Boat Noise Increases the Oxygen Consumption Rate of the Captive Juvenile Large Yellow Croaker, Larimichthys crocea
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Collection and Husbandry
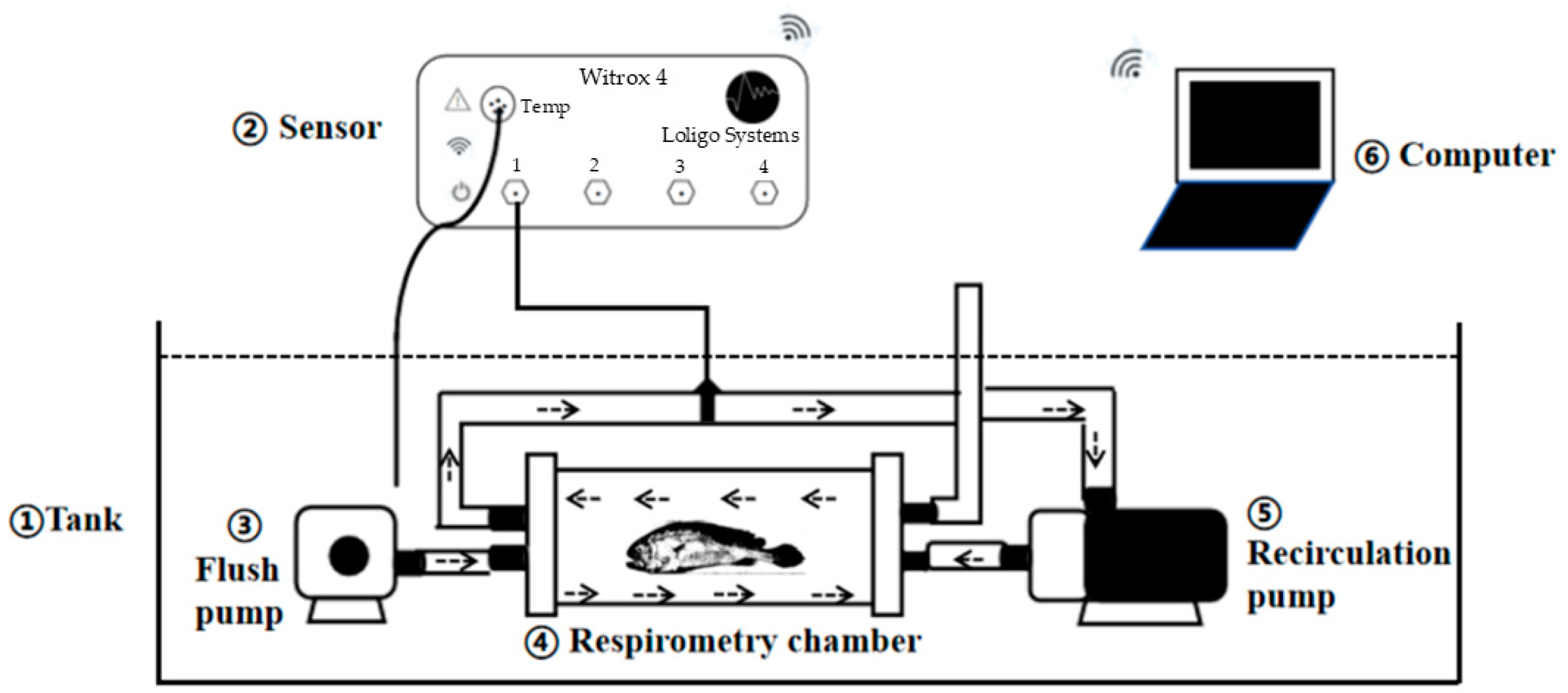
2.2. Intermittent (Stop-Flow) Respirometry
2.3. Experiment on Oxygen Consumption
2.4. Noise Exposure
2.5. Calculations and Analysis
2.6. Statistical Analysis
3. Results
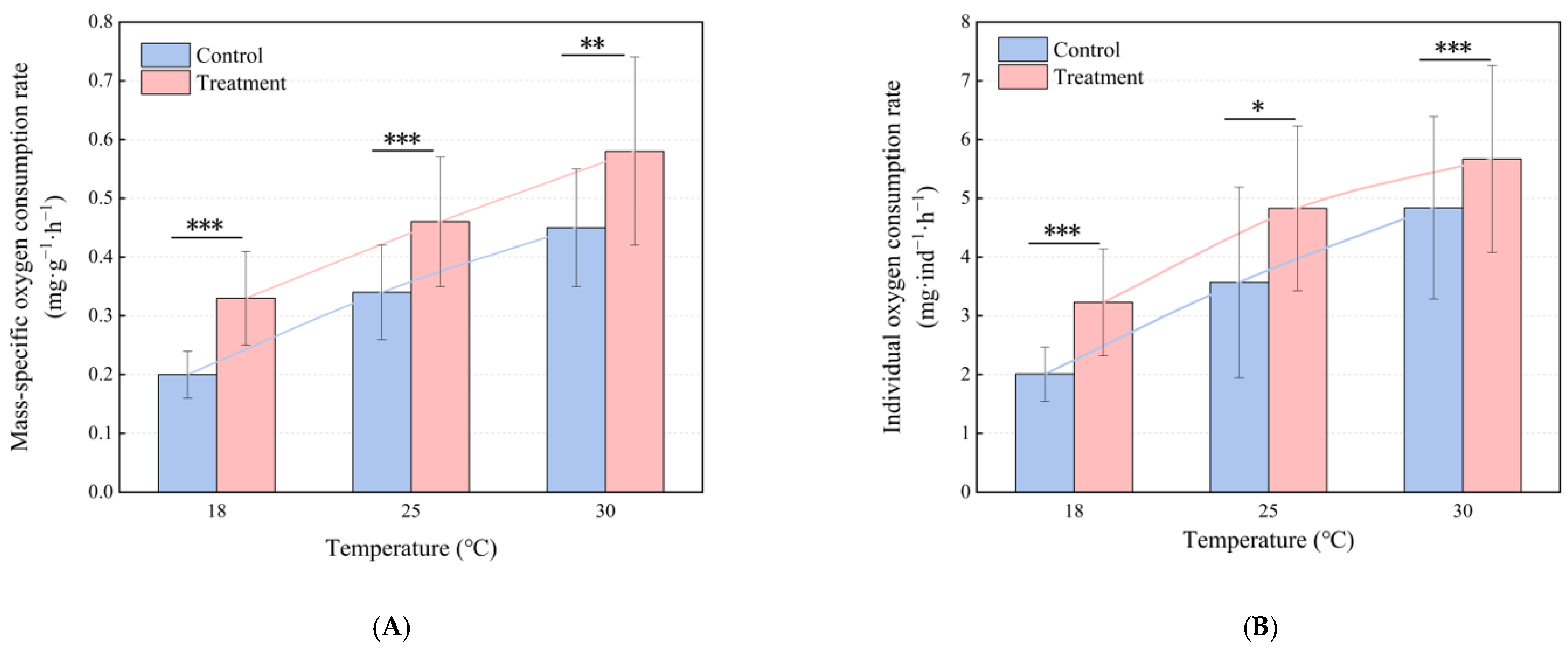
3.1. Effects of Temperature on Oxygen Consumption Rate
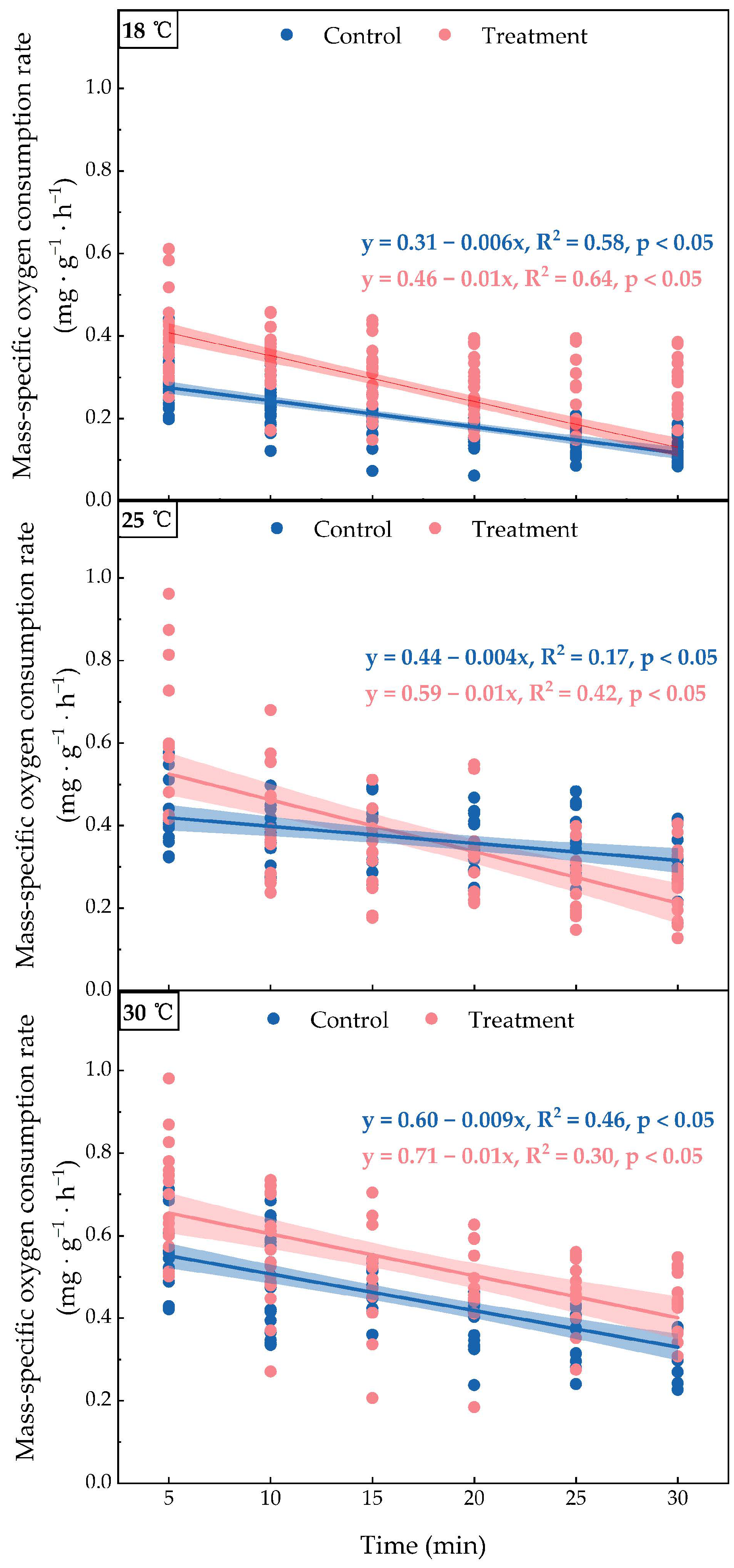
3.2. Changes in Oxygen Consumption Rate After Boat Noise Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Casper, B.M.; Halvorsen, M.B.; Matthews, F.; Carlson, T.J.; Popper, A.N. Recovery of barotrauma injuries resulting from exposure to pile driving sound in two sizes of hybrid striped bass. PLoS ONE 2013, 8, e73844. [Google Scholar] [CrossRef] [PubMed]
- Kight, C.R.; Swaddle, J.P. How and why environmental noise impacts animals: An integrative, mechanistic review. Ecol. Lett. 2011, 14, 1052–1061. [Google Scholar] [CrossRef] [PubMed]
- Wallenius, M.A. The interaction of noise stress and personal project stress on subjective health. J. Environ. Psychol. 2004, 24, 167–177. [Google Scholar] [CrossRef]
- Hildebrand, J.A. Anthropogenic and natural sources of ambient noise in the ocean. Mar. Ecol. Prog. Ser. 2009, 395, 5–20. [Google Scholar] [CrossRef]
- Neo, Y.Y.; Ufkes, E.; Kastelein, R.A.; Winter, H.V.; Ten, C.C.; Slabbekoorn, H. Impulsive sounds change European seabass swimming patterns: Influence of pulse repetition interval. Mar. Pollut. Bull. 2015, 97, 111–117. [Google Scholar] [CrossRef]
- Purser, J.; Radford, A.N. Acoustic noise induces attention shifts and reduces foraging performance in three-spined sticklebacks (Gasterosteus aculeatus). PLoS ONE 2011, 6, e17478. [Google Scholar] [CrossRef] [PubMed]
- Jong, K.D.; Forland, T.N.; Amorim, M.C.P.; Rieucau, G.; Slabbekoorn, H.; Sivle, L.D. Predicting the effects of anthropogenic noise on fish reproduction. Rev. Fish Biol. Fish. 2020, 30, 245–268. [Google Scholar] [CrossRef]
