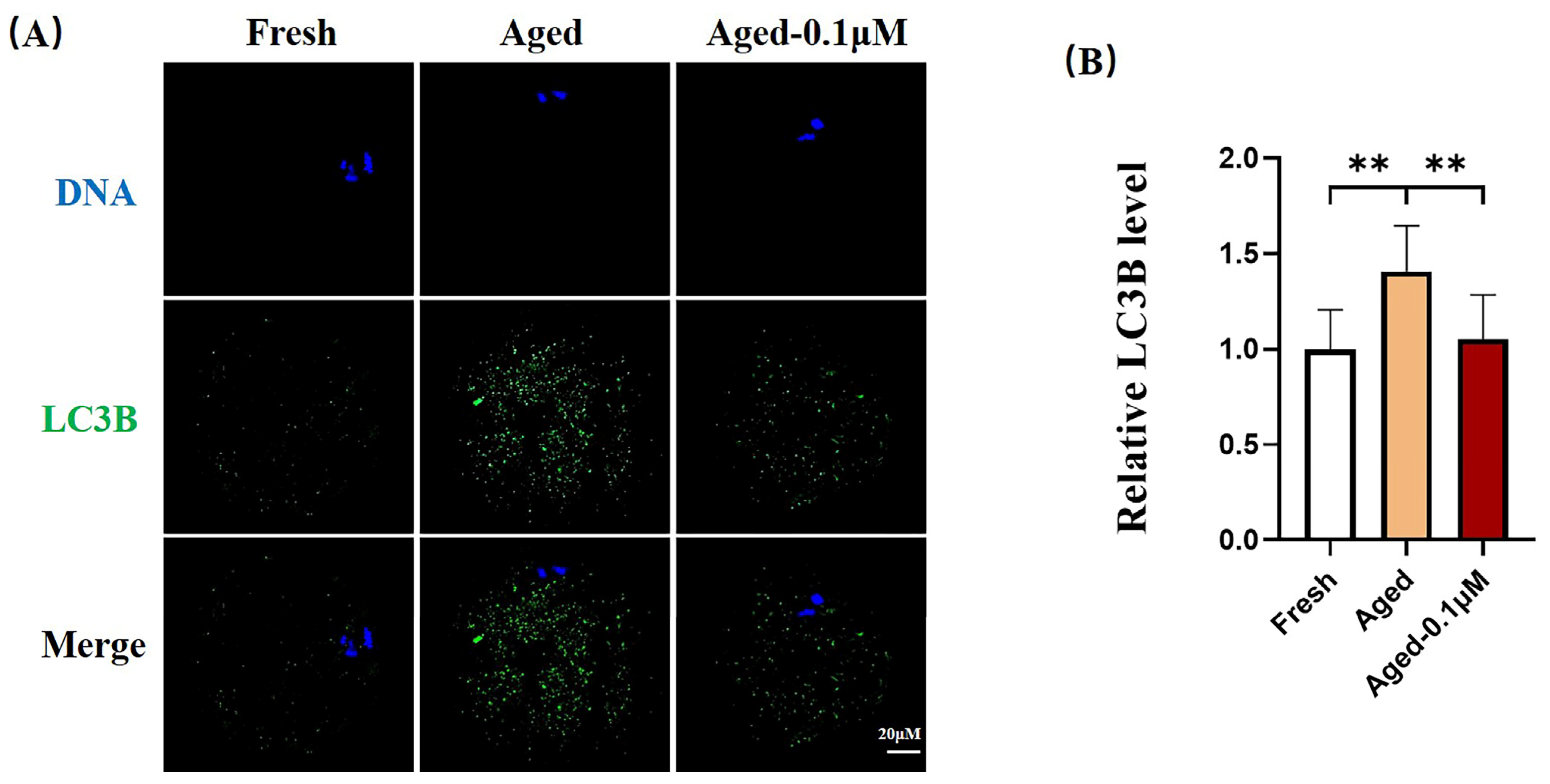
3.6. DIOS Supplementation Inhibits Autophagy in Senescent Oocytes
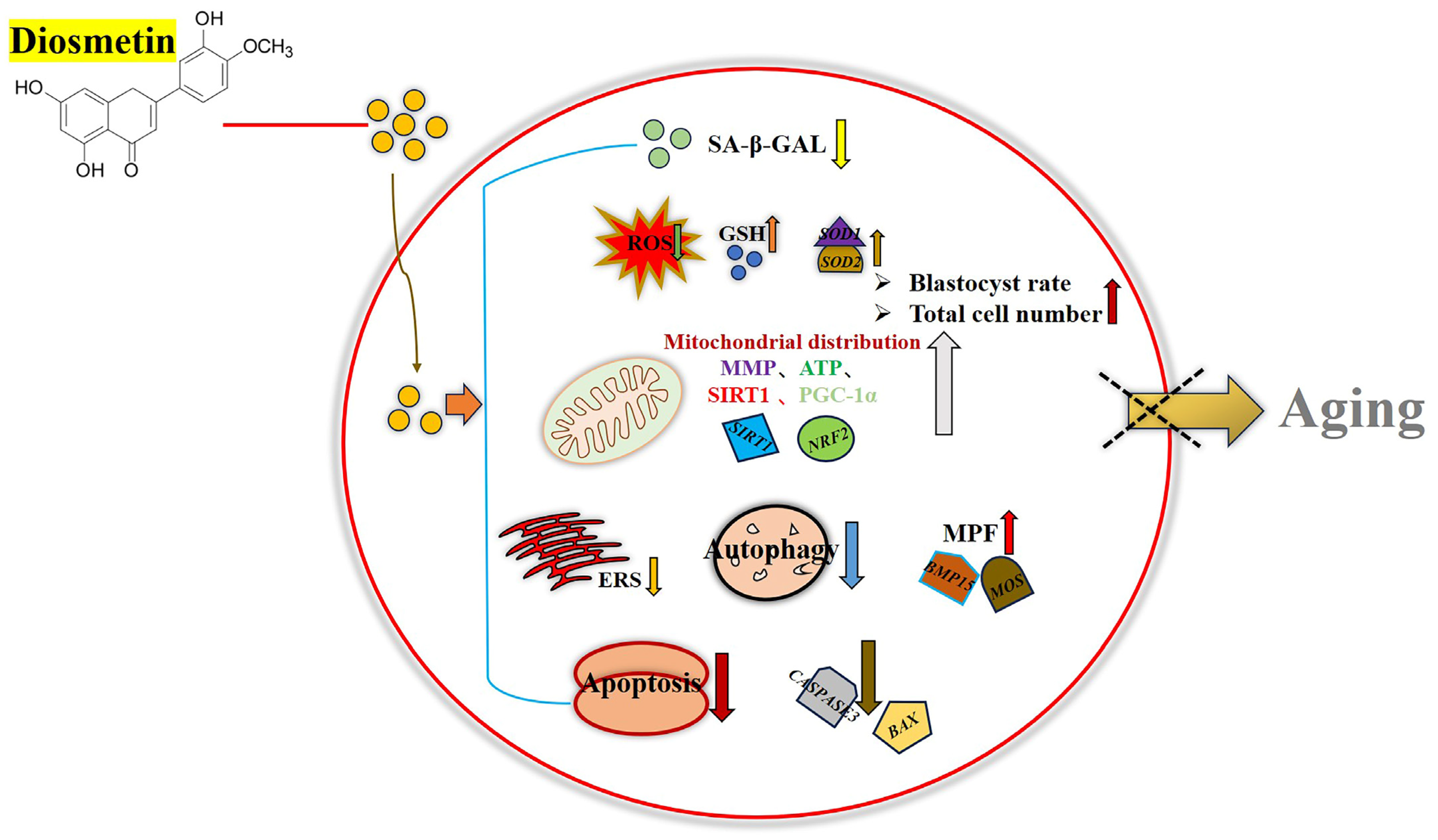
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, T.; Aitken, R.J. Oxidative stress and ageing of the post-ovulatory oocyte. Reproduction 2013, 146, R217–R227. [Google Scholar] [CrossRef] [PubMed]
- Di Nisio, V.; Antonouli, S.; Damdimopoulou, P.; Salumets, A.; Cecconi, S. In vivo and in vitro postovulatory aging: When time works against oocyte quality? J. Assist. Reprod. Genet. 2022, 39, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.-J.; Zhou, W.; Nie, Z.-W.; Zhou, D.; Xu, Y.-N.; Ock, S.A.; Yan, C.-G.; Cui, X.-S. Ubiquinol-10 delays postovulatory oocyte aging by improving mitochondrial renewal in pigs. Aging 2020, 12, 1256–1271. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.-M.; Li, J.; Tang, J.-X.; Hao, X.-X.; Wang, Z.-P.; Sun, T.-C.; Wang, X.-X.; Zhang, Y.; Chen, S.-R.; Liu, Y.-X. Merotelic kinetochore attachment in oocyte meiosis II causes sister chromatids segregation errors in aged mice. Cell Cycle 2017, 16, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, G.; Tomita, M.; Kataoka, M.; Kameyama, Y. Destabilization of spindle assembly checkpoint causes aneuploidy during meiosis II in murine post-ovulatory aged oocytes. J. Reprod. Dev. 2019, 65, 57–66. [Google Scholar] [CrossRef]
- Xu, D.; Jiang, X.; He, H.; Liu, D.; Yang, L.; Chen, H.; Wu, L.; Geng, G.; Li, Q. SIRT2 functions in aging, autophagy, and apoptosis in post-maturation bovine oocytes. Life Sci. 2019, 232, 116639. [Google Scholar] [CrossRef]
- Kasapoğlu, I.; Seli, E. Mitochondrial Dysfunction and Ovarian Aging. Endocrinology 2020, 161, bqaa001. [Google Scholar] [CrossRef]
- Kim, M.J.; Choi, K.H.; Seo, D.W.; Lee, H.R.; Kong, H.S.; Lee, C.H.; Lee, W.S.; Lee, H.T.; Ko, J.J.; Kim, J.H.; et al. Association Between Functional Activity of Mitochondria and Actin Cytoskeleton Instability in Oocytes from Advanced Age Mice. Reprod. Sci. 2020, 27, 1037–1046. [Google Scholar] [CrossRef]
- Takahashi, T.; Takahashi, E.; Igarashi, H.; Tezuka, N.; Kurachi, H. Impact of oxidative stress in aged mouse oocytes on calcium oscillations at fertilization. Mol. Reprod. Dev. 2003, 66, 143–152. [Google Scholar] [CrossRef]
- Takahashi, T.; Saito, H.; Hiroi, M.; Doi, K.; Takahashi, E. Effects of aging on inositol 1,4,5-triphosphate-induced Ca(2+) release in unfertilized mouse oocytes. Mol. Reprod. Dev. 2000, 55, 299–306. [Google Scholar] [CrossRef]
