Unlocking Gut Health: The Potent Role of Stilbenoids in Intestinal Homeostasis
Simple Summary
Abstract
1. Introduction
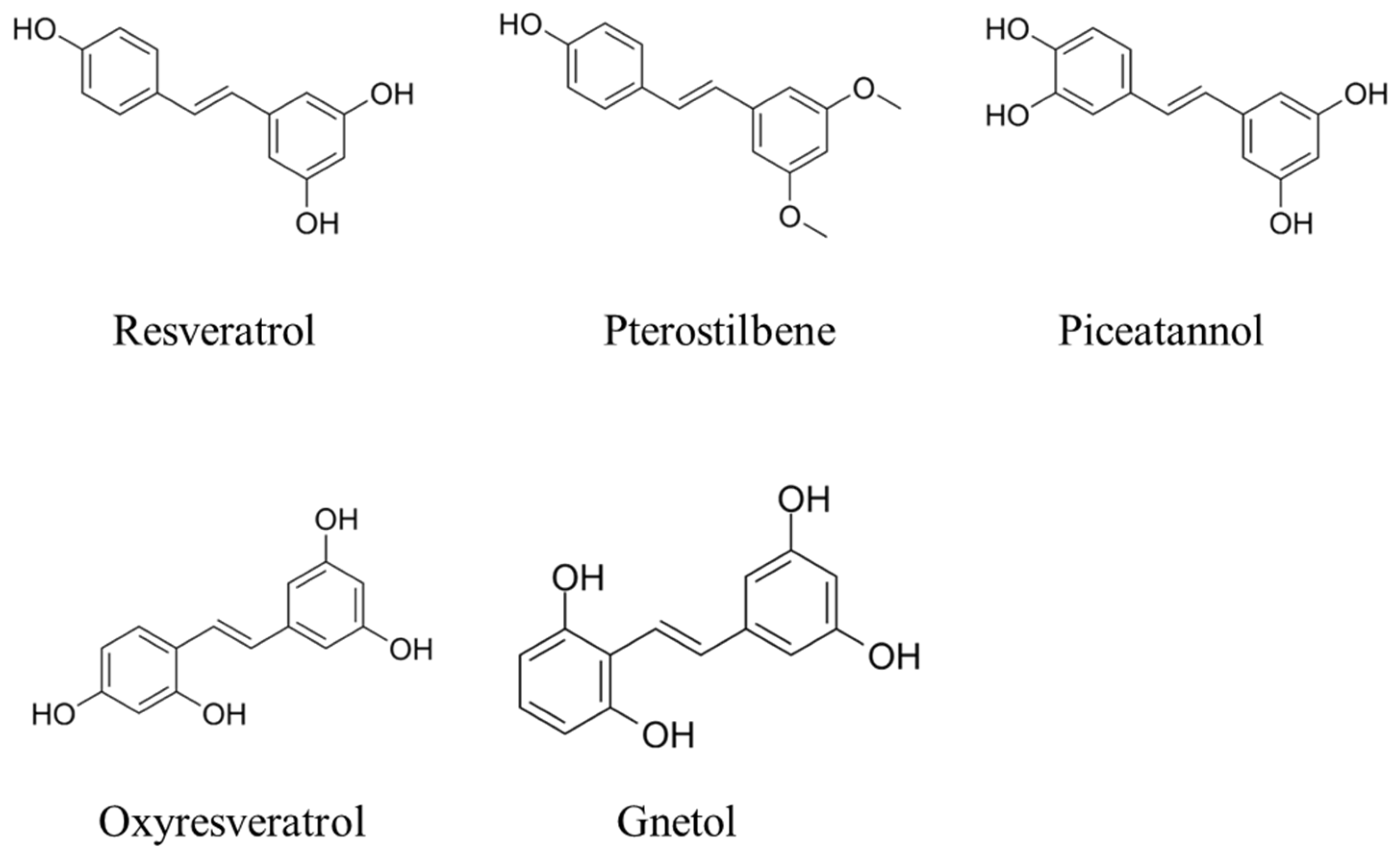
2. Chemistry of Stilbenoids
3. Biological Effects of Stilbenoids
3.1. Anti-Inflammatory and Antioxidant
3.1.1. RES
3.1.2. PTE
3.1.3. Piceatannol
3.1.4. ORES
3.2. Antimicrobial Activity
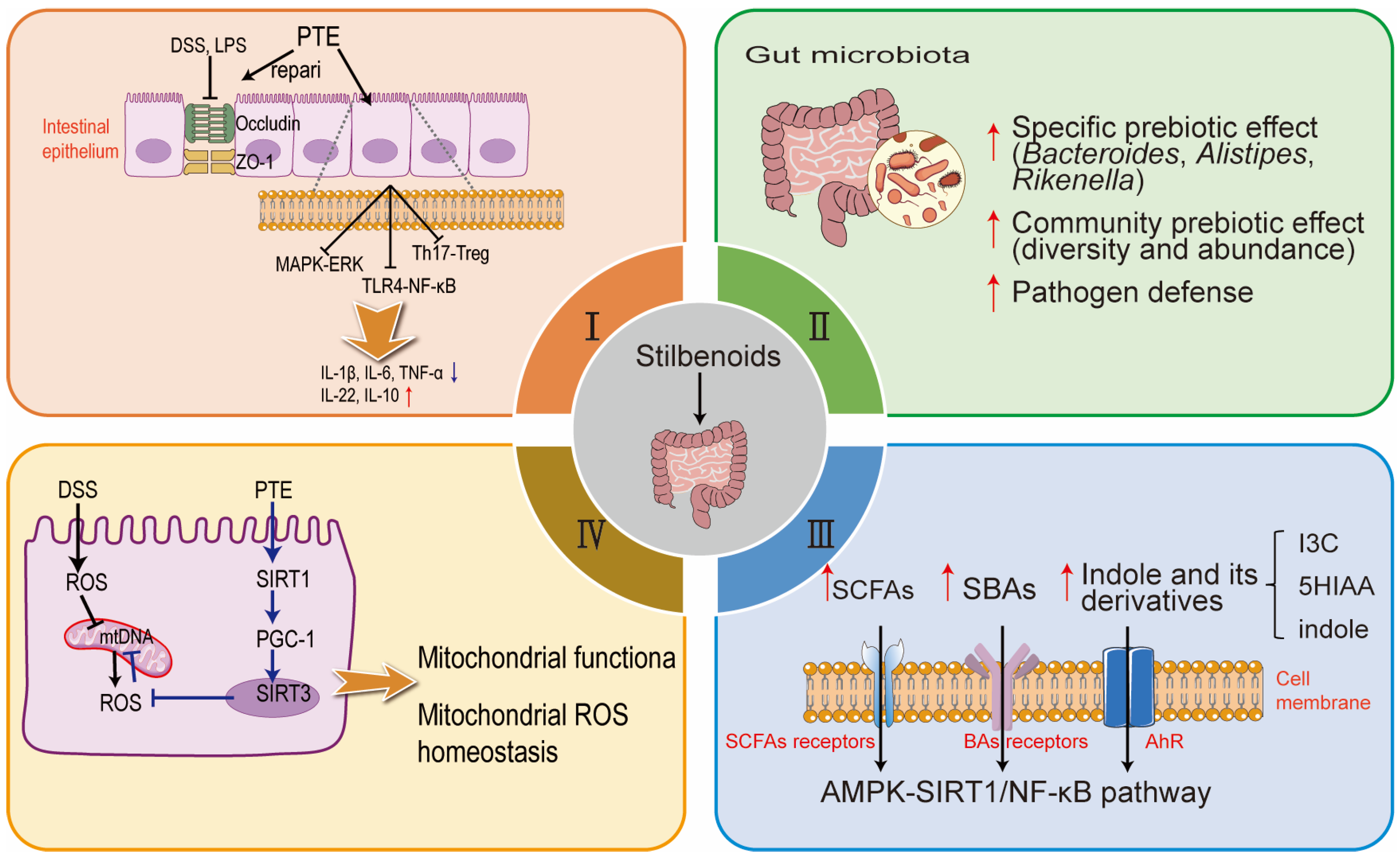
4. Multifaceted Roles of Stilbenoids in Gut Health and Function
4.1. Enhancement of Gut Morphology and Mucosal Function and Related Intestinal Inflammation
4.2. Modulation of Gut Microbiota Composition
4.3. Regulation of Metabolic Pathways and Gut Metabolome
4.4. Maintenance of Mitochondrial Health and Oxidative Balance
5. The Potential of Stilbenoids in Enhancing Livestock Gut Health
| Stilbenoids | Concentration | Model System | Observations | Ref. |
|---|---|---|---|---|
| RES | 90 mg/kg | Piglets under oxidative stress | ↑The genera Clostridium sensu stricto 1 and Lachnospiraceae unclassified, ↑indole-3-carbinol, ↑5-hydroxyindole-3-acetic acid, and ↑uridine | [108] |
| RES | 300 mg/kg | Deoxynivalenol-challenged piglets | ↓D-lactate levels, ↓pro-inflammatory markers (TNF-α, IL-1β), and↑ZO-1 expression | [135] |
| RES | 500 mg/kg | Cold-exposed broilers | ↑The activities of SOD and CAT and mRNA expression of anti-inflammatory genes, ↓concentrations of MDA and H2O2 and mRNA expression of ER stress, pyroptosis and proinflammatory genes | [12] |
| RES | 100 mg/kg | Diquat challenged piglets | ↑occludin, claudin-1, and ZO-1 proteins, improved redox status, ↓mitochondrial damage, and induced mitophagy | [132] |
| RES | 90 mg/kg | Diquat-challenged piglet | Protected intestinal integrity, ↓oxidative stress, and↑Akt/Nrf2 signaling pathway | [139] |
| RES | 400 mg/kg | Acute heat stressed ducks | ↑Villus height to crypt depth ratio, ↑goblet cell number, ↓histopathological damage in jejunum, and↑SIRT1 signaling pathway | [138] |
| RES | 400 mg/kg | LPS-induced broilers | ↑Average daily gain, ↓spleen index, ↓IgM, ↓secretory immunoglobulin A levels; ↓D-lactic acid, ↑occludin mRNA expression, ↓TLR4, ↓NF-κB, and ↓TNF-α levels | [140] |
| RES | 400 mg/kg | Heat stressed broilers | ↑Body weight, ↑average daily gain, ↑relative jejunum weight and length, ↑villus height, ↑GPX and glutathione S-transferase activities, ↑Nrf2 and SOD1 mRNA levels, and ↓Keap1 mRNA expression | [141] |
| RES | 150 and 300 mg/kg | Weaning piglets | ↑Serum IgG, ↑GPX activity, ↓MDA content, ↑villus height to crypt depth ratio, ↑jejunum villus height, ↓crypt depth, and ↑IL-10 and ZO-1 mRNA levels | [136] |
| RES | 300 mg/kg | Weaning piglets | ↑The proportion of butyrate-producing bacteria, include Flavonifractor, Odoribacter, and Oscillibacter | [137] |
| PTE | 400 mg/kg | Broiler chickens with immunological stress | ↑Body weight, ↑villus height to crypt depth ratio, ↑ZO-1 and occluding mRNA levels, and ↓the nuclear translocation of NF-κB p65 | [96] |
| PTE | 400 mg/kg | Broilers under diquat-induced stress | ↓Intestinal permeability, ↓jejunal apoptosis rate, ↑jejunal villus height, ↑villus height to crypt depth ratio, ↓ROS, ↑SOD2, ↑occludin, ↑ZO-1, and ↑Nrf2 pathway activation | [97] |
6. Summary and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Teka, T.; Zhang, L.; Ge, X.; Li, Y.; Han, L.; Yan, X. Stilbenes: Source plants, chemistry, biosynthesis, pharmacology, application and problems related to their clinical Application—A comprehensive review. Phytochemistry 2022, 197, 113128. [Google Scholar] [CrossRef] [PubMed]
- Duta-Bratu, C.-G.; Nitulescu, G.M.; Mihai, D.P.; Olaru, O.T. Resveratrol and Other Natural Oligomeric Stilbenoid Compounds and Their Therapeutic Applications. Plants 2023, 12, 2935. [Google Scholar] [CrossRef] [PubMed]
- Akinwumi, B.C.; Bordun, K.M.; Anderson, H.D. Biological Activities of Stilbenoids. Int. J. Mol. Sci. 2018, 19, 792. [Google Scholar] [CrossRef]
- Tsai, H.-Y.; Ho, C.-T.; Chen, Y.-K. Biological actions and molecular effects of resveratrol, pterostilbene, and 3′-hydroxypterostilbene. J. Food Drug Anal. 2017, 25, 134–147. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- He, S.; Yu, Q.; He, Y.; Hu, R.; Xia, S.; He, J. Dietary resveratrol supplementation inhibits heat stress-induced high-activated innate immunity and inflammatory response in spleen of yellow-feather broilers. Poult. Sci. 2019, 98, 6378–6387. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.; Deng, J.; Xiao, D.; Arowolo, M.A.; Liu, C.; Chen, L.; Deng, W.; He, S.; He, J. Protective Effects and Potential Mechanisms of Dietary Resveratrol Supplementation on the Spleen of Broilers Under Heat Stress. Front. Nutr. 2022, 9, 821272. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-F.; Yao, C.-S. Naturally active oligostilbenes. J. Asian Nat. Prod. Res. 2015, 18, 376–407. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Huang, J.C.; Chen, Y.L.; Huang, M.; Zhou, G.H. Identification and characterization of antioxidant peptides from enzymatic hydrolysates of duck meat. J. Agric. Food Chem. 2015, 63, 3437–3444. [Google Scholar] [CrossRef]