- Francis, J.; Kieran, C.; Lawrence, B.; Sarah, D. The effect of anthropogenic and biological noise on fish behavior and physiology: A meta-analysis. J. Acoust. Soc. Am. 2017, 141, 3862. [Google Scholar] [CrossRef]
- Cox, K.; Brennan, L.P.; Gerwing, T.G.; Dudas, S.E.; Juanes, F. Sound the alarm: A meta-analysis on the effect of aquatic noise on fish behavior and physiology. Glob. Change Biol. 2018, 24, 3105–3116. [Google Scholar] [CrossRef]
- Zhang, X.G.; Tang, X.M.; Zou, L.H. Transcriptome analysis reveals dysfunction of the endoplasmic reticulum protein processing in the sonic muscle of small yellow croaker (Larimichthys polyactis) following noise exposure. Mar. Environ. Res. 2024, 194, 106299. [Google Scholar] [CrossRef]
- Popper, A.N.; Hawkins, A.D. An overview of fish bioacoustics and the impacts of anthropogenic sounds on fishes. J. Fish Biol. 2019, 94, 692–713. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Lau, L.H.; Gordillo-Martinez, F.; Vasconcelos, R.O. The effect of time regime in noise exposure on the auditory system and behavioural stress in the zebrafish. Sci. Rep. 2022, 12, 15353. [Google Scholar] [CrossRef] [PubMed]
- Neo, Y.Y.; Seitz, J.; Kastelein, R.A.; Winter, H.V.; Cate, C.T.; Slabbekoorn, H. Temporal structure of sound affects behavioural recovery from noise impact in European seabass. Biol. Conserv. 2014, 178, 65–73. [Google Scholar] [CrossRef]
- Hubert, J.; Neo, Y.Y.; Winter, H.V.; Slabbekoorn, H. The role of ambient sound levels, signal-to-noise ratio, and stimulus pulse rate on behavioural disturbance of seabass in a net pen. Behav. Processes 2020, 170, 103992. [Google Scholar] [CrossRef]
- Lara, R.A.; Vasconcelos, R.O. Impact of noise on development, physiological stress and behavioural patterns in larval zebrafish. Sci. Rep. 2021, 11, 6615. [Google Scholar] [CrossRef]
- Cai, H.W.; Ross, L.G.; Telfer, T.C.; Wu, C.W.; Zhu, A.Y.; Zhao, S.; Xu, M.Y. Modelling the nitrogen loadings from large yellow croaker (Larimichthys crocea) cage aquaculture. Environ. Sci. Pollut. Res. Int. 2016, 23, 7529–7542. [Google Scholar] [CrossRef]
