Semen Quality, Testicular Cell Apoptosis, and Transcriptome Analysis Following Mild Scrotal Heat Stress in Wugu–Hu Crossbred and Hu Rams
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Welfare and Experimental Design
2.2. Scrotal Insulation
2.3. Temperature Records and Temperature–Humidity Index (THI)
2.4. Scrotal Circumference
2.5. Semen Collection and Evaluation
2.6. Testicular Sample Collection for Transcriptome Analysis and Preparation of Paraffin Tissue Sections
2.7. RNA Extraction and Library Preparation
2.8. Quality Control and Gene Expression Quantification
2.9. Function Enrichment Analysis
2.10. Real-Time Quantitative Polymerase Chain Reaction
2.11. TUNEL Assay for Detecting Apoptosis
2.12. Statistical Analysis
3. Results
3.1. Surface Temperature of the Scrotum
3.2. Housing and Ram Scrotal Insulation THI
3.3. Scrotal Circumference and Semen Concentration
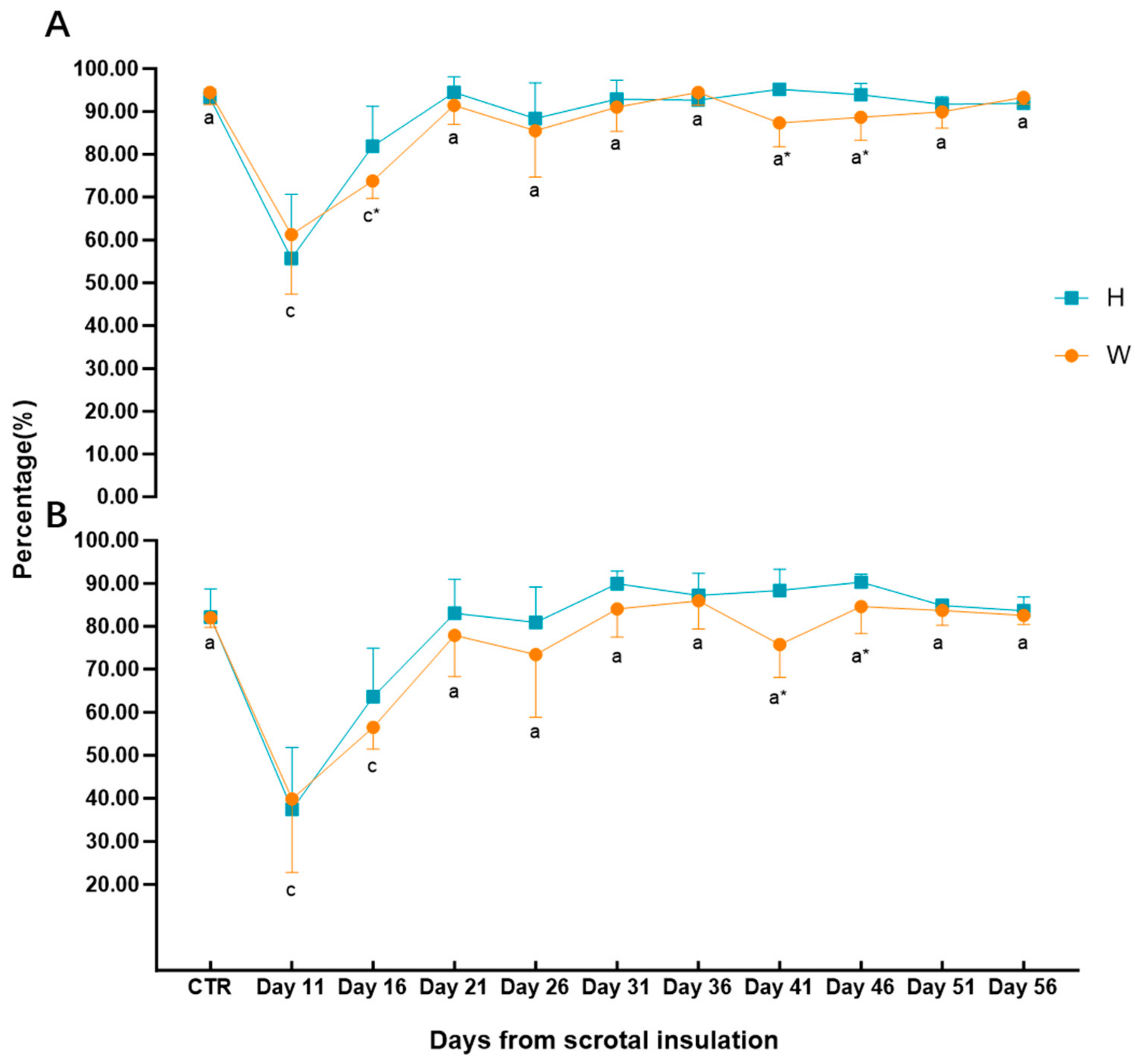
3.4. Sperm Motility
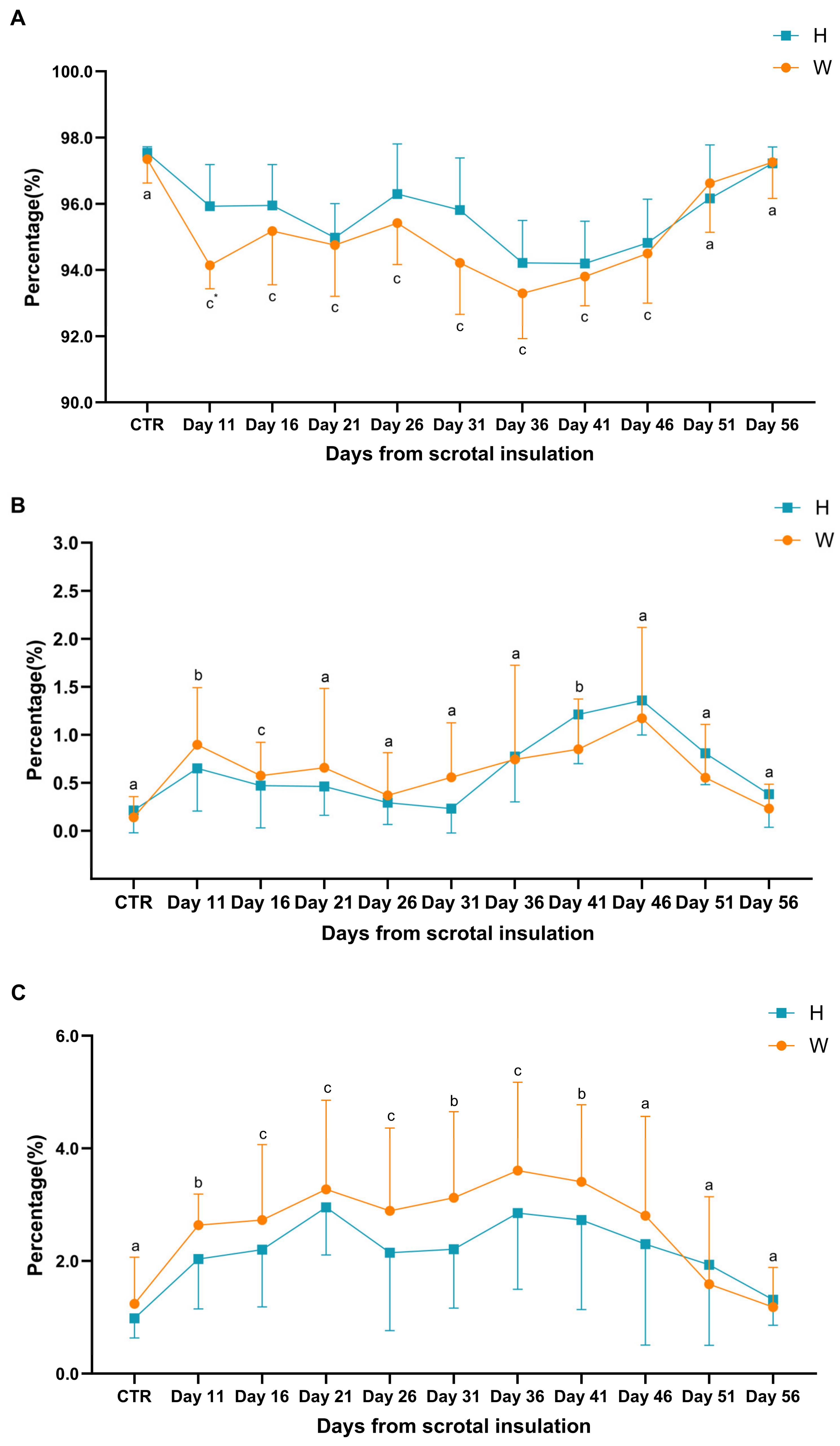
3.5. Sperm Morphology
3.6. RT-qPCR Validation
3.7. Transcriptome Characteristics of Ram Testis
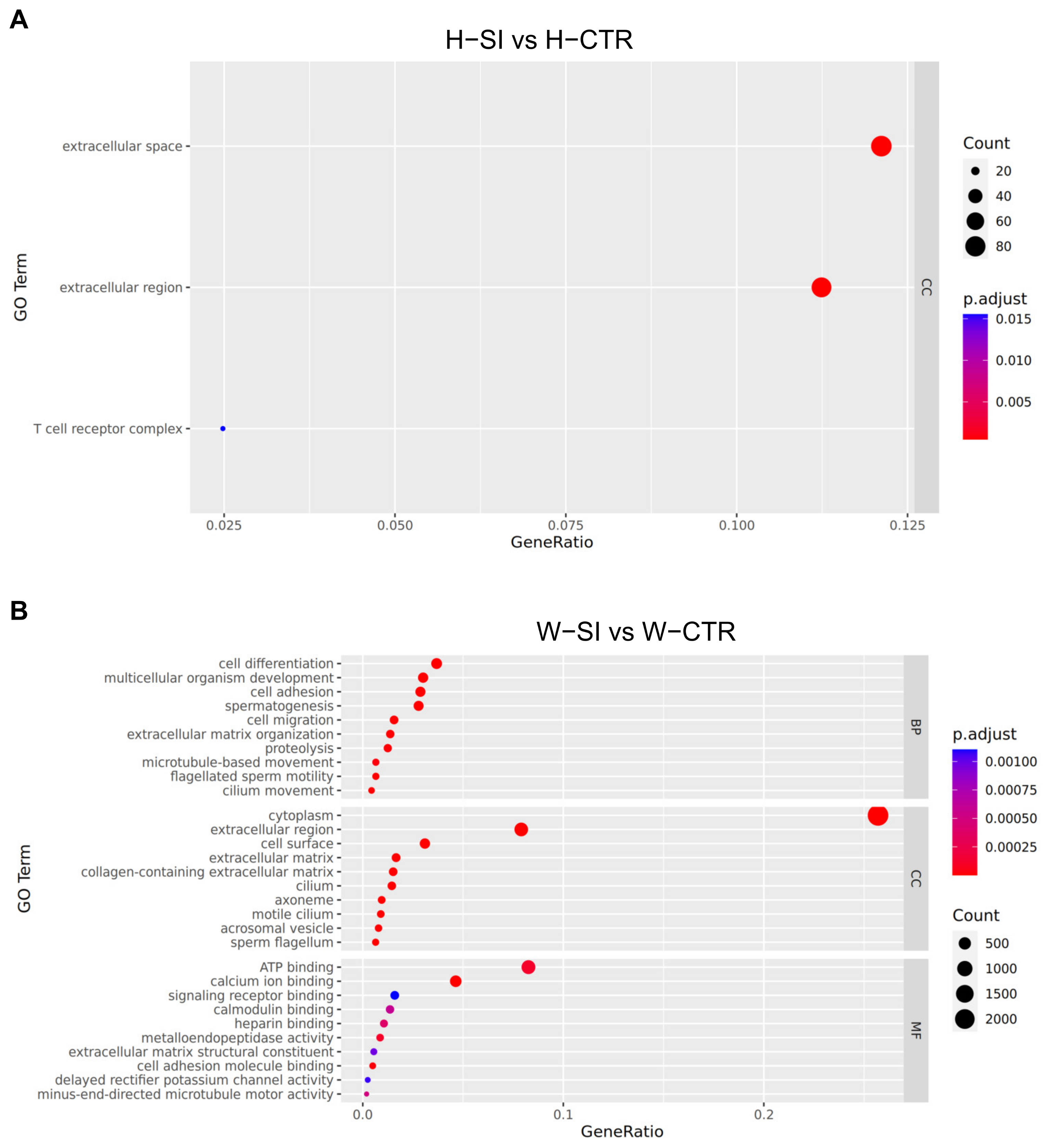
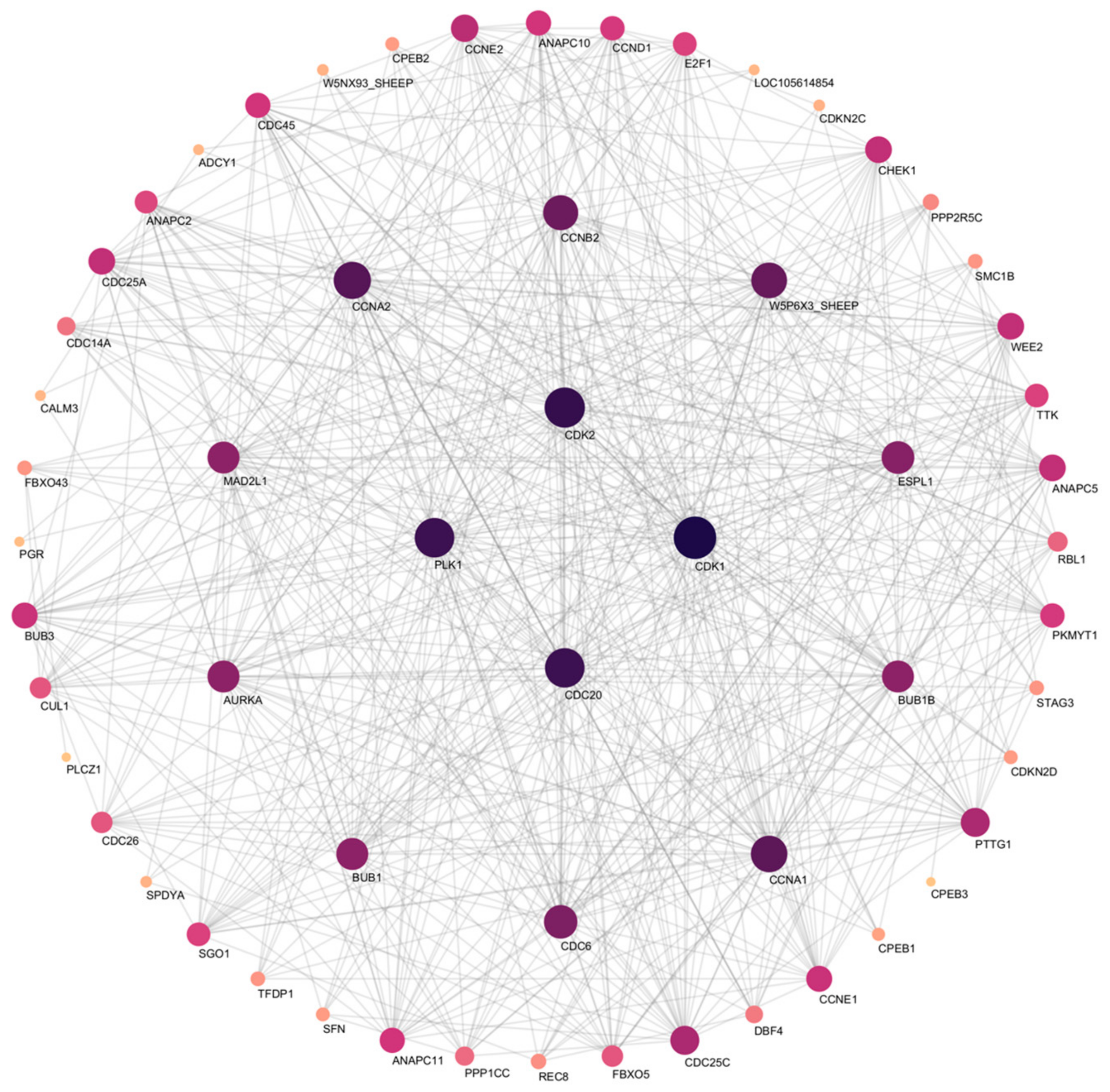
3.8. Function Analysis of DEGs
3.9. The Effect of Scrotal Heat Stress on Testicular Cell Apoptosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AV | Artificial vagina |