- Piotrowska, H.; Kucinska, M.; Murias, M. Biological activity of piceatannol: Leaving the shadow of resveratrol. Mutat. Res. 2012, 750, 60–82. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Li, H.; Miao, D.; Wang, H.; Liu, Y.; Xing, L.; Bao, J.; Li, J. Dietary resveratrol supplementation alleviates cold exposure-induced pyroptosis and inflammation in broiler heart by modulating oxidative stress and endoplasmic reticulum stress. Poult. Sci. 2024, 103, 104203. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, F.; Raza, S.H.A.; Wu, Z.; Su, Q.; Ji, Q.; He, T.; Zhu, K.; Zhang, Y.; Hou, S.; et al. Immune, Oxidative, and Morphological Changes in the Livers of Tibetan Sheep after Feeding Resveratrol and β-Hydroxy-β-methyl Butyric Acid: A Transcriptome–Metabolome Integrative Analysis. Int. J. Mol. Sci. 2024, 25, 9865. [Google Scholar] [CrossRef] [PubMed]
- Monmai, C.; Baek, S.-H. Anti-Inflammatory Effects of the Combined Treatment of Resveratrol- and Protopanaxadiol-Enriched Rice Seed Extract on Lipopolysaccharide-Stimulated RAW264.7 Cells. Molecules 2024, 29, 4343. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; You, Y.; Lu, J.; Chen, X.; Yang, Z. Recent Advances in Synthesis, Bioactivity, and Pharmacokinetics of Pterostilbene, an Important Analog of Resveratrol. Molecules 2020, 25, 5166. [Google Scholar] [CrossRef]
- Lin, W.-S.; Leland, J.V.; Ho, C.-T.; Pan, M.-H. Occurrence, Bioavailability, Anti-inflammatory, and Anticancer Effects of Pterostilbene. J. Agric. Food Chem. 2020, 68, 12788–12799. [Google Scholar] [CrossRef]
- Dellinger, R.W.; Gomez Garcia, A.M.; Meyskens, F.L. Differences in the Glucuronidation of Resveratrol and Pterostilbene: Altered Enzyme Specificity and Potential Gender Differences. Drug Metab. Pharmacokinet. 2014, 29, 112–119. [Google Scholar] [CrossRef]
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, oral bioavailability, and metabolic profile of resveratrol and its dimethylether analog, pterostilbene, in rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601. [Google Scholar] [CrossRef]
- Lange, K.W.; Li, S. Resveratrol, pterostilbene, and dementia. BioFactors 2017, 44, 83–90. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Ji, S.; Jia, P.; Chen, Y.; Li, Y.; Wang, T. Resveratrol and its derivative pterostilbene attenuate oxidative stress-induced intestinal injury by improving mitochondrial redox homeostasis and function via SIRT1 signaling. Free. Radic. Biol. Med. 2021, 177, 1–14. [Google Scholar] [CrossRef]
- Chen, X.; Song, Q.L.; Li, Z.H.; Ji, R.; Wang, J.Y.; Cao, M.L.; Mu, X.F.; Zhang, Y.; Guo, D.Y.; Yang, J. Pterostilbene ameliorates oxidative damage and ferroptosis in human ovarian granulosa cells by regulating the Nrf2/HO-1 pathway. Arch. Biochem. Biophys. 2023, 738, 109561. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ci, X.; Ma, X.; Yu, Q.; Cui, Y.; Zhen, Y.; Li, S. Pterostilbene Activates the Nrf2-Dependent Antioxidant Response to Ameliorate Arsenic-Induced Intracellular Damage and Apoptosis in Human Keratinocytes. Front. Pharmacol. 2019, 10, 497. [Google Scholar] [CrossRef] [PubMed]
- Ullah, O.; Li, Z.; Ali, I.; Xu, L.; Liu, H.; Jin, H.-Z.; Fang, Y.-Y.; Jin, Q.-G.; Fang, N. Pterostilbene exerts a protective effect via regulating tunicamycin-induced endoplasmic reticulum stress in mouse preimplantation embryos. In Vitro Cell. Dev. Biol.-Anim. 2018, 55, 82–93. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, Q.; Xiao, Y.; Cai, X.; Yang, Z.; Zeng, W.; Mi, Y.; Zhang, C. Pterostilbene, a Resveratrol Derivative, Improves Ovary Function by Upregulating Antioxidant Defenses in the Aging Chickens via Increased SIRT1/Nrf2 Expression. Antioxidants 2024, 13, 935. [Google Scholar] [CrossRef]
- Hou, Y.; Li, N.; Xie, G.; Wang, J.; Yuan, Q.; Jia, C.; Liu, X.; Li, G.; Tang, Y.; Wang, B. Pterostilbene exerts anti-neuroinflammatory effect on lipopolysaccharide-activated microglia via inhibition of MAPK signalling pathways. J. Funct. Foods 2015, 19, 676–687. [Google Scholar] [CrossRef]
- Sireesh, D.; Ganesh, M.-R.; Dhamodharan, U.; Sakthivadivel, M.; Sivasubramanian, S.; Gunasekaran, P.; Ramkumar, K.M. Role of pterostilbene in attenuating immune mediated devastation of pancreatic beta cells via Nrf2 signaling cascade. J. Nutr. Biochem. 2017, 44, 11–21. [Google Scholar] [CrossRef]
- Zhang, L.; Jian, X.; Yu, J.; Yu, J. Pterostilbene Interferes With Lipopolysaccharide-Induced Myocardial Injury Through Oxidative Stress and Inflammasome Pathways. Front. Physiol. 2022, 13, 862187. [Google Scholar] [CrossRef]
- Eräsalo, H.; Hämäläinen, M.; Leppänen, T.; Mäki-Opas, I.; Laavola, M.; Haavikko, R.; Yli-Kauhaluoma, J.; Moilanen, E. Natural Stilbenoids Have Anti-Inflammatory Properties in Vivo and Down-Regulate the Production of Inflammatory Mediators NO, IL6, and MCP1 Possibly in a PI3K/Akt-Dependent Manner. J. Nat. Prod. 2018, 81, 1131–1142. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y. Pterostilbene, a novel natural plant conduct, inhibits high fat-induced atherosclerosis inflammation via NF-κB signaling pathway in Toll-like receptor 5 (TLR5) deficient mice. Biomed. Pharmacother. 2016, 81, 345–355. [Google Scholar] [CrossRef]