- Ramcharitar, J.; Gannon, D.P.; Popper, A.N. Bioacoustics of fishes of the family sciaenidae (croakers and drums). Trans. Am. Fish. Soc. 2006, 135, 1409–1431. [Google Scholar] [CrossRef]
- Guillen, A.C.; Borges, M.E.; Herrerias, T.; Kandalski, P.K.; Souza, M.R.D.P.D.; Donatti, L. Gradual increase of temperature trigger metabolic and oxidative responses in plasma and body tissues in the Antarctic fish Notothenia rossii. Fish Physiol. Biochem. 2022, 48, 337–354. [Google Scholar] [CrossRef]
- Egorova, V.I.; Sveshnikova, E.V.; Naumova, V.V.; Kiryanov, D.A.; Smirnova, A.N. Influence of water temperature on metabolic energy structure in fish. Vestn. Astrakhan State Tech. Univ. Ser. Fish. Ind. 2019, 2019, 110–116. [Google Scholar] [CrossRef]
- Ern, R.; Andreassen, A.H.; Jutfelt, F. Physiological mechanisms of acute upper thermal tolerance in fish. Physiology 2023, 38, 141–158. [Google Scholar] [CrossRef]
- Schreck, C.B. Stress and fish reproduction: The roles of allostasis and hormesis. Gen. Comp. Endocrinol. 2010, 165, 549–556. [Google Scholar] [CrossRef]
- Petitjean, Q.; Jean, S.; Gandar, A.; Cte, J.; Jacquin, L. Stress responses in fish: From molecular to evolutionary processes. Sci. Total Environ. 2019, 20, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Ao, J.Q.; Mu, Y.N.; Xiang, L.X.; Fan, D.D.; Feng, M.J.; Zhang, S.C.; Shi, Q.; Zhu, L.Y.; Li, T.; Ding, Y.; et al. Genome sequencing of the perciform fish Larimichthys crocea provides insights into molecular and genetic mechanisms of stress adaptation. PLoS Genet. 2015, 11, e1005118. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, D.L.; van den Thillart, G.E.; Witte, F.; de Bakker, M.A.; Besser, J.; Richardson, M.K.; Spaink, H.P.; Leito, J.T.; Bagowski, C.P. Gene expression profiling of the long-term adaptive response to hypoxia in the gills of adult zebrafish. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, 1512–1519. [Google Scholar] [CrossRef] [PubMed]
- Khater, E.S.; Bahnasawy, A.; El-Ghobashy, H.; Shaban, Y.; Elsheikh, F.; El-Reheem, S.A.; Aboegela, M. Mathematical model for predicting oxygen concentration in tilapia fish farms. Sci. Rep. 2021, 11, 24130. [Google Scholar] [CrossRef]