| BP | Biological Process |
| CASA | Computer-aided sperm analysis |
| CC | Cellular Component |
| CTR | Control |
| CI | Confidence interval |
| DEG | Differentially expressed gene |
| DNA | Deoxyribonucleic acid |
| EDTA | Ethylenediaminetetraacetic acid |
| FC | Fold change |
| FSH | Follicle-stimulating hormone |
| GO | Gene Ontology |
| H-SI | Hu ram with scrotal insulation |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LH | Luteinizing hormone |
| MF | Molecular Function |
| mRNA | Messenger ribonucleic acid |
| NCBI | National Center for Biotechnology Information |
| PBS | Phosphate-buffered saline |
| PCR | Polymerase chain reaction |
| PMN | Percentage morphologically normal |
| PPI | Protein-protein interaction |
| qPCR | Quantitative polymerase chain reaction |
| RNA | Ribonucleic acid |
| ROS | Reactive oxygen species |
| SE | Standard error |
| SI | Scrotal insulation |
| THI | Temperature–Humidity Index |
| W-SI | Wugu–Hu ram with scrotal insulation |
References
- Kennedy, J.; Trewin, B.; Betts, R.; Thorne, P.; Foster, P.; Siegmund, P.; Ziese, M.; Mishra, S.; Uhlenbrook, S.; Alvar-Beltran, J. State of the Climate 2024. Update for COP29. 2024. Available online: https://mural.maynoothuniversity.ie/id/eprint/19179/1/State-Climate-2024-Update-COP29_en.pdf (accessed on 26 February 2025).