- Hsu, C.-L.; Lin, Y.-J.; Ho, C.-T.; Yen, G.-C. The Inhibitory Effect of Pterostilbene on Inflammatory Responses during the Interaction of 3T3-L1 Adipocytes and RAW 264.7 Macrophages. J. Agric. Food Chem. 2013, 61, 602–610. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, H.; Wang, L.; Tao, Y.; Du, G.; Guan, W.; Liu, J.; Brennan, C.; Ho, C.-T.; Li, S. Effects of Selected Resveratrol Analogues on Activation and Polarization of Lipopolysaccharide-Stimulated BV-2 Microglial Cells. J. Agric. Food Chem. 2020, 68, 3750–3757. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.T.; Deng, S.M.; Chen, C.; He, Q.H.; Peng, X.W.; Liang, Q.F.; Zhuang, G.D.; Wang, S.M.; Tang, D. Pterostilbene could alleviate diabetic cognitive impairment by suppressing TLR4/NF-кB pathway through microbiota-gut-brain axis. Phytother. Res. 2023, 37, 3522–3542. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Bai, L.; Wei, H.; Guo, Y.; Sun, G.; Sun, H.; Shi, B. Dietary supplementation with pterostilbene activates the PI3K-AKT-mTOR signalling pathway to alleviate progressive oxidative stress and promote placental nutrient transport. J. Anim. Sci. Biotechnol. 2024, 15, 133. [Google Scholar] [CrossRef]
- Matsui, Y.; Sugiyama, K.; Kamei, M.; Takahashi, T.; Suzuki, T.; Katagata, Y.; Ito, T. Extract of Passion Fruit (Passiflora edulis) Seed Containing High Amounts of Piceatannol Inhibits Melanogenesis and Promotes Collagen Synthesis. J. Agric. Food Chem. 2010, 58, 11112–11118. [Google Scholar] [CrossRef]
- Murias, M.; Jäger, W.; Handler, N.; Erker, T.; Horvath, Z.; Szekeres, T.; Nohl, H.; Gille, L. Antioxidant, prooxidant and cytotoxic activity of hydroxylated resveratrol analogues: Structure–activity relationship. Biochem. Pharmacol. 2005, 69, 903–912. [Google Scholar] [CrossRef]
- Dvorakova, M.; Landa, P. Anti-inflammatory activity of natural stilbenoids: A review. Pharmacol. Res. 2017, 124, 126–145. [Google Scholar] [CrossRef]
- Szekeres, T.; Saiko, P.; Fritzer-Szekeres, M.; Djavan, B.; Jäger, W. Chemopreventive effects of resveratrol and resveratrol derivatives. Ann. New York Acad. Sci. 2011, 1215, 89–95. [Google Scholar] [CrossRef]
- Suh, K.S.; Chon, S.; Choi, E.M. Protective effects of piceatannol on methylglyoxal-induced cytotoxicity in MC3T3-E1 osteoblastic cells. Free. Radic. Res. 2018, 52, 712–723. [Google Scholar] [CrossRef]
- Tang, Q.; Feng, Z.; Tong, M.; Xu, J.; Zheng, G.; Shen, L.; Shang, P.; Zhang, Y.; Liu, H. Piceatannol inhibits the IL-1β-induced inflammatory response in human osteoarthritic chondrocytes and ameliorates osteoarthritis in mice by activating Nrf2. Food Funct. 2017, 8, 3926–3937. [Google Scholar] [CrossRef]
- Kil, J.-S.; Jeong, S.-O.; Chung, H.-T.; Pae, H.-O. Piceatannol attenuates homocysteine-induced endoplasmic reticulum stress and endothelial cell damage via heme oxygenase-1 expression. Amino Acids 2016, 49, 735–745. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Kim, H.-H.; Kim, E.-J.; Katakura, Y.; Lee, W.-S.; Kim, G.-S.; Ryu, C.-H. Piceatannol inhibits mast cell-mediated allergic inflammation. Int. J. Mol. Med. 2013, 31, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mai, P.; Yang, Z.; Wang, Z.; Yang, W.; Wang, Z. Piceatannol Protects PC-12 Cells against Oxidative Damage and Mitochondrial Dysfunction by Inhibiting Autophagy via SIRT3 Pathway. Nutrients 2023, 15, 2973. [Google Scholar] [CrossRef] [PubMed]
- Hijona, E.; Aguirre, L.; Pérez-Matute, P.; Villanueva-Millán, M.J.; Mosqueda-Solis, A.; Hasnaoui, M.; Nepveu, F.; Senard, J.M.; Bujanda, L.; Aldámiz-Echevarría, L.; et al. Limited beneficial effects of piceatannol supplementation on obesity complications in the obese Zucker rat: Gut microbiota, metabolic, endocrine, and cardiac aspects. J. Physiol. Biochem. 2016, 72, 567–582. [Google Scholar] [CrossRef]
- Son, Y.; Chung, H.T.; Pae, H.O. Differential effects of resveratrol and its natural analogs, piceatannol and 3,5,4′-trans-trimethoxystilbene, on anti-inflammatory heme oxigenase-1 expression in RAW264.7 macrophages. BioFactors 2013, 40, 138–145. [Google Scholar] [CrossRef]
- Li, H.; Shi, Y.; Wang, X.; Li, P.; Zhang, S.; Wu, T.; Yan, Y.; Zhan, Y.; Ren, Y.; Rong, X.; et al. Piceatannol alleviates inflammation and oxidative stress via modulation of the Nrf2/HO-1 and NF-κB pathways in diabetic cardiomyopathy. Chem.-Biol. Interact. 2019, 310, 108754. [Google Scholar] [CrossRef]
- Liu, D.; Kim, D.-H.; Park, J.-M.; Na, H.-K.; Surh, Y.-J. Piceatannol Inhibits Phorbol Ester-Induced NF-κ B Activation and COX-2 Expression in Cultured Human Mammary Epithelial Cells. Nutr. Cancer 2009, 61, 855–863. [Google Scholar] [CrossRef]
