Genetic Evaluation of Resilience Indicators in Holstein Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
- yijklm is the evaluated indicator, i.e., Var, LnVar, r-auto, or skew;
- μ is population mean;
- Pi × agej is the fixed effect of parity i (five levels) combined with the age at calving j (three classes according to quantiles 10, 90, and the rest), with fifteen levels;
- HYSk is the fixed effect of herd–year–season k (10 herds, 3 years, and 3 seasons—38 levels in total);
- pem is the random effect of the permanent environment of the cow m (3080 levels);
- al is the random effect of individual l, connected with 3-generation pedigree (31,799 levels);
- eijklm is the random residual.
3. Results
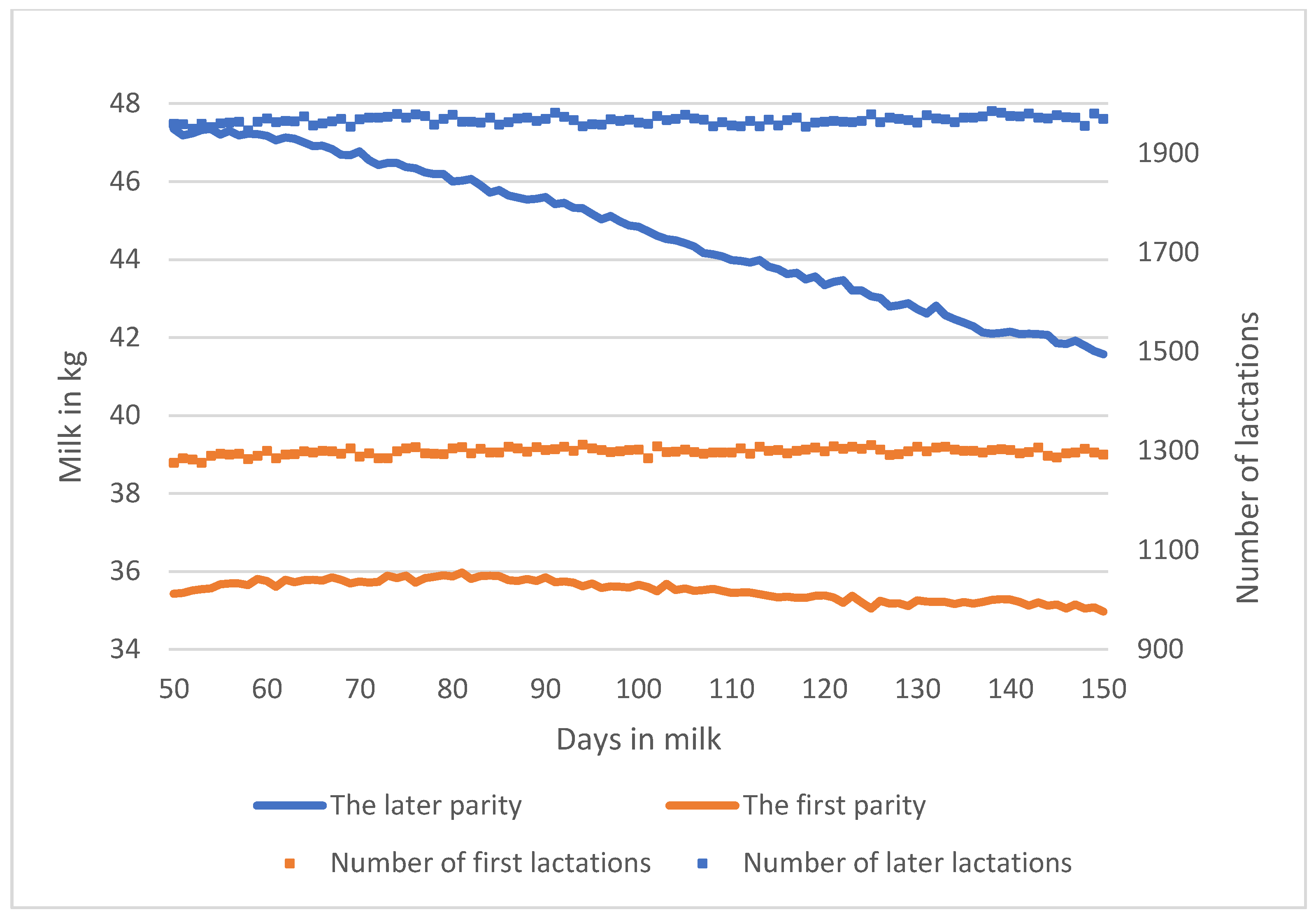