- Wu, J.; Han, Z.; Li, R.; Xu, Y.; Shi, Y. Changes of extreme climate events and related risk exposures in Huang-Huai-Hai river basin under 1.5–2 °C global warming targets based on high resolution combined dynamical and statistical downscaling dataset. Int. J. Climatol. 2021, 41, 1383–1401. [Google Scholar] [CrossRef]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.; Snelling, E.P.; Hetem, R.S.; Maloney, S.K.; Strauss, W.M.; Fuller, A. Revisiting concepts of thermal physiology: Predicting responses of mammals to climate change. J. Anim. Ecol. 2018, 87, 956–973. [Google Scholar] [CrossRef] [PubMed]
- Shahat, A.M.; Thundathil, J.C.; Kastelic, J.P. Melatonin improves post-thaw sperm quality after mild testicular heat stress in rams. Reprod. Domest. Anim. 2023, 58, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, T.R.; Mendes, C.M.; de Castro, L.S.; de Assis, P.M.; Siqueira, A.F.; Delgado Jde, C.; Goissis, M.D.; Muino-Blanco, T.; Cebrian-Perez, J.A.; Nichi, M.; et al. Evaluation of Lasting Effects of Heat Stress on Sperm Profile and Oxidative Status of Ram Semen and Epididymal Sperm. Oxid. Med. Cell. Longev. 2016, 2016, 1687657. [Google Scholar] [CrossRef]
- Van Wettere, W.; Kind, K.L.; Gatford, K.L.; Swinbourne, A.M.; Leu, S.T.; Hayman, P.T.; Kelly, J.M.; Weaver, A.C.; Kleemann, D.O.; Walker, S.K. Review of the impact of heat stress on reproductive performance of sheep. J. Anim. Sci. Biotechnol. 2021, 12, 26. [Google Scholar] [CrossRef]
- Rizzoto, G.; Kastelic, J.P. A new paradigm regarding testicular thermoregulation in ruminants? Theriogenology 2020, 147, 166–175. [Google Scholar] [CrossRef]
- Paul, C.; Murray, A.A.; Spears, N.; Saunders, P.T. A single, mild, transient scrotal heat stress causes DNA damage, subfertility and impairs formation of blastocysts in mice. Reproduction 2008, 136, 73–84. [Google Scholar] [CrossRef]
- Barragán Sierra, A.; Avendaño-Reyes, L.; Hernández Rivera, J.A.; Vicente-Pérez, R.; Correa-Calderón, A.; Mellado, M.; Meza-Herrera, C.A.; Macías-Cruz, U. Thermoregulation and reproductive responses of rams under heat stress. Review. Rev. Mex. Cienc. Pecu. 2021, 12, 910–931. [Google Scholar] [CrossRef]
- Perez-Crespo, M.; Pintado, B.; Gutierrez-Adan, A. Scrotal heat stress effects on sperm viability, sperm DNA integrity, and the offspring sex ratio in mice. Mol. Reprod. Dev. 2008, 75, 40–47. [Google Scholar] [CrossRef]
- Suhair, S.M.; Abdalla, M.A. Effects of seasonal changes and shearing on thermoregulation, blood constituents and semen characteristics of desert rams (Ovis aries). Pak. J. Biol. Sci. 2013, 16, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.R.; Javvaji, P.K.; Dhali, A.; Kolte, A.P.; Roy, S.C.; Giridhar, K.; Sejian, V. Seasonal variations in quality, preservability and fertilizing ability of ovine spermatozoa. Biol. Rhythm. Res. 2020, 51, 951–962. [Google Scholar] [CrossRef]
- Panyaboriban, S.; Suwimonteerabutr, J.; Swangchan-Uthai, T.; Tharasanit, T.; Phutikanit, N.; Techakumphu, M. Effect of heat stress on reproductive performance of an imported dorper ram: A case study in Thailand. Thai J. Vet. Med. 2016, 46, 671–677. [Google Scholar] [CrossRef]
- Júnior, C.A.C.; Lucci, C.M.; Peripolli, V.; Tanure, C.B.; Ribeiro, L.M.C.S.; Barbosa, T.M.; Ramos, A.F.; Louvandini, H.; McManus, C. Laser and thermographic infrared temperatures associated with heat tolerance in adult rams. Small Rumin. Res. 2015, 132, 86–91. [Google Scholar] [CrossRef]
- Arman, C.; Quintana Casares, P.I.; Sanchez-Partida, L.G.; Setchell, B.P. Ram sperm motility after intermittent scrotal insulation evaluated by manual and computer-assisted methods. Asian J. Androl. 2006, 8, 411–418. [Google Scholar] [CrossRef]
- Moore, C.R.; Oslund, R. Experiments on the sheep testis—Cryptorchidism, vasectomy and scrotal insulation. Am. J. Physiol.-Leg. Content 1924, 67, 595–607. [Google Scholar] [CrossRef]
- Ross, A.D.; Entwistle, K.W. The effect of scrotal insulation on spermatozoal morphology and the rates of spermatogenesis and epididymal passage of spermatozoa in the bull. Theriogenology 1979, 11, 111–129. [Google Scholar] [CrossRef]