- Gordo, A.C.; Rodrigues, P.; Kurokawa, M.; Jellerette, T.; Exley, G.E.; Warner, C.; Fissore, R. Intracellular Calcium Oscillations Signal Apoptosis Rather than Activation in In Vitro Aged Mouse Eggs1. Biol. Reprod. 2002, 66, 1828–1837. [Google Scholar] [CrossRef] [PubMed]
- Gordo, A.C.; Wu, H.; He, C.L.; Fissore, R.A. Injection of Sperm Cytosolic Factor Into Mouse Metaphase II Oocytes Induces Different Developmental Fates According to the Frequency of [Ca2+]i Oscillations and Oocyte Age1. Biol. Reprod. 2000, 62, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Balakier, H.; Sojecki, A.; Motamedi, G.; Librach, C. Time-dependent capability of human oocytes for activation and pronuclear formation during metaphase II arrest. Hum. Reprod. 2004, 19, 982–987. [Google Scholar] [CrossRef] [PubMed]
- Goud, A.P.; Goud, P.T.; Diamond, M.P.; Gonik, B.; Abu-Soud, H.M. Reactive oxygen species and oocyte aging: Role of superoxide, hydrogen peroxide, and hypochlorous acid. Free. Radic. Biol. Med. 2008, 44, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Free radicals in aging. Mol. Cell. Biochem. 1988, 84, 155–161. [Google Scholar] [CrossRef]
- Babayev, E.; Wang, T.; Szigeti-Buck, K.; Lowther, K.; Taylor, H.S.; Horvath, T.; Seli, E. Reproductive aging is associated with changes in oocyte mitochondrial dynamics, function, and mtDNA quantity. Maturitas 2016, 93, 121–130. [Google Scholar] [CrossRef]
- Tarín, J.J.; Pérez-Albalá, S.; Pérez-Hoyos, S.; Cano, A. Postovulatory Aging of Oocytes Decreases Reproductive Fitness and Longevity of Offspring1. Biol. Reprod. 2002, 66, 495–499. [Google Scholar] [CrossRef]
- Warburton, D. Biological aging and the etiology of aneuploidy. Cytogenet. Genome Res. 2005, 111, 266–272. [Google Scholar] [CrossRef]
- Zhang, H.; Li, C.; Wen, D.; Li, R.; Lu, S.; Xu, R.; Tang, Y.; Sun, Y.; Zhao, X.; Pan, M.; et al. Melatonin improves the quality of maternally aged oocytes by maintaining intercellular communication and antioxidant metabolite supply. Redox Biol. 2021, 49, 102215. [Google Scholar] [CrossRef]
- Yin, Y.-J.; Zhang, Y.-H.; Wang, Y.; Jiang, H.; Zhang, J.-B.; Liang, S.; Yuan, B. Ferulic acid ameliorates the quality of in vitro-aged bovine oocytes by suppressing oxidative stress and apoptosis. Aging 2023, 15, 12497–12512. [Google Scholar] [CrossRef]