3.1. Phenotypic Characteristics of Resilience Indicators
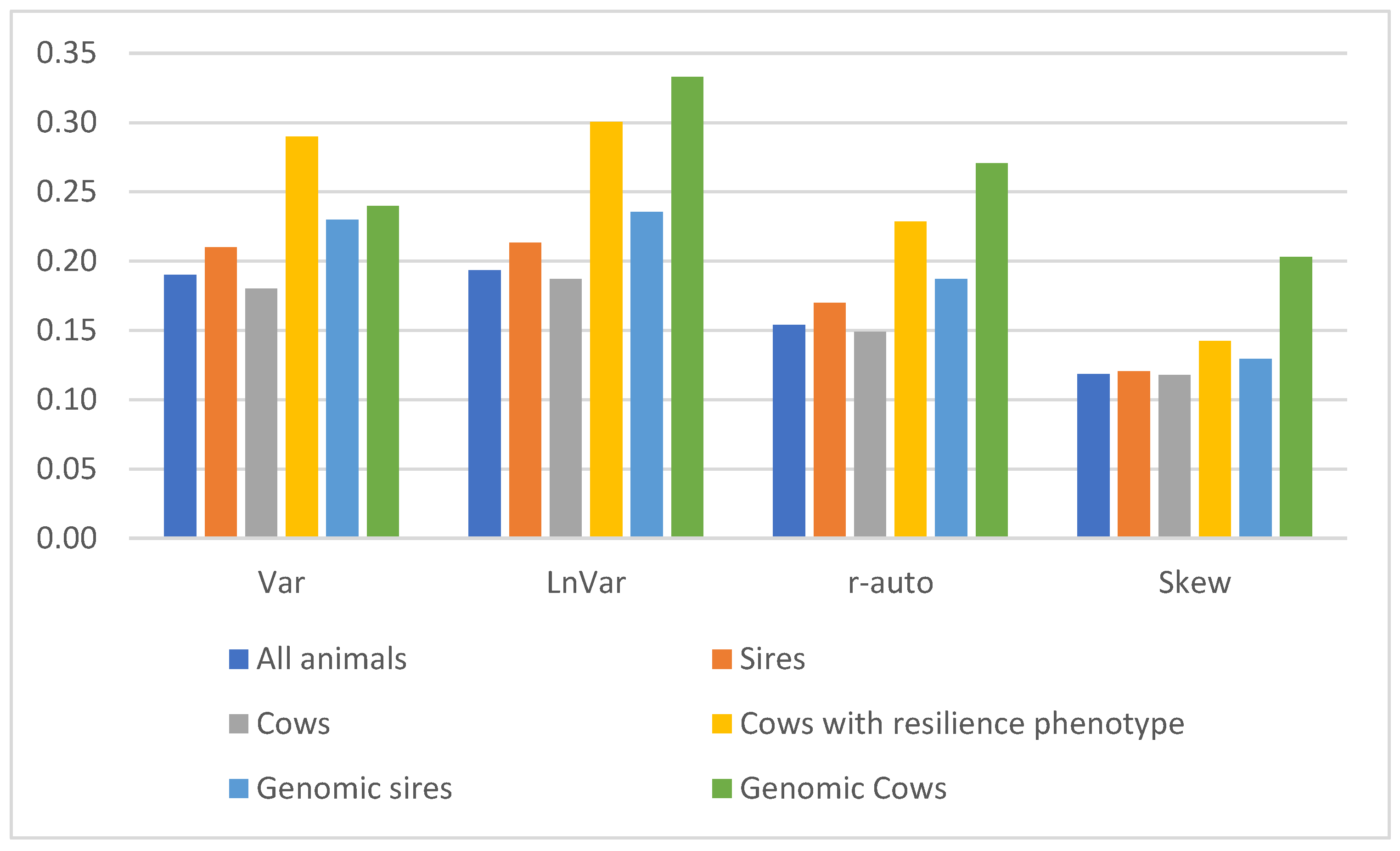
3.2. Genetic Parameters
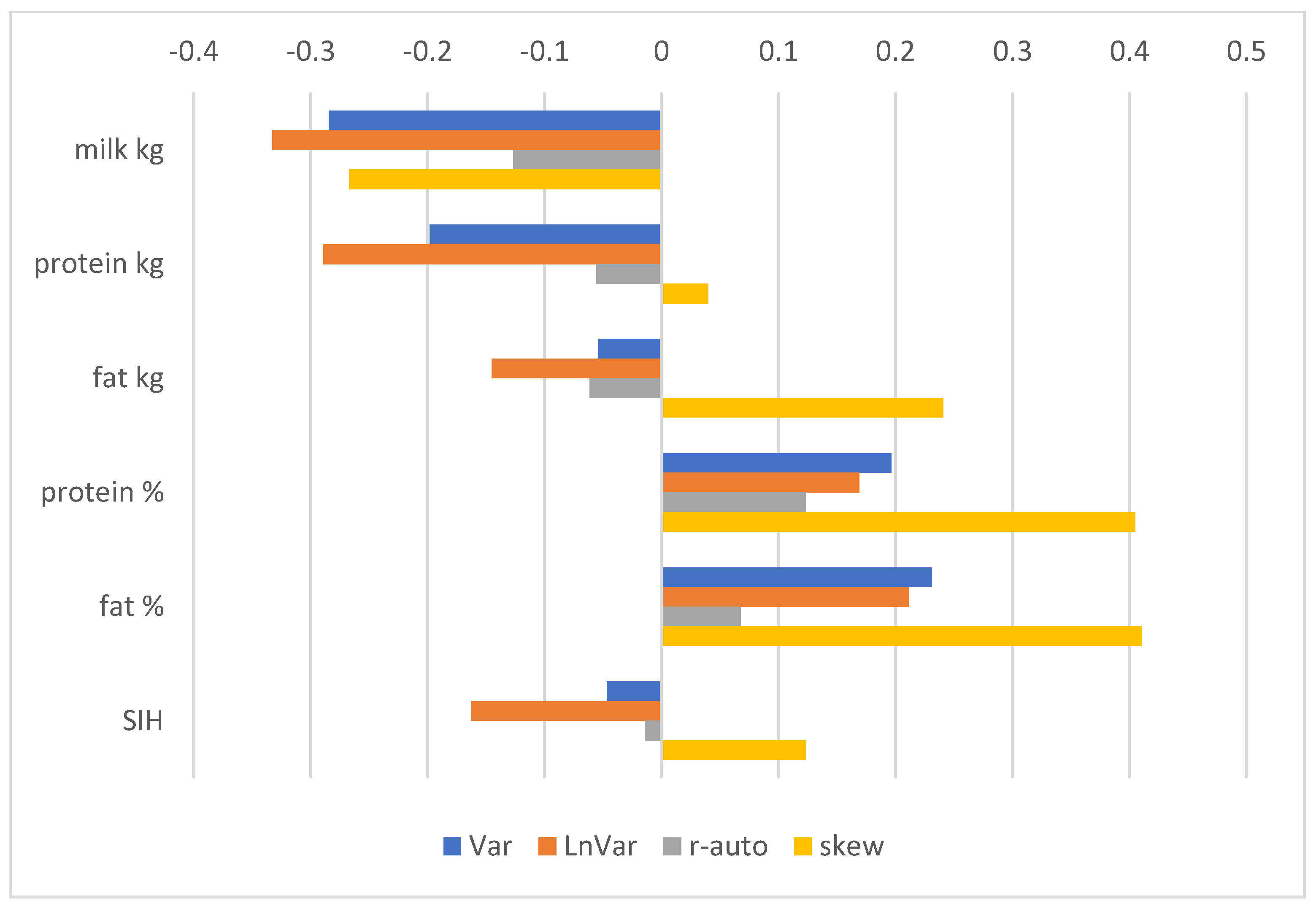
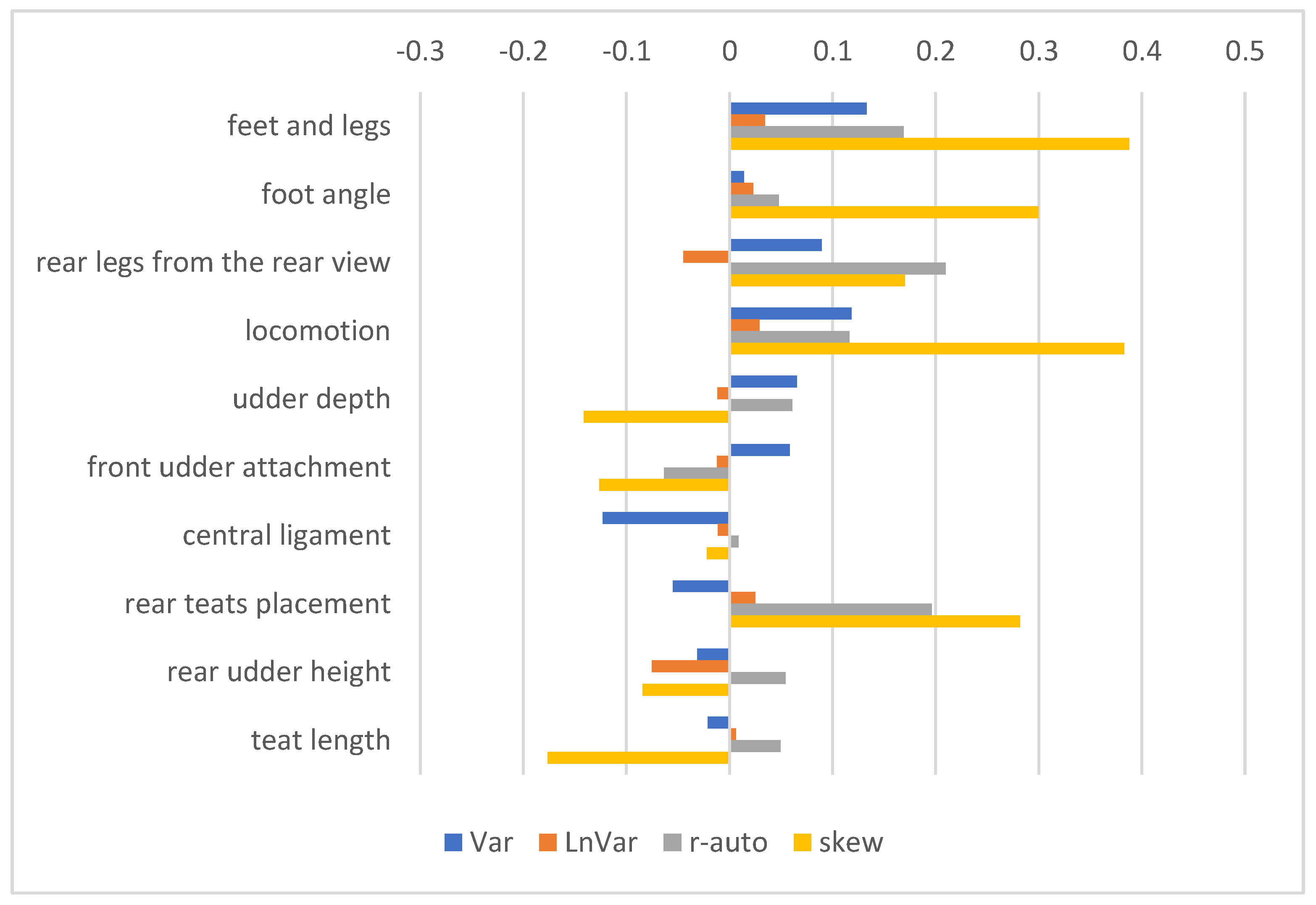
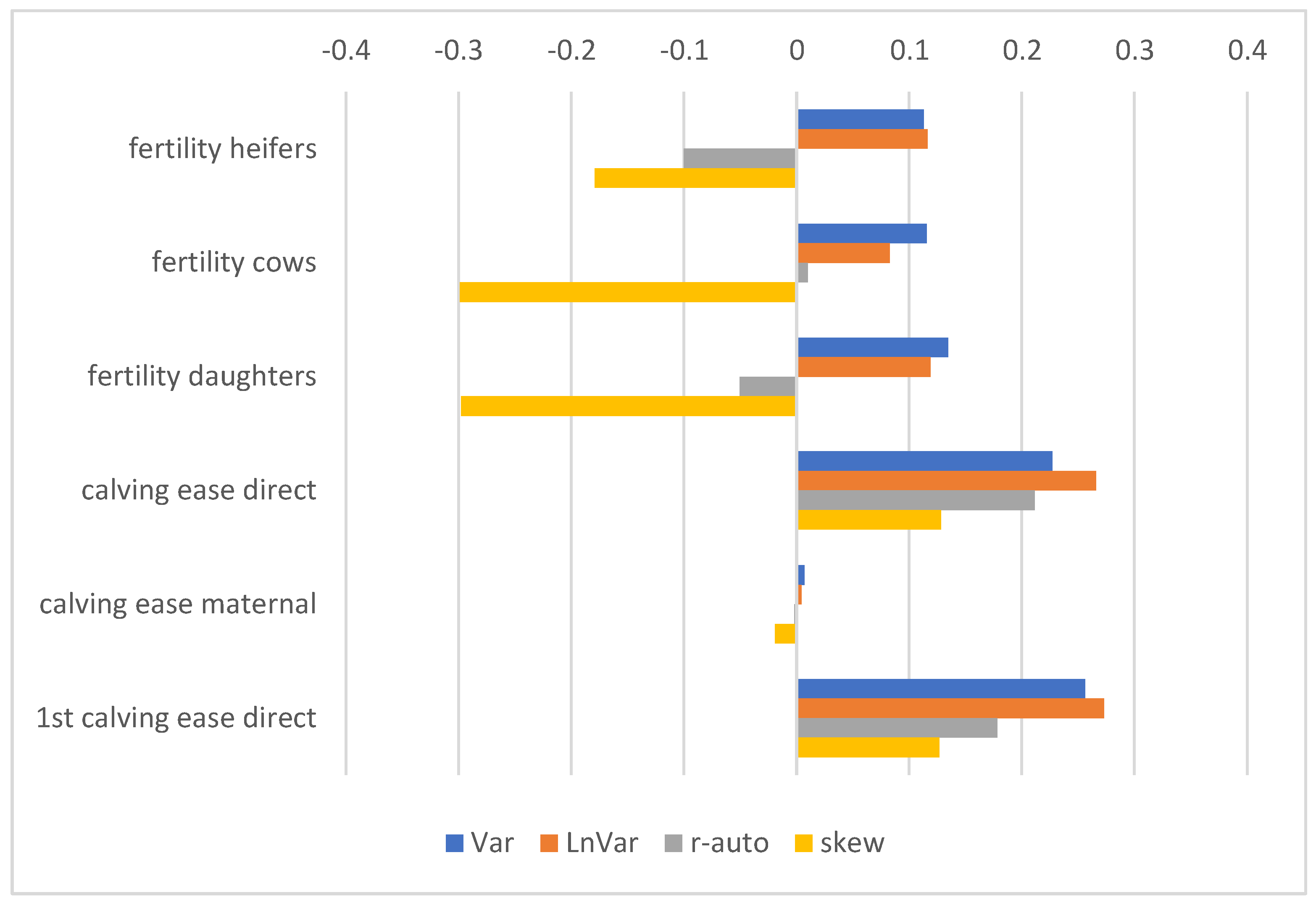
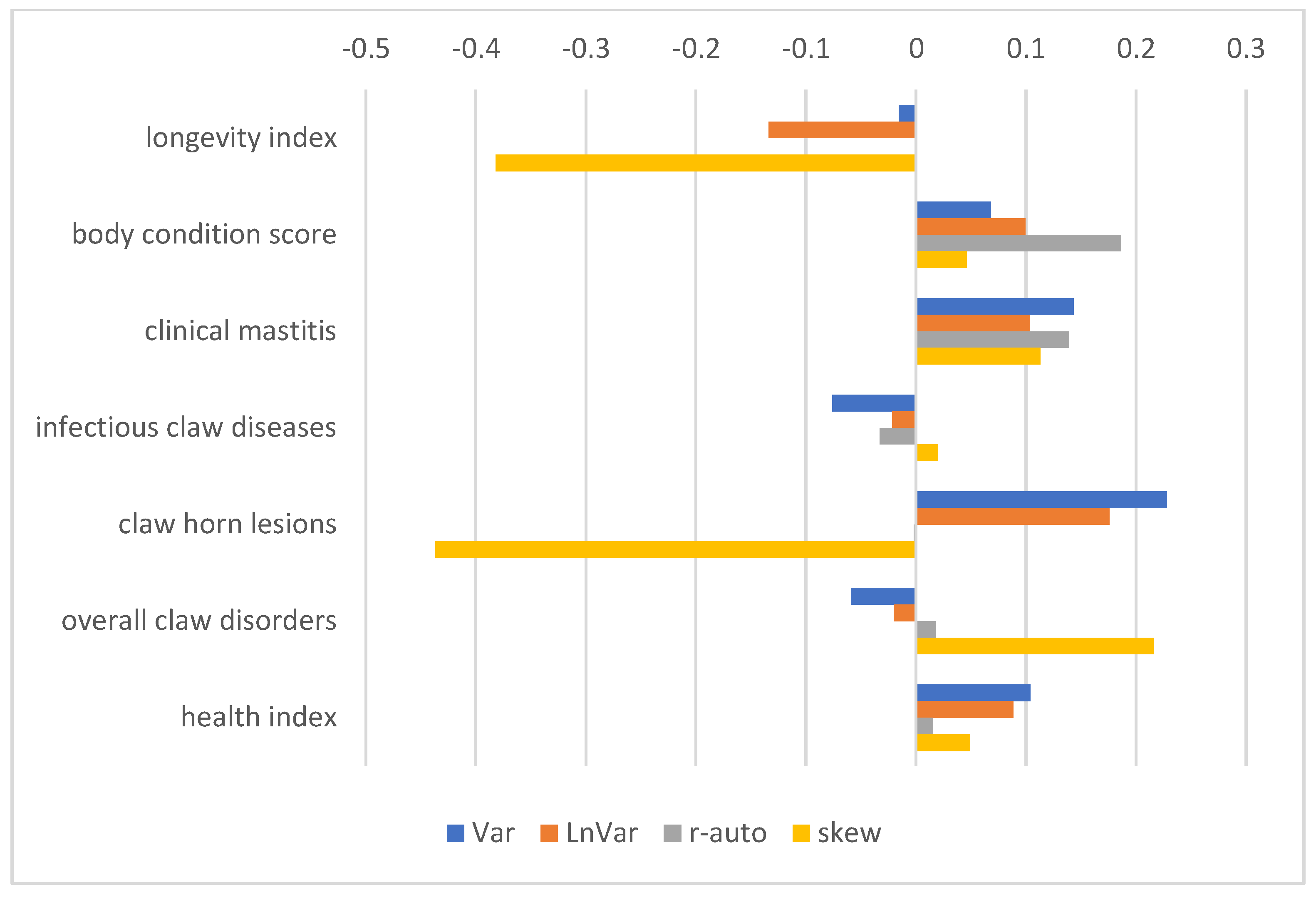
3.3. GEBV and Correlations with Production, Exterior, Reproduction, and Health Traits
4. Discussion
4.1. Genetic Parameters of Resilience Indicators
4.2. Corelations of Resilience Indicators with Other Traits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Food and Agriculture Organization. Climate Change and the Global Dairy Cattle Sector: The Role of the Dairy Sector in a Low-Carbon Future; FAO: Rome, Italy, 2019; ISBN 978-92-5-131232-2. [Google Scholar]