- Shahat, A.M.; Thundathil, J.C.; Kastelic, J.P. Melatonin improves testicular hemodynamics and sperm quality in rams subjected to mild testicular heat stress. Theriogenology 2022, 188, 163–169. [Google Scholar] [CrossRef]
- Shahat, A.M.; Thundathil, J.C.; Kastelic, J.P. Scrotal subcutaneous temperature is increased by scrotal insulation or whole-body heating, but not by scrotal neck insulation; however, all three heat-stress models decrease sperm quality in bulls and rams. J. Therm. Biol. 2021, 100, 103064. [Google Scholar] [CrossRef]
- Júnior, C.C.; Lucci, C.; Peripolli, V.; Silva, A.; Menezes, A.; Morais, S.; Araújo, M.; Ribeiro, L.; Mattos, R.; McManus, C. Effects of testicle insulation on seminal traits in rams: Preliminary study. Small Rumin. Res. 2015, 130, 157–165. [Google Scholar] [CrossRef]
- Cruz, J.; Lucci, C.M.; Peripolli, V.; Tanure, C.B.; da Silva, A.F.; de Menezes, A.M.; Ramos, A.F.; McManus, C. Breed comparison for heat adaptation in rams using multivariate analysis. Biosci. J. 2016, 32, 178–190. [Google Scholar] [CrossRef]
- Lisboa Neto, A.F.d.S.; Silva, P.H.F.; Santos, A.C.d.; Santos, I.G.C.d.; Santos, G.V.d.; Santos, N.P.d.S.; Silva, I.P.d.; Rocha, H.B.d.; Silva Filho, M.L.d.; Assis Neto, A.C.d. Effect of racial crossing on the seminal parameters of rams submitted to heat stress. Biosci. J. 2020, 36, 1700–1704. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, A.; Wang, B.; Geng, X.; Zheng, A. Research Progress on Mechanism Melanin Deposition and Related Candidate Gemes in Black-bone Sheep. China Anim. Husb. Vet. Med. 2021, 48, 4588–4596. [Google Scholar]
- Mao, H.; Deng, W.; Sun, S.; Shu, W.; Yang, S. Studies on the Specific Characteristics of Yunnan Black-bone Sheep. J. Yunnan Agric. Univ. 2005, 20, 89–93. [Google Scholar]
- Chong, Y.; Xiong, H.; Gao, Z.; Lu, Y.; Hong, J.; Wu, J.; He, X.; Xi, D.; Tu, X.; Deng, W. Genomic and Transcriptomic Landscape to Decipher the Genetic Basis of Hyperpigmentation in Lanping Black-Boned Sheep (Ovis aries). BMC Genom. 2024, 25, 845. [Google Scholar] [CrossRef]
- Lowe, R.; Shirley, N.; Bleackley, M.; Dolan, S.; Shafee, T. Transcriptomics technologies. PLoS Comput. Biol. 2017, 13, e1005457. [Google Scholar] [CrossRef]
- Yadav, S.K.; Pandey, A.; Kumar, L.; Devi, A.; Kushwaha, B.; Vishvkarma, R.; Maikhuri, J.P.; Rajender, S.; Gupta, G. The thermo-sensitive gene expression signatures of spermatogenesis. Reprod. Biol. Endocrinol. 2018, 16, 56. [Google Scholar] [CrossRef]
- Mailin, G.; Yang, Y.; Liu, C.; Jing, Y.; Wang, Y.; Ma, J.; Liao, T.; Shen, L.; Zhu, L. The RNA-seq mapping of Testicular Development after Heat Stress in Sexually Mature Mice. Sci. Data 2024, 11, 913. [Google Scholar] [CrossRef]
- Liu, Y.; Cai, H.; Guo, X.; Aierken, A.; Hua, J.; Ma, B.; Peng, S. Melatonin alleviates heat stress-induced testicular damage in dairy goats by inhibiting the PI3K/AKT signaling pathway. Stress Biol. 2022, 2, 47. [Google Scholar] [CrossRef]
- Alves, M.B.; Andrade, A.F.; Arruda, R.P.; Batissaco, L.; Florez-Rodriguez, S.A.; Oliveira, B.M.; Torres, M.A.; Lanconi, R.; Ravagnani, G.M.; Prado Filho, R.R.; et al. Recovery of normal testicular temperature after scrotal heat stress in rams assessed by infrared thermography and its effects on seminal characteristics and testosterone blood serum concentration. Theriogenology 2016, 86, 795–805.e2. [Google Scholar] [CrossRef]
- Habeeb, A.A.; Gad, A.E.; Atta, M.A. Temperature-humidity indices as indicators to heat stress of climatic conditions with relation to production and reproduction of farm animals. Int. J. Biotechnol. Recent Adv. 2018, 1, 35–50. [Google Scholar] [CrossRef]
- Jin, Z.R.; Cao, Y.L.; Luo, Z.C.; Zhao, Q.C.; Xi, Y.; Weng, J.M.; Zhang, Z.; Jiang, H. Therapeutic Effects of Xianlu Oral Solution on Rats with Oligoasthenozoospermia through Alleviating Apoptosis and Oxidative Stress. Evid.-Based Complement. Altern. Med. 2022, 2022, 1269530. [Google Scholar] [CrossRef] [PubMed]
- Auger, J.; Jouannet, P.; Eustache, F. Another look at human sperm morphology. Hum. Reprod. 2016, 31, 10–23. [Google Scholar] [CrossRef] [PubMed]
- China Meteorological Administration. China Meteorological Data Service Centre. Available online: https://data.cma.cn/en (accessed on 26 February 2025).