- Yao, X.; Guo, P.; Li, Y.-H.; Guo, H.; Jin, Z.; Lui, W.; Yuan, J.; Gao, Q.; Wang, L.; Li, Y.; et al. Apigenin delays postovulatory oocyte aging by reducing oxidative stress through SIRT1 upregulation. Theriogenology 2024, 218, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Ning, Z.; Chen, L.; Wei, Q.; Yuan, E.; Yang, J.; Ren, J. Intracellular Antioxidant Detoxifying Effects of Diosmetin on 2,2-Azobis(2-amidinopropane) Dihydrochloride (AAPH)-Induced Oxidative Stress through Inhibition of Reactive Oxygen Species Generation. J. Agric. Food Chem. 2014, 62, 8648–8654. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.W.; Liu, X.; Yepes, M.; Shepherd, K.R.; Miller, G.W.; Liu, Y.; Wilson, W.D.; Xiao, G.; Blanchi, B.; Sun, Y.E.; et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc. Natl. Acad. Sci. USA 2010, 107, 2687–2692. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Luo, C.; Huang, P.; Cheng, Y.; Ma, Y.; Gao, J.; Ding, H. Diosmetin Ameliorates HFD-induced Cognitive Impairments via Inhibiting Metabolic Disorders, Mitochondrial Dysfunction and Neuroinflammation in Male SD Rats. Mol. Neurobiol. 2024, 61, 8069–8085. [Google Scholar] [CrossRef]
- Lord, T.; Martin, J.H.; Aitken, R.J. Accumulation of Electrophilic Aldehydes During Postovulatory Aging of Mouse Oocytes Causes Reduced Fertility, Oxidative Stress, and Apoptosis1. Biol. Reprod. 2015, 92, 33. [Google Scholar] [CrossRef]
- Miao, Y.; Cui, Z.; Gao, Q.; Rui, R.; Xiong, B. Nicotinamide Mononucleotide Supplementation Reverses the Declining Quality of Maternally Aged Oocytes. Cell Rep. 2020, 32, 107987. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Fan, L.-H.; Jing, Y.; Li, J.; Ouyang, Y.-C.; Wang, Z.-B.; Hou, Y.; Sun, Q.-Y. N-acetyl-L-cysteine (NAC) delays post-ovulatory oocyte aging in mouse. Aging 2019, 11, 2020–2030. [Google Scholar] [CrossRef]
- Yamauchi, N.; Sasada, H.; Sugawara, S.; Nagai, T. Effect of culture conditions on artificial activation of porcine oocytes matured in vitro. Reprod. Fertil. Dev. 1996, 8, 1153–1156. [Google Scholar] [CrossRef]
- Lin, T.; Lee, J.E.; Kang, J.W.; Oqani, R.K.; Cho, E.S.; Kim, S.B.; Jin, D.I. Melatonin supplementation during prolonged in vitro maturation improves the quality and development of poor-quality porcine oocytes via anti-oxidative and anti-apoptotic effects. Mol. Reprod. Dev. 2018, 85, 665–681. [Google Scholar] [CrossRef]