- Green, L.; Jutfelt, F. Elevated carbon dioxide alters the plasma composition and behaviour of a shark. Biol. Lett. 2014, 10, 1127–1132. [Google Scholar] [CrossRef]
- Bickler, P.E.; Buck, L.T. Hypoxia tolerance in reptiles, amphibians, and fishes: Life with variable oxygen availability. Annu. Rev. Physiol. 2007, 69, 145–170. [Google Scholar] [CrossRef]
- Nour, E.; Wang, H.P. Transcriptional stress responses to environmental and husbandry stressors in aquaculture species. Rev. Aquac. 2016, 8, 61–88. [Google Scholar] [CrossRef]
- Meskendahl, L.; Fontes, R.P.; Herrmann, J.P.; Temming, A. Metabolic costs of spontaneous swimming in Sprattus sprattus L., at different water temperatures. PLoS ONE 2019, 14, e0225568. [Google Scholar] [CrossRef]
- Borowiec, B.G.; Firth, B.L.; Craig, P.M. Oxygen consumption rate during recovery from loss of equilibrium induced by warming, hypoxia, or exhaustive exercise in rainbow darter (Etheostoma caeruleum). J. Fish Biol. 2024, 105, 23–33. [Google Scholar] [CrossRef]
- Yan, Y.L.; Yuan, X.C.; Shi, Y.H.; Liu, Y.H.; Xie, Y.D. Effect of salinity on growth, oxygen consumption rate and ammonia excretion rate of false kelpfish (Sebastiscus marmoratus). J. Dalian Ocean Univ. 2019, 34, 545–551. [Google Scholar] [CrossRef]
- Jing, R.; Song, X.; Liu, R.; Guo, X. Effects of Temperature on Growth and Oxygen Consumption Rate in Juvenile Goldfish (Carassius auratus). Hebei Fish. 2015, 1–3. [Google Scholar]
- Damsgaard, C.; Baliga, V.B.; Bates, E.; Burggren, W.; McKenzie, D.J.; Taylor, E.; Wright, P.A. Evolutionary and cardio-respiratory physiology of air-breathing and amphibious fishes. Acta Physiol. 2020, 228, e13406. [Google Scholar] [CrossRef] [PubMed]
- Reid, S.G.; Sundin, L.; Milsom, W.K. The cardiorespiratory system in tropical fishes: Structure, function, and control. Fish Physiol. 2005, 21, 225–275. [Google Scholar] [CrossRef]
- Wang, Q.F.; Shen, W.L.; Liu, C.; Mu, D.L.; Wu, X.F.; Guo, N.G.; Zhu, J.Q. Effects of multi-environmental factors on physiological and biochemical responses of large yellow croaker, Larimichthys crocea. Chemosphere 2017, 184, 907–915. [Google Scholar] [CrossRef]