- Gauly, M.; Bollwein, H.; Breves, G.; Brügemann, K.; Dänicke, S.; Daş, G.; Demeler, J.; Hansen, H.; Isselstein, J.; König, S.; et al. Future Consequences and Challenges for Dairy Cow Production Systems Arising from Climate Change in Central Europe—A Review. Animal 2013, 7, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Giannone, C.; Bovo, M.; Ceccarelli, M.; Torreggiani, D.; Tassinari, P. Review of the Heat Stress-Induced Responses in Dairy Cattle. Animals 2023, 13, 3451. [Google Scholar] [CrossRef] [PubMed]
- Colditz, I.G.; Hine, B.C. Resilience in Farm Animals: Biology, Management, Breeding and Implications for Animal Welfare. Anim. Prod. Sci. 2016, 56, 1961–1983. [Google Scholar] [CrossRef]
- Berghof, T.V.L.; Poppe, M.; Mulder, H.A. Opportunities to Improve Resilience in Animal Breeding Programs. Front. Genet. 2019, 9, 692. [Google Scholar] [CrossRef] [PubMed]
- Adriaens, I.; Friggens, N.C.; Ouweltjes, W.; Scott, H.; Aernouts, B.; Statham, J. Productive Life Span and Resilience Rank Can Be Predicted from On-Farm First-Parity Sensor Time Series but Not Using a Common Equation across Farms. J. Dairy Sci. 2020, 103, 7155–7171. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, C.; Thomasen, J.R.; Kargo, M.; Bouquet, A.; Slagboom, M. Emphasis on Resilience in Dairy Cattle Breeding: Possibilities and Consequences. J. Dairy Sci. 2022, 105, 7588–7599. [Google Scholar] [CrossRef] [PubMed]
- Elgersma, G.G.; De Jong, G.; Van Der Linde, R.; Mulder, H.A. Fluctuations in Milk Yield Are Heritable and Can Be Used as a Resilience Indicator to