- Wójciak, M.; Feldo, M.; Borowski, G.; Kubrak, T.; Płachno, B.J.; Sowa, I. Antioxidant Potential of Diosmin and Diosmetin against Oxidative Stress in Endothelial Cells. Molecules 2022, 27, 8232. [Google Scholar] [CrossRef]
- Liu, M.-N.; Zhang, K.; Xu, T.-M. The role of BMP15 and GDF9 in the pathogenesis of primary ovarian insufficiency. Hum. Fertil. 2019, 24, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Gode, F.; Gulekli, B.; Dogan, E.; Korhan, P.; Dogan, S.; Bige, O.; Cimrin, D.; Atabey, N. Influence of follicular fluid GDF9 and BMP15 on embryo quality. Fertil. Steril. 2011, 95, 2274–2278. [Google Scholar] [CrossRef]
- Gong, Y.; Li-Ling, J.; Xiong, D.; Wei, J.; Zhong, T.; Tan, H. Age-related decline in the expression of GDF9 and BMP15 genes in follicle fluid and granulosa cells derived from poor ovarian responders. J. Ovarian Res. 2021, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Verlhac, M.-H.; Kubiak, J.Z.; Weber, M.; Géraud, G.; Colledge, W.H.; Evans, M.J.; Maro, B. Mos is required for MAP kinase activation and is involved in microtubule organization during meiotic maturation in the mouse. Development 1996, 122, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Sagata, N. What does Mos do in oocytes and somatic cells? BioEssays 1997, 19, 13–21. [Google Scholar] [CrossRef]
- Jałocha, I.; Gabrys, M.; Bal, J. The crucial role of the proto-oncogene c-mos in regulation of oocyte maturation. Postep. Hig. I Med. Dosw. 2010, 64, 636–641. [Google Scholar]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Ge, H.; Tollner, T.L.; Hu, Z.; Dai, M.; Li, X.; Guan, H.; Shan, D.; Zhang, X.; Lv, J.; Huang, C.; et al. The importance of mitochondrial metabolic activity and mitochondrial DNA replication during oocyte maturation in vitro on oocyte quality and subsequent embryo developmental competence. Mol. Reprod. Dev. 2012, 79, 392–401. [Google Scholar] [CrossRef]
- Van Blerkom, J.; Caltrider, K. Sperm attachment and penetration competence in the human oocyte: A possible aetiology of fertilization failure involving the organization of oolemmal lipid raft microdomains influenced by the ΔΨm of subplasmalemmal mitochondria. Reprod. Biomed. Online 2013, 27, 690–701. [Google Scholar] [CrossRef]
- Dalton, C.M.; Szabadkai, G.; Carroll, J. Measurement of ATP in Single Oocytes: Impact of Maturation and Cumulus Cells on Levels and Consumption. J. Cell. Physiol. 2013, 229, 353–361. [Google Scholar] [CrossRef]