- Rubalcaba, J.G. Metabolic responses to cold and warm extremes in the ocean. PLoS Biol. 2024, 22, e3002479. [Google Scholar] [CrossRef]
- Cai, L.; Yin, J.; Yan, X.; Zhou, Y.; Tang, R.; Yu, M. The environmental analysis and site selection of mussel and large yellow croaker aquaculture areas based on high resolution remote sensing. Acta Oceanol. Sin. 2024, 43, 66–86. [Google Scholar] [CrossRef]
- Liu, J.F. Large Yellow Croaker Aquaculture and Biology; Xiamen University Press: Xiamen, China, 2013. [Google Scholar]
- Li, M.Y.; Miao, L.; Chen, J.; Chen, Q.J.; Yu, C. Establishment and discussion on the new model of simulated ecological and grading aquaculture for large yellow croaker. J. Ningbo Univ. Nat. Sci. Eng. Ed. 2017, 30, 1–5. [Google Scholar] [CrossRef]
- Khan, F.U.; Younas, W.; Shang, Y.; Tu, Z.; Khan, M.I.; Zuberi, A.; Wang, Y. Effects of acclimation temperature on growth, physiology and thermal tolerance of the juvenile grass carp Ctenopharyngodon idella. Aquaculture 2024, 581, 740421. [Google Scholar] [CrossRef]
- Jayasundara, N.; Somero, G.N. Physiological plasticity of cardiorespiratory function in a eurythermal marine teleost, the longjaw mudsucker, Gillichthys mirabilis. J. Exp. Biol. 2013, 216, 2111–2121. [Google Scholar] [CrossRef]
- Carter, M.J.; Cortes, P.A.; Rezende, E.L. Temperature variability and metabolic adaptation in terrestrial and aquatic ectotherms. J. Therm. Biol. 2023, 115, 103565. [Google Scholar] [CrossRef] [PubMed]
- Sandblom, E.; Gräns, A.; Axelsson, M.; Seth, H. Temperature acclimation rate of aerobic scope and feeding metabolism in fishes: Implications in a thermally extreme future. Proc. Biol. Sci. 2014, 281, 4482–4500. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Yao, C.W.; Cui, K.; Hao, T.T.; Yin, Z.Y.; Xu, W.X.; Huang, W.X.; Mai, K.S.; Ai, Q.H. Nutritional programming of large yellow croaker (Larimichthys crocea) larvae by dietary vegetable oil: Effects on growth performance, lipid metabolism and antioxidant capacity. Br. J. Nutr. 2022, 129, 967–980. [Google Scholar] [CrossRef]