- Son, P.-S.; Park, S.-A.; Na, H.-K.; Jue, D.-M.; Kim, S.; Surh, Y.-J. Piceatannol, a catechol-type polyphenol, inhibits phorbol ester-induced NF-κB activation and cyclooxygenase-2 expression in human breast epithelial cells: Cysteine 179 of IKKβ as a potential target. Carcinogenesis 2010, 31, 1442–1449. [Google Scholar] [CrossRef]
- Liu, L.; Li, J.; Kundu, J.K.; Surh, Y.-J. Piceatannol inhibits phorbol ester-induced expression of COX-2 and iNOS in HR-1 hairless mouse skin by blocking the activation of NF-κB and AP-1. Inflamm. Res. 2014, 63, 1013–1021. [Google Scholar] [CrossRef]
- Shaik, R.A.; Eid, B.G. Piceatannol Affects Gastric Ulcers Induced by Indomethacin: Association of Antioxidant, Anti-Inflammatory, and Angiogenesis Mechanisms in Rats. Life 2022, 12, 356. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Hao, Y.; Wang, Z.; Liu, J.; Wang, J. Piceatannol alleviate ROS-mediated PC-12 cells damage and mitochondrial dysfunction through SIRT3/FOXO3a signaling pathway. J. Food Biochem. 2021, 46, e13820. [Google Scholar] [CrossRef]
- Khan, I.; Preeti, K.; Kumar, R.; Kumar Khatri, D.; Bala Singh, S. Piceatannol promotes neuroprotection by inducing mitophagy and mitobiogenesis in the experimental diabetic peripheral neuropathy and hyperglycemia-induced neurotoxicity. Int. Immunopharmacol. 2023, 116, 109793. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Y.; Zhang, C.; Gao, L.; Li, J. Piceatannol pretreatment alleviates acute cardiac injury via regulating PI3K-Akt-eNOS signaling in H9c2 cells. Biomed. Pharmacother. 2019, 109, 886–891. [Google Scholar] [CrossRef] [PubMed]
- Likhitwitayawuid, K. Oxyresveratrol: Sources, Productions, Biological Activities, Pharmacokinetics, and Delivery Systems. Molecules 2021, 26, 4212. [Google Scholar] [CrossRef] [PubMed]
- Abbas, Z.; Tong, Y.; Wang, J.; Zhang, J.; Wei, X.; Si, D.; Zhang, R. Potential Role and Mechanism of Mulberry Extract in Immune Modulation: Focus on Chemical Compositions, Mechanistic Insights, and Extraction Techniques. Int. J. Mol. Sci. 2024, 25, 5333. [Google Scholar] [CrossRef]
- Hsu, J.-H.; Yang, C.-S.; Chen, J.-J. Antioxidant, Anti-α-Glucosidase, Antityrosinase, and Anti-Inflammatory Activities of Bioactive Components from Morus alba. Antioxidants 2022, 11, 2222. [Google Scholar] [CrossRef]
- Li, J.; Lin, Z.; Tang, X.; Liu, G.; Chen, Y.; Zhai, X.; Huang, Q.; Cao, Y. Oxyresveratrol extracted from Artocarpus heterophyllus Lam. inhibits tyrosinase and age pigments in vitro and in vivo. Food Funct. 2020, 11, 6595–6607. [Google Scholar] [CrossRef]
- Liang, C.; Lim, J.H.; Kim, S.H.; Kim, D.S. Dioscin: A synergistic tyrosinase inhibitor from the roots of Smilax china. Food Chem. 2012, 134, 1146–1148. [Google Scholar] [CrossRef]
- Tran, H.G.; Shuayprom, A.; Kueanjinda, P.; Leelahavanichkul, A.; Wongsinkongman, P.; Chaisomboonpan, S.; Tawatsin, A.; Ruchusatsawat, K.; Wongpiyabovorn, J. Oxyresveratrol Attenuates Inflammation in Human Keratinocyte via Regulating NF-kB Signaling and Ameliorates Eczematous Lesion in DNCB-Induced Dermatitis Mice. Pharmaceutics 2023, 15, 1709. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Jourdes, M.; Da Costa, G.; Courtois, A.; Gabaston, J.; Teissedre, P.-L.; Richard, T.; Krisa, S. Oxyresveratrol and Gnetol Glucuronide Metabolites: Chemical Production, Structural Identification, Metabolism by Human and Rat Liver Fractions, and In Vitro Anti-inflammatory Properties. J. Agric. Food Chem. 2022, 70, 13082–13092. [Google Scholar] [CrossRef]
- Ban, J.Y.; Cho, S.O.; Choi, S.-H.; Ju, H.S.; Kim, J.Y.; Bae, K.; Song, K.-S.; Seong, Y.H. Neuroprotective Effect of Smilacis chinae Rhizome on NMDA-Induced Neurotoxicity In Vitro and Focal Cerebral Ischemia In Vivo. J. Pharmacol. Sci. 2008, 106, 68–77. [Google Scholar] [CrossRef]
- Aziz, R.S.; Siddiqua, A.; Shahzad, M.; Shabbir, A.; Naseem, N. Oxyresveratrol ameliorates ethanol-induced gastric ulcer via downregulation of IL-6, TNF-α, NF-ĸB, and COX-2 levels, and upregulation of TFF-2 levels. Biomed. Pharmacother. 2019, 110, 554–560. [Google Scholar] [CrossRef]
- Chung, K.-O.; Kim, B.-Y.; Lee, M.-H.; Kim, Y.-R.; Chung, H.-Y.; Park, J.-H.; Moon, J.-O. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J. Pharm. Pharmacol. 2003, 55, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Suriyaprom, S.; Srisai, P.; Intachaisri, V.; Kaewkod, T.; Pekkoh, J.; Desvaux, M.; Tragoolpua, Y. Antioxidant and Anti-Inflammatory Activity on LPS-Stimulated RAW 264.7 Macrophage Cells of White Mulberry (Morus alba L.) Leaf Extracts. Molecules 2023, 28, 4395. [Google Scholar] [CrossRef] [PubMed]
- Kutil, Z.; Kvasnicova, M.; Temml, V.; Schuster, D.; Marsik, P.; Cusimamani, E.F.; Lou, J.-D.; Vanek, T.; Landa, P. Effect of Dietary Stilbenes on 5-Lipoxygenase and Cyclooxygenases Activities In Vitro. Int. J. Food Prop. 2014, 18, 1471–1477. [Google Scholar] [CrossRef]
- Lu, H.P.; Jia, Y.N.; Peng, Y.L.; Yu, Y.; Sun, S.L.; Yue, M.T.; Pan, M.H.; Zeng, L.S.; Xu, L. Oxyresveratrol, a Stilbene Compound from Morus alba L. Twig Extract Active Against Trichophyton rubrum. Phytother. Res. 2017, 31, 1842–1848. [Google Scholar] [CrossRef]