- Dai, X.; Lu, Y.; Zhang, M.; Miao, Y.; Zhou, C.; Cui, Z.; Xiong, B. Melatonin improves the fertilization ability of post-ovulatory aged mouse oocytes by stabilizing ovastacin and Juno to promote sperm binding and fusion. Hum. Reprod. 2017, 32, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Bentov, Y.; Casper, R.F. The aging oocyte--can mitochondrial function be improved? Fertil. Steril. 2013, 99, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhou, M.; Ge, Y.; Wang, X. SIRT1 and aging related signaling pathways. Mech. Ageing Dev. 2020, 187, 111215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-N.; Zhang, M.-Q.; Yu, F.-L.; Han, B.; Bao, M.-Y.; He, Y.; Li, X.; Zhang, Y. Peroxisom proliferator-activated receptor-γ coactivator-1α in neurodegenerative disorders: A promising therapeutic target. Biochem. Pharmacol. 2023, 215, 115717. [Google Scholar] [CrossRef]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Lomb, D.J.; Laurent, G.; Haigis, M.C. Sirtuins regulate key aspects of lipid metabolism. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2010, 1804, 1652–1657. [Google Scholar] [CrossRef]
- Anderson, R.M.; Barger, J.L.; Edwards, M.G.; Braun, K.H.; O’connor, C.E.; Prolla, T.A.; Weindruch, R. Dynamic regulation of PGC-1α localization and turnover implicates mitochondrial adaptation in calorie restriction and the stress response. Aging Cell 2007, 7, 101–111. [Google Scholar] [CrossRef]
- Bulbiankova, D.; Díaz-Puertas, R.; Álvarez-Martínez, F.J.; Herranz-López, M.; Barrajón-Catalán, E.; Micol, V. Hallmarks and Biomarkers of Skin Senescence: An Updated Review of Skin Senotherapeutics. Antioxidants 2023, 12, 444. [Google Scholar] [CrossRef]
- Li, X.L.; Xu, M.; Yu, F.; Fu, C.L.; Yu, X.; Cheng, M.; Gao, H.Q. Effects of D-pinitol on myocardial apoptosis and fibrosis in streptozocin-induced aging-accelerated mice. J. Food Biochem. 2021, 45, e13669. [Google Scholar] [CrossRef]
- Khatun, H.; Wada, Y.; Konno, T.; Tatemoto, H.; Yamanaka, K.-I. Endoplasmic reticulum stress attenuation promotes bovine oocyte maturation in vitro. Reproduction 2020, 159, 361–370. [Google Scholar] [CrossRef]