- Kastelic, J.P.; Cook, R.B.; Coulter, G.H. Contribution of the scrotum and testes to scrotal and testicular thermoregulation in bulls and rams. J. Reprod. Fertil. 1996, 108, 81–85. [Google Scholar] [CrossRef]
- Ben Moula, A.; Moussafir, Z.; Hamidallah, N.; El Amiri, B. Heat stress and ram semen production and preservation: Exploring impacts and effective strategies. J. Therm. Biol. 2024, 119, 103794. [Google Scholar] [CrossRef]
- Carreau, S.; Hess, R.A. Oestrogens and spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010, 365, 1517–1535. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A. Spermatogenesis: An overview. In Sperm Chromatin: Biological and Clinical Applications in Male Infertility and Assisted Reproduction; Springer: New York, NY, USA, 2011; pp. 19–44. [Google Scholar]
- Durairajanayagam, D.; Agarwal, A.; Ong, C. Causes, effects and molecular mechanisms of testicular heat stress. Reprod. Biomed. Online 2015, 30, 14–27. [Google Scholar] [CrossRef]
- Cai, H.; Qin, D.; Peng, S. Responses and coping methods of different testicular cell types to heat stress: Overview and perspectives. Biosci. Rep. 2021, 41, BSR20210443. [Google Scholar] [CrossRef]
- Boshoff, N.; Lambrechts, H.; Maree, L.; Cloete, S.; Horst, G. A novel flush technique to simulate natural dispersal of spermatozoa in the female reproductive tract and expedite motility assessment of fresh ejaculated Merino (Ovis aries) sperm. S. Afr. J. Anim. Sci. 2018, 48, 469–476. [Google Scholar] [CrossRef]
- Rocha, D.R.; Martins, J.A.; van Tilburg, M.F.; Oliveira, R.V.; Moreno, F.B.; Monteiro-Moreira, A.C.; Moreira, R.A.; Araujo, A.A.; Moura, A.A. Effect of increased testicular temperature on seminal plasma proteome of the ram. Theriogenology 2015, 84, 1291–1305. [Google Scholar] [CrossRef]
- Li, T.; Wang, H.; Luo, R.; An, X.; Li, Q.; Su, M.; Shi, H.; Chen, H.; Zhang, Y.; Ma, Y. Proteome Informatics in Tibetan Sheep (Ovis aries) testes suggest the crucial proteins related to development and functionality. Front. Vet. Sci. 2022, 9, 923789. [Google Scholar] [CrossRef] [PubMed]
- Andrade, O.; Vázquez, R.; Orihuela, A. Sexual Behaviour of Hair Rams (Ovis aries) Subjected to Frequent Collection and Free from Habituation to the Female. J. Appl. Anim. Res. 2010, 37, 121–124. [Google Scholar] [CrossRef]
- Kanter, M.; Aktas, C.; Erboga, M. Heat stress decreases testicular germ cell proliferation and increases apoptosis in short term: An immunohistochemical and ultrastructural study. Toxicol. Ind. Health 2013, 29, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Hikim, A.P.; Lue, Y.; Yamamoto, C.M.; Vera, Y.; Rodriguez, S.; Yen, P.H.; Soeng, K.; Wang, C.; Swerdloff, R.S. Key apoptotic pathways for heat-induced programmed germ cell death in the testis. Endocrinology 2003, 144, 3167–3175. [Google Scholar] [CrossRef]
- Deng, C.C.; Zhang, J.P.; Huo, Y.N.; Xue, H.Y.; Wang, W.; Zhang, J.J.; Wang, X.Z. Melatonin alleviates the heat stress-induced impairment of Sertoli cells by reprogramming glucose metabolism. J. Pineal Res. 2022, 73, e12819. [Google Scholar] [CrossRef]
- Marai, I.F.; EL-Darawany, A.H.A.; Ismail, E.S.A.-F.; Abdel-Hafez, M.A. Tunica dartos index as a parameter for measurement of adaptability of rams to subtropical conditions of Egypt. Anim. Sci. J. 2006, 77, 487–494. [Google Scholar] [CrossRef]
- Kastelic, J.; Wilde, R.; Rizzoto, G.; Thundathil, J. Hyperthermia and not hypoxia may reduce sperm motility and morphology following testicular hyperthermia. Vet. Med. 2017, 62, 437–442. [Google Scholar] [CrossRef]
- Banks, S.; King, S.A.; Irvine, D.S.; Saunders, P.T. Impact of a mild scrotal heat stress on DNA integrity in murine spermatozoa. Reproduction 2005, 129, 505–514. [Google Scholar] [CrossRef]
- Viana Neto, A.M.; Guerreiro, D.D.; Martins, J.A.M.; Vasconcelos, F.á.R.; Melo, R.é.B.F.; Velho, A.L.M.C.S.; Neila-Montero, M.; Montes-Garrido, R.; Nagano, C.S.; Araújo, A.A.; et al. Sperm traits and seminal plasma proteome of locally adapted hairy rams subjected to intermittent scrotal insulation. Anim. Reprod. Sci. 2024, 263, 107439. [Google Scholar] [CrossRef]