- Chen, Y.L.; Huang, W.Q.; Shan, X.J.; Chen, J.; Wang, H.B. Growth characteristics of cage-cultured large yellow croaker Larimichthys crocea. Aquac. Rep. 2020, 16, 100242. [Google Scholar] [CrossRef]
- Kullgren, A.; Jutfelt, F.; Fontanillas, R.; Sundell, K.; Samuelsson, L.M.; Wiklander, K.; Kling, P.; Koppe, W.; Larsson, D.G.; Björnsson, B.T.; et al. The impact of temperature on the metabolome and endocrine metabolic signals in atlantic salmon (Salmo salar). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 164, 44–53. [Google Scholar] [CrossRef]
- Kathleen, M.G.; Brittany, B. Social buffering of the stress response: Insights from fishes. Biol. Lett. 2022, 18, 20220332. [Google Scholar] [CrossRef]
- Zotin, A.A.; Klemenov, S.Y. Endogenous biorhythms of the oxygen consumption rate in individual development of Lymnaea stagnalis (Lymnaeidae, Gastropoda). Biol. Bull. Russ. Acad. Sci. 2013, 40, 500–507. [Google Scholar] [CrossRef]
- Slabbekoorn, N.; Bouton, H. Soundscape orientation: A new field in need of sound investigation. Anim. Behav. 2008, 76, e5–e8. [Google Scholar] [CrossRef]
- Melvin, G.D.; Kloser, R.J.; Honkalehto, T. The adaptation of acoustic data from commercial fishing vessels in resource assessment and ecosystem monitoring. Fish. Res. 2016, 178, 13–25. [Google Scholar] [CrossRef]
- Parsons, M.; Meekan, M. Acoustic characteristics of small research vessels. J. Mar. Sci. Eng. 2020, 8, 970. [Google Scholar] [CrossRef]
- Jain-Schlaepfer, S.; Fakan, E.; Rummer, J.L.; Simpson, S.D.; Mccormick, M.I. Impact of motorboats on fish embryos depends on engine type. Conserv. Physiol. 2018, 6, coy014. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.I.; Allan, B.J.M.; Harding, H.; Simpson, S.D. Boat noise impacts risk assessment in a coral reef fish but effects depend on engine type. Sci. Rep. 2018, 8, 3847. [Google Scholar] [CrossRef] [PubMed]
- McCormick, M.I.; Fakan, E.P.; Nedelec, S.L.; Allan, B.J.M. Effects of boat noise on fish fast-start escape response depend on engine type. Sci. Rep. 2019, 9, 6554. [Google Scholar] [CrossRef] [PubMed]