- Han, L.; Xu, Y.; Shi, Y. Molecular Mechanism of the ATF6α/S1P/S2P Signaling Pathway in Hippocampal Neuronal Apoptosis in SPS Rats. J. Mol. Neurosci. 2021, 71, 2487–2499. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Chi, R.; Qin, F.; Guo, X. Distinct changes of myocyte autophagy during myocardial hypertrophy and heart failure: Association with oxidative stress. Exp. Physiol. 2016, 101, 1050–1063. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Ha, H.; Lee, B.S.; Kim, B.H.; Song, H.K.; Kim, Y.K. LC3B is an RNA-binding protein to trigger rapid mRNA degradation during autophagy. Nat. Commun. 2022, 13, 1436. [Google Scholar] [CrossRef] [PubMed]



| Genes | Primer Sequences (5′-3′) | Base |
|---|---|---|
| GAPDH | F: TTCCACGGCACAGTCAAG R: ATACTCAGCACCAGCATCG | 18 19 |
| BMP15 | F: CCTCCATCCTTTCCAAGTCA R: GTGTAGTACCCGAGGGCAGA | 20 20 |
| MOS | F: TGGGAAGAAACTGGAGGACA R: TTCGGGTCAGCCCAGTTCA | 20 19 |
| SOD1 | F: CAAAGGATCAAGAGAGGCACG R: CGAGAGGGCGATCACAGAAT | 21 20 |
| SOD2 | F: TTCTGGACAAATCTGAGCCCTAACG R: CGACGGATACAGCGGTCAACTTC | 25 23 |
| NRF2 | F: AGCGGATTGCTCGTAGACAG R: TTCAGTCGCTTCACGTCGG | 20 19 |
| SIRT1 | F: ACAGGTTGCAGGAATCCAGAG R: TAGGACATCGAGGAACCACCT | 21 21 |
| CASP3 | F: AGAATTGGACTGTGGGATTGAGACG R: GCCAGGAATAGTAACCAGGTGCTG | 25 24 |
| BAX | F: GGACTTCCTTCGAGATCGGC R: GCGTCCCAAAGTAGGAGAGG | 20 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, J.-J.; Yuan, X.-W.; Zhang, Y.-H.; Meng, Z.-L.; Liang, X.-W.; Kim, N.-H.; Xu, Y.-N.; Li, Y.-H. Diosmetin Delays In Vitro Aging of Porcine Oocytes by Improving Mitochondrial Function and Reducing Oxidative Stress. Animals 2025, 15, 291. https://doi.org/10.3390/ani15030291
Ren J-J, Yuan X-W, Zhang Y-H, Meng Z-L, Liang X-W, Kim N-H, Xu Y-N, Li Y-H. Diosmetin Delays In Vitro Aging of Porcine Oocytes by Improving Mitochondrial Function and Reducing Oxidative Stress. Animals. 2025; 15(3):291. https://doi.org/10.3390/ani15030291
Chicago/Turabian StyleRen, Jia-Jun, Xiu-Wen Yuan, Yu-Hao Zhang, Zi-Long Meng, Xing-Wei Liang, Nam-Hyung Kim, Yong-Nan Xu, and Ying-Hua Li. 2025. "Diosmetin Delays In Vitro Aging of Porcine Oocytes by Improving Mitochondrial Function and Reducing Oxidative Stress" Animals 15, no. 3: 291. https://doi.org/10.3390/ani15030291
APA StyleRen, J.-J., Yuan, X.-W., Zhang, Y.-H., Meng, Z.-L., Liang, X.-W., Kim, N.-H., Xu, Y.-N., & Li, Y.-H. (2025). Diosmetin Delays In Vitro Aging of Porcine Oocytes by Improving Mitochondrial Function and Reducing Oxidative Stress. Animals, 15(3), 291. https://doi.org/10.3390/ani15030291