- Yin, G.; Pan, C.; Liu, H.; Dong, C.; Chang, X.; Zhou, W.; Wang, S.; Du, Z. Oxyresveratrol Improves Cognitive Impairments and Episodic-like Memory through Modulating Neuroinflammation and PI3K-Akt Signaling Pathway in LPS-Induced Mice. Molecules 2024, 29, 1272. [Google Scholar] [CrossRef]
- Jeandet, P.; Delaunois, B.; Conreux, A.; Donnez, D.; Nuzzo, V.; Cordelier, S.; Clément, C.; Courot, E. Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. BioFactors 2010, 36, 331–341. [Google Scholar] [CrossRef]
- Chong, J.; Poutaraud, A.; Hugueney, P. Metabolism and roles of stilbenes in plants. Plant Sci. 2009, 177, 143–155. [Google Scholar] [CrossRef]
- Mahady, G.B.; Pendland, S.L. Resveratrol inhibits the growth of Helicobacter pylori in vitro. Am. J. Gastroenterol. 2000, 95, 1849. [Google Scholar] [CrossRef]
- Martini, S.; Bonechi, C.; Rossi, C.; Figura, N. Increased susceptibility to resveratrol of Helicobacter pylori strains isolated from patients with gastric carcinoma. J. Nat. Prod. 2011, 74, 2257–2260. [Google Scholar] [CrossRef]
- Augustine, N.; Goel, A.K.; Sivakumar, K.C.; Kumar, R.A.; Thomas, S. Resveratrol--a potential inhibitor of biofilm formation in Vibrio cholerae. Phytomedicine 2014, 21, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Abedini, E.; Khodadadi, E.; Zeinalzadeh, E.; Moaddab, S.R.; Asgharzadeh, M.; Mehramouz, B.; Dao, S.; Samadi Kafil, H.; Hu, W. A Comprehensive Study on the Antimicrobial Properties of Resveratrol as an Alternative Therapy. Evid.-Based Complement. Altern. Med. 2021, 2021, 8866311. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.; Ingmer, H. Antibacterial and antifungal properties of resveratrol. Int. J. Antimicrob. Agents 2019, 53, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Vaňková, E.; Paldrychová, M.; Kašparová, P.; Lokočová, K.; Kodeš, Z.; Maťátková, O.; Kolouchová, I.; Masák, J. Natural antioxidant pterostilbene as an effective antibiofilm agent, particularly for gram-positive cocci. World J. Microbiol. Biotechnol. 2020, 36, 101. [Google Scholar] [CrossRef]
- Xu, D.; Qiao, F.; Xi, P.; Lin, Z.; Jiang, Z.; Romanazzi, G.; Gao, L. Efficacy of pterostilbene suppression of postharvest gray mold in table grapes and potential mechanisms. Postharvest Biol. Technol. 2022, 183, 111745. [Google Scholar] [CrossRef]
- Chalal, M.; Klinguer, A.; Echairi, A.; Meunier, P.; Vervandier-Fasseur, D.; Adrian, M. Antimicrobial Activity of Resveratrol Analogues. Molecules 2014, 19, 7679–7688. [Google Scholar] [CrossRef]
- Mikstacka, R.; Przybylska, D.; Rimando, A.M.; Baer-Dubowska, W. Inhibition of human recombinant cytochromes P450 CYP1A1 and CYP1B1 by trans-resveratrol methyl ethers. Mol. Nutr. Food Res. 2007, 51, 517–524. [Google Scholar] [CrossRef]
- Zakova, T.; Rondevaldova, J.; Bernardos, A.; Landa, P.; Kokoska, L. The relationship between structure and in vitro antistaphylococcal effect of plant-derived stilbenes. Acta Microbiol. Immunol. Hung. 2018, 65, 467–476. [Google Scholar] [CrossRef]
- Joung, D.-K.; Choi, S.-H.; Kang, O.-H.; Kim, S.-B.; Mun, S.-H.; Seo, Y.-S.; Kang, D.-H.; Gong, R.; Shin, D.-W.; Kim, Y.-C.; et al. Synergistic effects of oxyresveratrol in conjunction with antibiotics against methicillin-resistant Staphylococcus aureus. Mol. Med. Rep. 2015, 12, 663–667. [Google Scholar] [CrossRef]
- Mazimba, O. Antioxidant and antibacterial constituents from Morus nigra. Afr. J. Pharm. Pharmacol. 2011, 5, 751–754. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Raorane, C.J.; Ryu, S.Y.; Shim, J.-J.; Lee, J. The anti-biofilm and anti-virulence activities of trans-resveratrol and oxyresveratrol against uropathogenic Escherichia coli. Biofouling 2019, 35, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.-Y.; Chen, T.-T.; Tan, X.-J.; Chen, T.; Jia, A.-Q. The quorum-sensing inhibiting effects of stilbenoids and their potential structure–activity relationship. Bioorganic Med. Chem. Lett. 2015, 25, 5217–5220. [Google Scholar] [CrossRef] [PubMed]
- Basset, C.; Rodrigues, A.M.S.; Eparvier, V.; Silva, M.R.R.; Lopes, N.P.; Sabatier, D.; Fonty, E.; Espindola, L.S.; Stien, D. Secondary metabolites from Spirotropis longifolia (DC) Baill and their antifungal activity against human pathogenic fungi. Phytochemistry 2012, 74, 166–172. [Google Scholar] [CrossRef]
- Perrot, T.; Schwartz, M.; Saiag, F.; Salzet, G.; Dumarçay, S.; Favier, F.; Gérardin, P.; Girardet, J.-M.; Sormani, R.; Morel-Rouhier, M.; et al. Fungal Glutathione Transferases as Tools to Explore the Chemical Diversity of Amazonian Wood Extractives. ACS Sustain. Chem. Eng. 2018, 6, 13078–13085. [Google Scholar] [CrossRef]
- Meng, T.; Liu, C.; Chen, Y.; Yu, M.; He, J.; Tan, B.; Fu, X.; He, J.; Xiao, D. Dietary Chito-oligosaccharide attenuates LPS-challenged intestinal inflammation via regulating mitochondrial apoptotic and MAPK signaling pathway. Int. Immunopharmacol. 2023, 126, 111153. [Google Scholar] [CrossRef]