- Erbe, C.; Reichmuth, C.; Cunningham, K.; Lucke, K.; Dooling, R. Communication masking in marine mammals: A review and research strategy. Mar. Pollut. Bull. 2016, 103, 15–38. [Google Scholar] [CrossRef]
- Filiciotto, F.; Cecchini, S.; Buscaino, G.; Maccarrone, V.; Piccione, G.; Fazio, F. Impact of aquatic acoustic noise on oxidative status and some immune parameters in gilthead sea bream Sparus aurata (Linnaeus, 1758) juveniles. Aquac. Res. 2017, 48, 1895–1903. [Google Scholar] [CrossRef]

| Temperature (°C) | Weight (g) | Mass-Specific Oxygen Consumption Rate (mg·g−1·h−1) | Individual Oxygen Consumption Rate (mg·ind−1·h−1) | |||
|---|---|---|---|---|---|---|
| Control | Noise | Control | Noise | Control | Noise | |
| 18.58 ± 0.64 | 10.25 ± 1.25 Aa | 9.99 ± 1.29 Aa | 0.20 ± 0.04 Aa | 0.33 ± 0.08 Ba | 2.01 ± 0.46 Aa | 3.23 ± 0.91 Ba |
| 25.26 ± 0.02 | 10.25 ± 3.16 Aa | 10.35 ± 1.53 Aa | 0.34 ± 0.08 Ab | 0.46 ± 0.11 Bb | 3.57 ± 1.62 Ab | 4.83 ± 1.40 Bb |
| 30.07 ± 0.09 | 10.75 ± 2.02 Aa | 10.03 ± 2.05 Aa | 0.45 ± 0.10 Ac | 0.58 ± 0.16 Bc | 4.84 ± 1.55 Ac | 5.67 ± 1.59 Bc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, R.; Yang, S.; Li, Y.; Zhang, X.; Tang, X. Boat Noise Increases the Oxygen Consumption Rate of the Captive Juvenile Large Yellow Croaker, Larimichthys crocea. Animals 2025, 15, 714. https://doi.org/10.3390/ani15050714
Xu R, Yang S, Li Y, Zhang X, Tang X. Boat Noise Increases the Oxygen Consumption Rate of the Captive Juvenile Large Yellow Croaker, Larimichthys crocea. Animals. 2025; 15(5):714. https://doi.org/10.3390/ani15050714
Chicago/Turabian StyleXu, Ruijie, Shouguo Yang, Yiyu Li, Xuguang Zhang, and Xianming Tang. 2025. "Boat Noise Increases the Oxygen Consumption Rate of the Captive Juvenile Large Yellow Croaker, Larimichthys crocea" Animals 15, no. 5: 714. https://doi.org/10.3390/ani15050714
APA StyleXu, R., Yang, S., Li, Y., Zhang, X., & Tang, X. (2025). Boat Noise Increases the Oxygen Consumption Rate of the Captive Juvenile Large Yellow Croaker, Larimichthys crocea. Animals, 15(5), 714. https://doi.org/10.3390/ani15050714