Breed Healthy Cows. J. Dairy Sci. 2018, 101, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Keßler, F.; Wellmann, R.; Chagunda, M.G.G.; Bennewitz, J. Resilience Indicator Traits in 3 Dairy Cattle Breeds in Baden-Württemberg. J. Dairy Sci. 2024, 107, 3780–3793. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Veerkamp, R.F.; Van Pelt, M.L.; Mulder, H.A. Exploration of Variance, Autocorrelation, and Skewness of Deviations from Lactation Curves as Resilience Indicators for Breeding. J. Dairy Sci. 2020, 103, 1667–1684. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Brito, L.F.; Zhang, H.; Shi, R.; Zhu, L.; Liu, D.; Guo, G.; Wang, Y. Exploring Milk Loss and Variability during Environmental Perturbations across Lactation Stages as Resilience Indicators in Holstein Cattle. Front. Genet. 2022, 13, 1031557. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-Y.; Boerman, J.P.; Gloria, L.S.; Pedrosa, V.B.; Doucette, J.; Brito, L.F. Genomic-Based Genetic Parameters for Resilience across Lactations in North American Holstein Cattle Based on Variability in Daily Milk Yield Records. J. Dairy Sci. 2023, 106, 4133–4146. [Google Scholar] [CrossRef] [PubMed]
- Poppe, M.; Bonekamp, G.; Van Pelt, M.L.; Mulder, H.A. Genetic Analysis of Resilience Indicators Based on Milk Yield Records in Different Lactations and at Different Lactation Stages. J. Dairy Sci. 2021, 104, 1967–1981. [Google Scholar] [CrossRef] [PubMed]
- Krupová, Z.; Kašná, E.; Zavadilová, L.; Krupa, E.; Bauer, J.; Wolfová, M. Udder, Claw, and Reproductive Health in Genomic Selection of the Czech Holstein. Animals 2024, 14, 864. [Google Scholar] [CrossRef] [PubMed]
- Seymour, D.J.; Cant, J.P.; Osborne, V.R.; Chud, T.C.S.; Schenkel, F.S.; Miglior, F. A Novel Method of Estimating Milking Interval-Adjusted 24-h Milk Yields in Dairy Cattle Milked in Automated Milking Systems. Anim. Open Space 2022, 1, 100011. [Google Scholar] [CrossRef]
- Masuda, Y. Introduction to BLUPF90 Suite Programs. Available online: https://masuday.github.io/blupf90_tutorial/index.html (accessed on 15 January 2025).
- Misztal, I.; Lourenco, D.; Aguilar, I.; Legarra, A.; Vitezica, Z. Manual for BLUPF90 Family of Programs. Available online: https://nce.ads.uga.edu/html/projects/programs/docs/blupf90_all8.pdf (accessed on 15 January 2025).