- Ma, J.; Piao, X.; Mahfuz, S.; Long, S.; Wang, J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 2022, 9, 159–174. [Google Scholar] [CrossRef]
- Gu, Y.; Lou, Y.; Zhou, Z.; Zhao, X.; Ye, X.; Wu, S.; Li, H.; Ji, Y. Resveratrol for inflammatory bowel disease in preclinical studies: A systematic review and meta-analysis. Front. Pharmacol. 2024, 15, 1411566. [Google Scholar] [CrossRef]
- Gomes, M.J.C.; Kolba, N.; Agarwal, N.; Kim, D.; Eshel, A.; Koren, O.; Tako, E. Modifications in the Intestinal Functionality, Morphology and Microbiome Following Intra-Amniotic Administration (Gallus gallus) of Grape (Vitis vinifera) Stilbenes (Resveratrol and Pterostilbene). Nutrients 2021, 13, 3247. [Google Scholar] [CrossRef]
- Serreli, G.; Melis, M.P.; Zodio, S.; Naitza, M.R.; Casula, E.; Peñalver, P.; Lucas, R.; Loi, R.; Morales, J.C.; Deiana, M. Altered paracellular permeability in intestinal cell monolayer challenged with lipopolysaccharide: Modulatory effects of pterostilbene metabolites. Food Chem. Toxicol. 2020, 145, 111729. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Cai, X.; Gu, M.; Sun, J.; Qi, C.; Goulette, T.; Song, M.; Li, Z.; Xiao, H. Dietary resveratrol attenuated colitis and modulated gut microbiota in dextran sulfate sodium-treated mice. Food Funct. 2020, 11, 1063–1073. [Google Scholar] [CrossRef]
- Cai, T.T.; Ye, X.L.; Li, R.R.; Chen, H.; Wang, Y.Y.; Yong, H.J.; Pan, M.L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect Against Diabetic Nephropathy in Mice. Front. Pharmacol. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, D.; Ke, W.; Liang, D.; Hu, X.; Chen, F. Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 2020, 44, 213–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ocansey, D.K.W.; Pei, B.; Zhang, Y.; Wang, N.; Wang, Z.; Mao, F. Resveratrol alleviates DSS-induced IBD in mice by regulating the intestinal microbiota-macrophage-arginine metabolism axis. Eur. J. Med. Res. 2023, 28, 319. [Google Scholar] [CrossRef]
- Fan-Jiang, P.-Y.; Lee, P.-S.; Nagabhushanam, K.; Ho, C.-T.; Pan, M.-H. Pterostilbene Attenuates High-Fat Diet and Dextran Sulfate Sodium-Induced Colitis via Suppressing Inflammation and Intestinal Fibrosis in Mice. J. Agric. Food Chem. 2021, 69, 7093–7103. [Google Scholar] [CrossRef]
- Hu, X.-B.; Kang, R.-R.; Tang, T.-T.; Li, Y.-J.; Wu, J.-Y.; Wang, J.-M.; Liu, X.-Y.; Xiang, D.-X. Topical delivery of 3,5,4′-trimethoxy-trans-stilbene-loaded microemulsion-based hydrogel for the treatment of osteoarthritis in a rabbit model. Drug Deliv. Transl. Res. 2018, 9, 357–365. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Chen, Y.; Li, Y.; Jia, P.; Ji, S.; Zhou, Y.; Wang, T. Dietary pterostilbene supplementation attenuates intestinal damage and immunological stress of broiler chickens challenged with lipopolysaccharide. J. Anim. Sci. 2020, 98, skz373. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Li, Y.; Wang, T. Pterostilbene Confers Protection against Diquat-Induced Intestinal Damage with Potential Regulation of Redox Status and Ferroptosis in Broiler Chickens. Oxidative Med. Cell. Longev. 2023, 2023, 8258354. [Google Scholar] [CrossRef]
- Lv, K.; Song, J.; Wang, J.; Zhao, W.; Yang, F.; Feiya, J.; Bai, L.; Guan, W.; Liu, J.; Ho, C.T.; et al. Pterostilbene Alleviates Dextran Sodium Sulfate (DSS)-Induced Intestinal Barrier Dysfunction Involving Suppression of a S100A8-TLR-4-NF-κB Signaling Cascade. J. Agric. Food Chem. 2024, 72, 18489–18496. [Google Scholar] [CrossRef]
- Yashiro, T.; Yura, S.; Tobita, A.; Toyoda, Y.; Kasakura, K.; Nishiyama, C. Pterostilbene reduces colonic inflammation by suppressing dendritic cell activation and promoting regulatory T cell development. FASEB J. 2020, 34, 14810–14819. [Google Scholar] [CrossRef]
- Jo, H.; Hwang, D.; Kim, J.-K.; Lim, Y.-H. Oxyresveratrol improves tight junction integrity through the PKC and MAPK signaling pathways in Caco-2 cells. Food Chem. Toxicol. 2017, 108, 203–213. [Google Scholar] [CrossRef]
- Hwang, D.; Jo, H.; Hwang, S.; Kim, J.-K.; Kim, I.-H.; Lim, Y.-H. Conditioned medium from LS 174T goblet cells treated with oxyresveratrol strengthens tight junctions in Caco-2 cells. Biomed. Pharmacother. 2017, 85, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.; Ma, S.; Kim, J.-K.; Lim, Y.-H. Oxyresveratrol Ameliorates Dextran Sulfate Sodium-Induced Colitis in Rats by Suppressing Inflammation. Molecules 2021, 26, 2630. [Google Scholar] [CrossRef] [PubMed]
- Radapong, S.; Sarker, S.D.; Ritchie, K.J. Oxyresveratrol Possesses DNA Damaging Activity. Molecules 2020, 25, 2577. [Google Scholar] [CrossRef] [PubMed]
- Newberry, R.D.; Lorenz, R.G. Organizing a mucosal defense. Immunol. Rev. 2005, 206, 6–21. [Google Scholar] [CrossRef] [PubMed]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Soleas, G.J.; Angelini, M.; Grass, L.; Diamandis, E.P.; Goldberg, D.M. Absorption of trans-resveratrol in rats. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 2001; Volume 335, pp. 145–154. [Google Scholar]