- Jia, Y.; Castellanos, J.; Wang, C.; Sinha-Hikim, I.; Lue, Y.; Swerdloff, R.S.; Sinha-Hikim, A.P. Mitogen-activated protein kinase signaling in male germ cell apoptosis in the rat. Biol. Reprod. 2009, 80, 771–780. [Google Scholar] [CrossRef]
- Vera, Y.; Rodriguez, S.; Castanares, M.; Lue, Y.; Atienza, V.; Wang, C.; Swerdloff, R.S.; Sinha Hikim, A.P. Functional role of caspases in heat-induced testicular germ cell apoptosis. Biol. Reprod. 2005, 72, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, J.Z.; Weber, M.; Geraud, G.; Maro, B. Cell cycle modification during the transitions between meiotic M-phases in mouse oocytes. J. Cell Sci. 1992, 102 Pt 3, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Viera, A.; Rufas, J.S.; Martinez, I.; Barbero, J.L.; Ortega, S.; Suja, J.A. CDK2 is required for proper homologous pairing, recombination and sex-body formation during male mouse meiosis. J. Cell Sci. 2009, 122, 2149–2159. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Hamada, M.; Malureanu, L.; Jeganathan, K.B.; Zhou, W.; Morbeck, D.E.; van Deursen, J.M. Cdc20 is critical for meiosis I and fertility of female mice. PLoS Genet. 2010, 6, e1001147. [Google Scholar] [CrossRef]
- Kalous, J.; Aleshkina, D. Multiple Roles of PLK1 in Mitosis and Meiosis. Cells 2023, 12, 187. [Google Scholar] [CrossRef]
- Jia, L.; Li, B.; Yu, H. The Bub1-Plk1 kinase complex promotes spindle checkpoint signalling through Cdc20 phosphorylation. Nat. Commun. 2016, 7, 10818. [Google Scholar] [CrossRef]
- Nabti, I.; Marangos, P.; Bormann, J.; Kudo, N.R.; Carroll, J. Dual-mode regulation of the APC/C by CDK1 and MAPK controls meiosis I progression and fidelity. J. Cell Biol. 2014, 204, 891–900. [Google Scholar] [CrossRef]
- Pesin, J.A.; Orr-Weaver, T.L. Regulation of APC/C activators in mitosis and meiosis. Annu. Rev. Cell Dev. Biol. 2008, 24, 475–499. [Google Scholar] [CrossRef]
- Li, L.; Fan, L.; Peng, N.; Yang, L.; Mou, L.; Huang, W. R383C mutation of human CDC20 results in idiopathic non-obstructive azoospermia. Oncotarget 2017, 8, 99816. [Google Scholar] [CrossRef]
- Hietakangas, V.; Sistonen, L. Regulation of the heat shock response by heat shock transcription factors. In Chaperones: 16; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–34. [Google Scholar]
- Nixon, B.; Bromfield, E.G.; Cui, J.; De Iuliis, G.N. Heat shock protein A2 (HSPA2): Regulatory roles in germ cell development and sperm function. In The Role of Heat Shock Proteins in Reproductive System Development and Function; Springer: Cham, Switzerland, 2017; pp. 67–93. [Google Scholar]
- Li, T.; Wang, X.; Luo, R.; An, X.; Zhang, Y.; Zhao, X.; Ma, Y. Integrating miRNA and mRNA Profiling to Assess the Potential miRNA–mRNA Modules Linked With Testicular Immune Homeostasis in Sheep. Front. Vet. Sci. 2021, 8, 647153. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Jiang, M.; Wang, Y.; Pan, Q.; Annandale, H.; Irons, P.C.; Dong, H. Semen Quality, Testicular Cell Apoptosis, and Transcriptome Analysis Following Mild Scrotal Heat Stress in Wugu–Hu Crossbred and Hu Rams. Animals 2025, 15, 724. https://doi.org/10.3390/ani15050724
Chen S, Jiang M, Wang Y, Pan Q, Annandale H, Irons PC, Dong H. Semen Quality, Testicular Cell Apoptosis, and Transcriptome Analysis Following Mild Scrotal Heat Stress in Wugu–Hu Crossbred and Hu Rams. Animals. 2025; 15(5):724. https://doi.org/10.3390/ani15050724
Chicago/Turabian StyleChen, Shikun, Mingxu Jiang, Yanyun Wang, Qingjie Pan, Henry Annandale, Peter Charles Irons, and Huansheng Dong. 2025. "Semen Quality, Testicular Cell Apoptosis, and Transcriptome Analysis Following Mild Scrotal Heat Stress in Wugu–Hu Crossbred and Hu Rams" Animals 15, no. 5: 724. https://doi.org/10.3390/ani15050724
APA StyleChen, S., Jiang, M., Wang, Y., Pan, Q., Annandale, H., Irons, P. C., & Dong, H. (2025). Semen Quality, Testicular Cell Apoptosis, and Transcriptome Analysis Following Mild Scrotal Heat Stress in Wugu–Hu Crossbred and Hu Rams. Animals, 15(5), 724. https://doi.org/10.3390/ani15050724