- Schöpke, K.; Weidling, W.; Pjil, R.; Swalve, H.H. Relationships between Bovine Hoof Disorders, Body Condition Traits, and Test-Day Yields. J. Dairy Sci. 2013, 96, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Opgenorth, J.; Mayorga, E.J.; Abeyta, M.A.; Rodriguez-Jimenez, S.; Goetz, B.M.; Freestone, A.D.; Baumgard, L.H. Intravenous Lipopolysaccharide Challenge in Early- versus Mid-Lactation Dairy Cattle. II: The Production and Metabolic Responses. J. Dairy Sci. 2024, 107, 6240–6251. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, I.I.; Misztal, I.; Tsuruta, S. Genetic components of heat stress for dairy cattle with multiple lactations. J. Dairy Sci. 2009, 95, 5702–5711. [Google Scholar] [CrossRef] [PubMed]
- Barden, M.; Li, B.; Griffiths, B.E.; Anagnostopoulos, A.; Bedford, C.; Psifidi, A.; Banos, G.; Oikonomou, G. Genetic Parameters and Genome-Wide Association Study of Digital Cushion Thickness in Holstein Cows. J. Dairy Sci. 2022, 105, 8237–8256. [Google Scholar] [CrossRef] [PubMed]
- Endo, N. Possible Causes and Treatment Strategies for the Estrus and Ovulation Disorders in Dairy Cows. J. Reprod. Dev. 2022, 68, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Dobson, H.; Smith, R.; Royal, M.; Knight, C.; Sheldon, I. The High-producing Dairy Cow and Its Reproductive Performance. Reprod. Domest. Anim. 2007, 42, 17–23. [Google Scholar] [CrossRef] [PubMed]
| N | Minimum | Maximum | Mean | SD | Coefficient of Variation | |
|---|---|---|---|---|---|---|
| DMY (kg) | 331,589 | 5.05 | 80.90 | 41.04 | 74.59 | 21.05 |
| DMY conventional (kg) | 85,722 | 5.10 | 80.90 | 41.45 | 63.41 | 19.21 |
| DMY-AMS (kg) | 169,350 | 5.10 | 79.25 | 40.61 | 91.02 | 17.02 |
| DMY robotic parlour (kg) | 76,517 | 5.05 | 78.88 | 41.52 | 49.91 | 23.49 |
| DIM (days) | 331,589 | 50 | 150 | 100.03 | 849.60 | 29.14 |
| Indicator | No | Minimum | Maximum | Mean | Variance | Coefficient of Variation |
|---|---|---|---|---|---|---|
| Var | 3347 | –0.46 | 5.69 | 2.41 | 0.67 | 34.01 |
| LnVar | 3347 | –1.12 | 5.06 | 1.88 | 0.55 | 39.62 |
| r-auto | 3347 | 0.00 | 0.90 | 0.32 | 0.04 | 62.83 |
| skew | 3347 | –5.73 | 5.63 | –0.81 | 1.16 | –132.91 |
| Variance Component | Var | LnVar | r-Auto | Skew |
|---|---|---|---|---|
| 0.057 (0.015) | 0.051 (0.014) | 0.002 (0.0009) | 0.024 (0.012) | |
| 0.019 (0.020) | 0.036 (0.021) | 0.004 (0.0021) | 0.024 (0.042) | |
| 0.404 (0.023) | 0.317 (0.022) | 0.027 (0.0022) | 1.005 (0.050) | |
| 0.480 | 0.404 | 0.034 | 1.053 |
| Trait | Var | LnVar | r-Auto | Skew |
|---|---|---|---|---|
| Var | 12 | 87 | 56 | −43 |
| LnVar | 92 | 13 | 26 | −20 |
| r-auto | 17 | 13 | 7 | −73 |
| skew | 9 | 11 | −58 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kašná, E.; Zavadilová, L.; Vařeka, J. Genetic Evaluation of Resilience Indicators in Holstein Cows. Animals 2025, 15, 667. https://doi.org/10.3390/ani15050667
Kašná E, Zavadilová L, Vařeka J. Genetic Evaluation of Resilience Indicators in Holstein Cows. Animals. 2025; 15(5):667. https://doi.org/10.3390/ani15050667
Chicago/Turabian StyleKašná, Eva, Ludmila Zavadilová, and Jan Vařeka. 2025. "Genetic Evaluation of Resilience Indicators in Holstein Cows" Animals 15, no. 5: 667. https://doi.org/10.3390/ani15050667
APA StyleKašná, E., Zavadilová, L., & Vařeka, J. (2025). Genetic Evaluation of Resilience Indicators in Holstein Cows. Animals, 15(5), 667. https://doi.org/10.3390/ani15050667