- Jaimes, J.D.; Jarosova, V.; Vesely, O.; Mekadim, C.; Mrazek, J.; Marsik, P.; Killer, J.; Smejkal, K.; Kloucek, P.; Havlik, J. Effect of Selected Stilbenoids on Human Fecal Microbiota. Molecules 2019, 24, 744. [Google Scholar] [CrossRef]
- Fu, Q.; Tan, Z.; Shi, L.; Xun, W. Resveratrol Attenuates Diquat-Induced Oxidative Stress by Regulating Gut Microbiota and Metabolome Characteristics in Piglets. Front. Microbiol. 2021, 12, 695155. [Google Scholar] [CrossRef]
- Koh, Y.C.; Lee, P.S.; Kuo, Y.L.; Nagabhushanam, K.; Ho, C.T.; Pan, M.H. Dietary Pterostilbene and Resveratrol Modulate the Gut Microbiota Influenced by Circadian Rhythm Dysregulation. Mol. Nutr. Food Res. 2021, 65, e2100434. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Chen, Y.; Jia, P.; Ji, S.; Zhang, Y.; Wang, T. Resveratrol and its derivative pterostilbene ameliorate intestine injury in intrauterine growth-retarded weanling piglets by modulating redox status and gut microbiota. J. Anim. Sci. Biotechnol. 2021, 12, 70. [Google Scholar] [CrossRef]
- Shih, Y.H.; Tsai, P.J.; Chen, Y.L.; Pranata, R.; Chen, R.J. Assessment of the Antibacterial Mechanism of Pterostilbene against Bacillus cereus through Apoptosis-like Cell Death and Evaluation of Its Beneficial Effects on the Gut Microbiota. J. Agric. Food Chem. 2021, 69, 12219–12229. [Google Scholar] [CrossRef]
- Rui, Z.; Zhang, L.; Li, X.; Han, J.; Yuan, Y.; Ding, H.; Liu, Y.; Ding, X. Pterostilbene exert an anti-arthritic effect by attenuating inflammation, oxidative stress, and alteration of gut microbiota. J. Food Biochem. 2022, 46, e14011. [Google Scholar] [CrossRef] [PubMed]
- Gentile, C.L.; Weir, T.L. The gut microbiota at the intersection of diet and human health. Science 2018, 362, 776–780. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2020, 19, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Richard, M.L.; Leducq, V.; Pham, H.P.; Michel, M.L.; Da Costa, G.; Bridonneau, C.; Jegou, S.; Hoffmann, T.W.; Natividad, J.M.; et al. CARD9 impacts colitis by altering gut microbiota metabolism of tryptophan into aryl hydrocarbon receptor ligands. Nat. Med. 2016, 22, 598–605. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Liu, X.; Yin, J.; Li, X.; Zhang, X.; Xing, X.; Wang, J.; Wang, S. Differential Protective Effect of Resveratrol and Its Microbial Metabolites on Intestinal Barrier Dysfunction is Mediated by the AMPK Pathway. J. Agric. Food Chem. 2022, 70, 11301–11313. [Google Scholar] [CrossRef]
- Ban, W.; Jiang, X.; Lv, L.; Jiao, Y.; Huang, J.; Yang, Z.; You, Y. Illustrate the distribution and metabolic regulatory effects of pterostilbene in cerebral ischemia-reperfusion rat brain by mass spectrometry imaging and spatial metabolomics. Talanta 2024, 266, 125060. [Google Scholar] [CrossRef]
- Haque, P.S.; Kapur, N.; Barrett, T.A.; Theiss, A.L. Mitochondrial function and gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2024, 21, 537–555. [Google Scholar] [CrossRef]
- Ho, G.-t.; Theiss, A.L. Mitochondria and Inflammatory Bowel Diseases: Toward a Stratified Therapeutic Intervention. Annu. Rev. Physiol. 2022, 84, 435–459. [Google Scholar] [CrossRef]
- Hsieh, S.Y.; Shih, T.C.; Yeh, C.Y.; Lin, C.J.; Chou, Y.Y.; Lee, Y.S. Comparative proteomic studies on the pathogenesis of human ulcerative colitis. Proteomics 2006, 6, 5322–5331. [Google Scholar] [CrossRef]
- Chernyavskij, D.A.; Galkin, I.I.; Pavlyuchenkova, A.N.; Fedorov, A.V.; Chelombitko, M.A. Role of Mitochondria in Intestinal Epithelial Barrier Dysfunction in Inflammatory Bowel Disease. Mol. Biol. 2023, 57, 1024–1037. [Google Scholar] [CrossRef]
- Jackson, D.N.; Theiss, A.L. Gut bacteria signaling to mitochondria in intestinal inflammation and cancer. Gut Microbes 2020, 11, 285–304. [Google Scholar] [CrossRef] [PubMed]
- Sifroni, K.G.; Damiani, C.R.; Stoffel, C.; Cardoso, M.R.; Ferreira, G.K.; Jeremias, I.C.; Rezin, G.T.; Scaini, G.; Schuck, P.F.; Dal-Pizzol, F.; et al. Mitochondrial respiratory chain in the colonic mucosal of patients with ulcerative colitis. Mol. Cell. Biochem. 2010, 342, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, S.; Venkatraman, A.; Ramakrishna, B.S. Impairment of mitochondrial acetoacetyl CoA thiolase activity in the colonic mucosa of patients with ulcerative colitis. Gut 2007, 56, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- Figueira, T.R.; Barros, M.H.; Camargo, A.A.; Castilho, R.F.; Ferreira, J.C.B.; Kowaltowski, A.J.; Sluse, F.E.; Souza-Pinto, N.C.; Vercesi, A.E. Mitochondria as a Source of Reactive Oxygen and Nitrogen Species: From Molecular Mechanisms to Human Health. Antioxid. Redox Signal. 2013, 18, 2029–2074. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial ROS-induced ROS release: An update and review. Biochim. Biophys. Acta (BBA)-Bioenerg. 2006, 1757, 509–517. [Google Scholar] [CrossRef]
- Ho, G.T.; Aird, R.E.; Liu, B.; Boyapati, R.K.; Kennedy, N.A.; Dorward, D.A.; Noble, C.L.; Shimizu, T.; Carter, R.N.; Chew, E.T.S.; et al. MDR1 deficiency impairs mitochondrial homeostasis and promotes intestinal inflammation. Mucosal Immunol. 2018, 11, 120–130. [Google Scholar] [CrossRef]
- Saita, S.; Shirane, M.; Nakayama, K.I. Selective escape of proteins from the mitochondria during mitophagy. Nat. Commun. 2013, 4, 1410. [Google Scholar] [CrossRef]
- Vincent, G.; Novak, E.A.; Siow, V.S.; Cunningham, K.E.; Griffith, B.D.; Comerford, T.E.; Mentrup, H.L.; Stolz, D.B.; Loughran, P.; Ranganathan, S.; et al. Nix-Mediated Mitophagy Modulates Mitochondrial Damage During Intestinal Inflammation. Antioxid. Redox Signal. 2020, 33, 1–19. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Mizgier, M.L.; Speisky, H.; Gotteland, M. Differential protective effects of quercetin, resveratrol, rutin and epigallocatechin gallate against mitochondrial dysfunction induced by indomethacin in Caco-2 cells. Chem.-Biol. Interact. 2012, 195, 199–205. [Google Scholar] [CrossRef]
- Zheng, C.-M.; Hou, Y.-C.; Tsai, K.-W.; Hu, W.-C.; Yang, H.-C.; Liao, M.-T.; Lu, K.-C. Resveratrol Mitigates Uremic Toxin-Induced Intestinal Barrier Dysfunction in Chronic Kidney Disease by Promoting Mitophagy and Inhibiting Apoptosis Pathways. Int. J. Med. Sci. 2024, 21, 2437–2449. [Google Scholar] [CrossRef]
- Cao, S.; Shen, Z.; Wang, C.; Zhang, Q.; Hong, Q.; He, Y.; Hu, C. Resveratrol improves intestinal barrier function, alleviates mitochondrial dysfunction and induces mitophagy in diquat challenged piglets. Food Funct. 2019, 10, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Zhao, G.; Wei, S.; Guo, C.; Wu, X.; Zhao, R.C.; Di, G. Pterostilbene alleviates liver ischemia/reperfusion injury via PINK1-mediated mitophagy. J. Pharmacol. Sci. 2022, 148, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, Y.; Chen, Y.; Jia, P.; Ji, S.; Xu, J.; Li, Y.; Wang, T. Comparison of the effects of resveratrol and its derivative pterostilbene on hepatic oxidative stress and mitochondrial dysfunction in piglets challenged with diquat. Food Funct. 2020, 11, 4202–4215. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, J.; Wang, L.; Yang, X.; Gao, K.; Zhu, C.; Jiang, Z. Dietary resveratrol attenuation of intestinal inflammation and oxidative damage is linked to the alteration of gut microbiota and butyrate in piglets challenged with deoxynivalenol. J. Anim. Sci. Biotechnol. 2021, 12, 71. [Google Scholar] [CrossRef]
- Chen, X.; Zeng, Z.; Huang, Z.; Chen, D.; He, J.; Chen, H.; Yu, B.; Yu, J.; Luo, J.; Luo, Y.; et al. Effects of dietary resveratrol supplementation on immunity, antioxidative capacity and intestinal barrier function in weaning piglets. Anim. Biotechnol. 2021, 32, 240–245. [Google Scholar] [CrossRef]
- Meng, Q.; Sun, S.; Luo, Z.; Shi, B.; Shan, A.; Cheng, B. Maternal dietary resveratrol alleviates weaning-associated diarrhea and intestinal inflammation in pig offspring by changing intestinal gene expression and microbiota. Food Funct. 2019, 10, 5626–5643. [Google Scholar] [CrossRef]
- Yang, C.; Luo, P.; Chen, S.J.; Deng, Z.C.; Fu, X.L.; Xu, D.N.; Tian, Y.B.; Huang, Y.M.; Liu, W.J. Resveratrol sustains intestinal barrier integrity, improves antioxidant capacity, and alleviates inflammation in the jejunum of ducks exposed to acute heat stress. Poult. Sci. 2021, 100, 101459. [Google Scholar] [CrossRef]
- Xun, W.; Fu, Q.; Shi, L.; Cao, T.; Jiang, H.; Ma, Z. Resveratrol protects intestinal integrity, alleviates intestinal inflammation and oxidative stress by modulating AhR/Nrf2 pathways in weaned piglets challenged with diquat. Int. Immunopharmacol. 2021, 99, 107989. [Google Scholar] [CrossRef]
- Zhang, L.-Z.; Gong, J.-G.; Li, J.-H.; Hao, Y.-S.; Xu, H.-J.; Liu, Y.-C.; Feng, Z.-H. Dietary resveratrol supplementation on growth performance, immune function and intestinal barrier function in broilers challenged with lipopolysaccharide. Poult. Sci. 2023, 102, 102968. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, F.; Li, Z.; Jin, X.; Chen, X.; Geng, Z.; Hu, H.; Zhang, C. Effects of Resveratrol on Growth Performance, Intestinal Development, and Antioxidant Status of Broilers under Heat Stress. Animals 2021, 11, 1427. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, T.; Wen, Z.; Cheng, X.; Li, C.; Zhang, P.; Xiao, D.; Xu, Y. Unlocking Gut Health: The Potent Role of Stilbenoids in Intestinal Homeostasis. Animals 2025, 15, 417. https://doi.org/10.3390/ani15030417
Meng T, Wen Z, Cheng X, Li C, Zhang P, Xiao D, Xu Y. Unlocking Gut Health: The Potent Role of Stilbenoids in Intestinal Homeostasis. Animals. 2025; 15(3):417. https://doi.org/10.3390/ani15030417
Chicago/Turabian StyleMeng, Tiantian, Ziwei Wen, Xiaofang Cheng, Cencen Li, Pengpeng Zhang, Dingfu Xiao, and Yongjie Xu. 2025. "Unlocking Gut Health: The Potent Role of Stilbenoids in Intestinal Homeostasis" Animals 15, no. 3: 417. https://doi.org/10.3390/ani15030417
APA StyleMeng, T., Wen, Z., Cheng, X., Li, C., Zhang, P., Xiao, D., & Xu, Y. (2025). Unlocking Gut Health: The Potent Role of Stilbenoids in Intestinal Homeostasis. Animals, 15(3), 417. https://doi.org/10.3390/ani15030417





