Dolphin Pituitary Gland: Immunohistochemistry and Ultrastructural Cell Characterization Following a Novel Anatomical Dissection Protocol and Non-Invasive Imaging (MRI)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
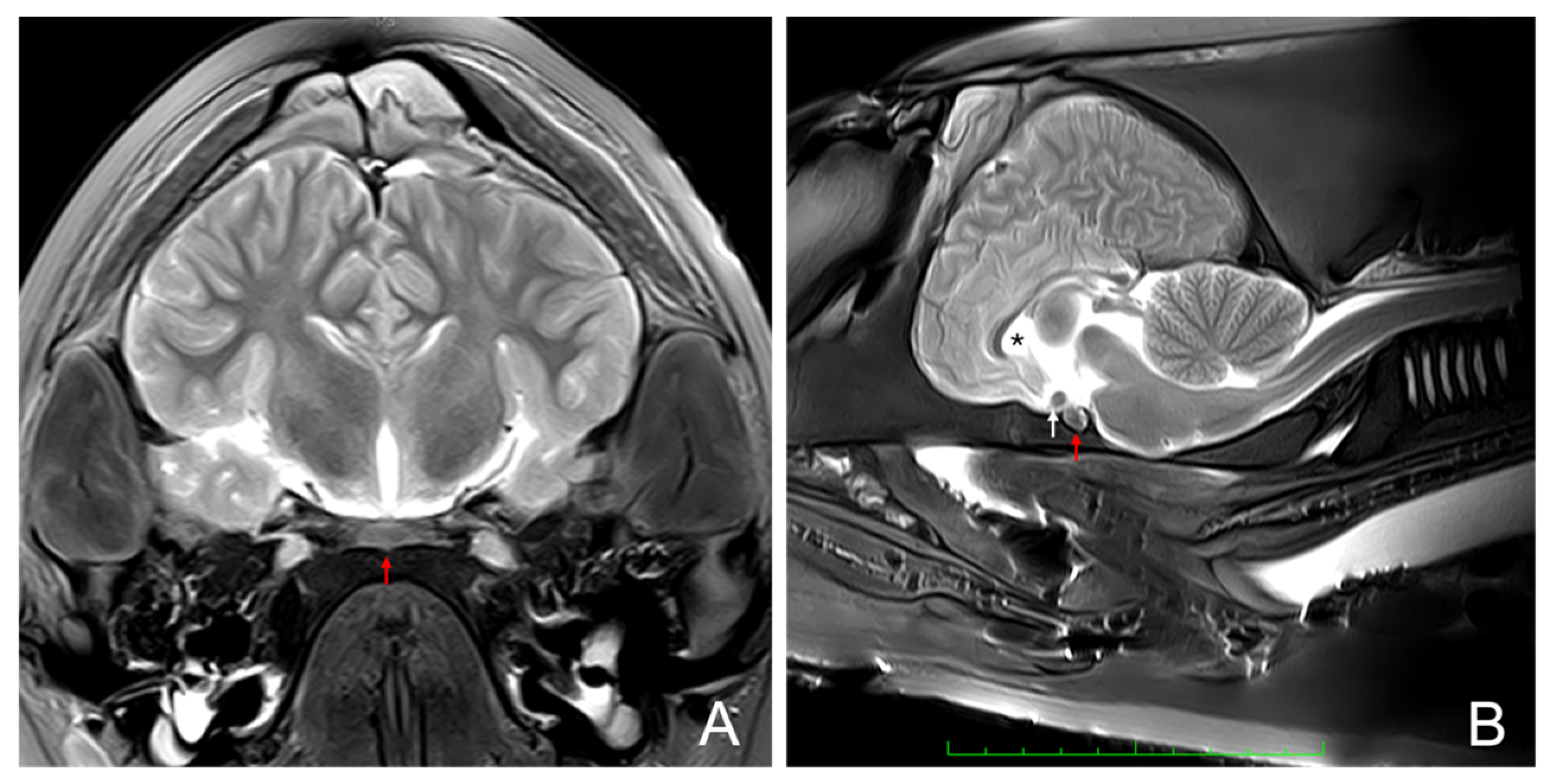
2.2. Anatomical Identification of the Pituitary Gland Using Magnetic Resonance Imaging (MRI)
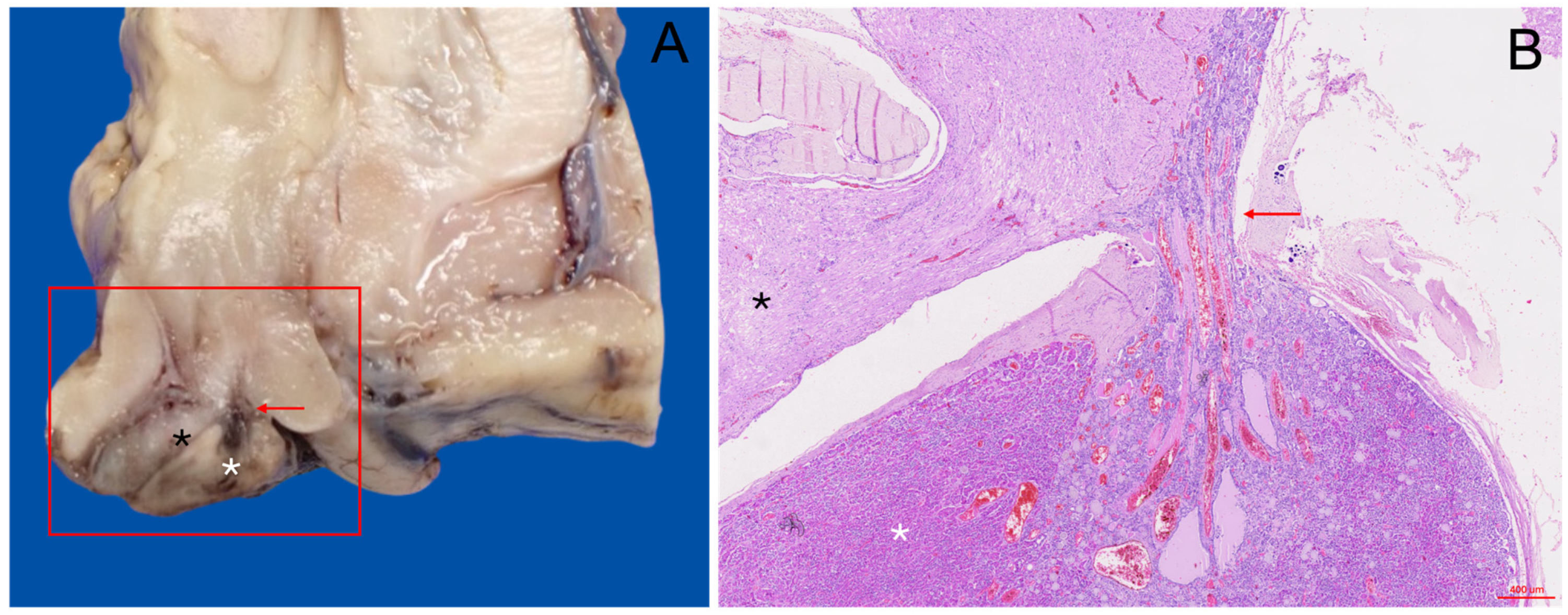
2.3. Pituitary Gland Extraction and Sampling
- Dorsal line: a line parallel to the nuchal ridge (crista occipitalis externa), 1 cm caudal to it;
- Lateral lines: two perpendicular lines extending from the dorsal line through the parietal and squamosal bones in the temporal fossa. These bilateral lines continued to the outermost lateral angles of the occipital condyles;
- Ventral continuation: with the head turned upside down (ventral view), the lateral lines were extended, forming a V-shaped pattern. These lines started at the lateral angles of the occipital condyles and converged just caudal to the basioccipital crest.
2.4. Histochemical Study
2.5. Immunohistochemical Study
2.6. Ultrastructural Study
3. Results
3.1. Magnetic Resonance Imaging
3.2. Gross Anatomical Description
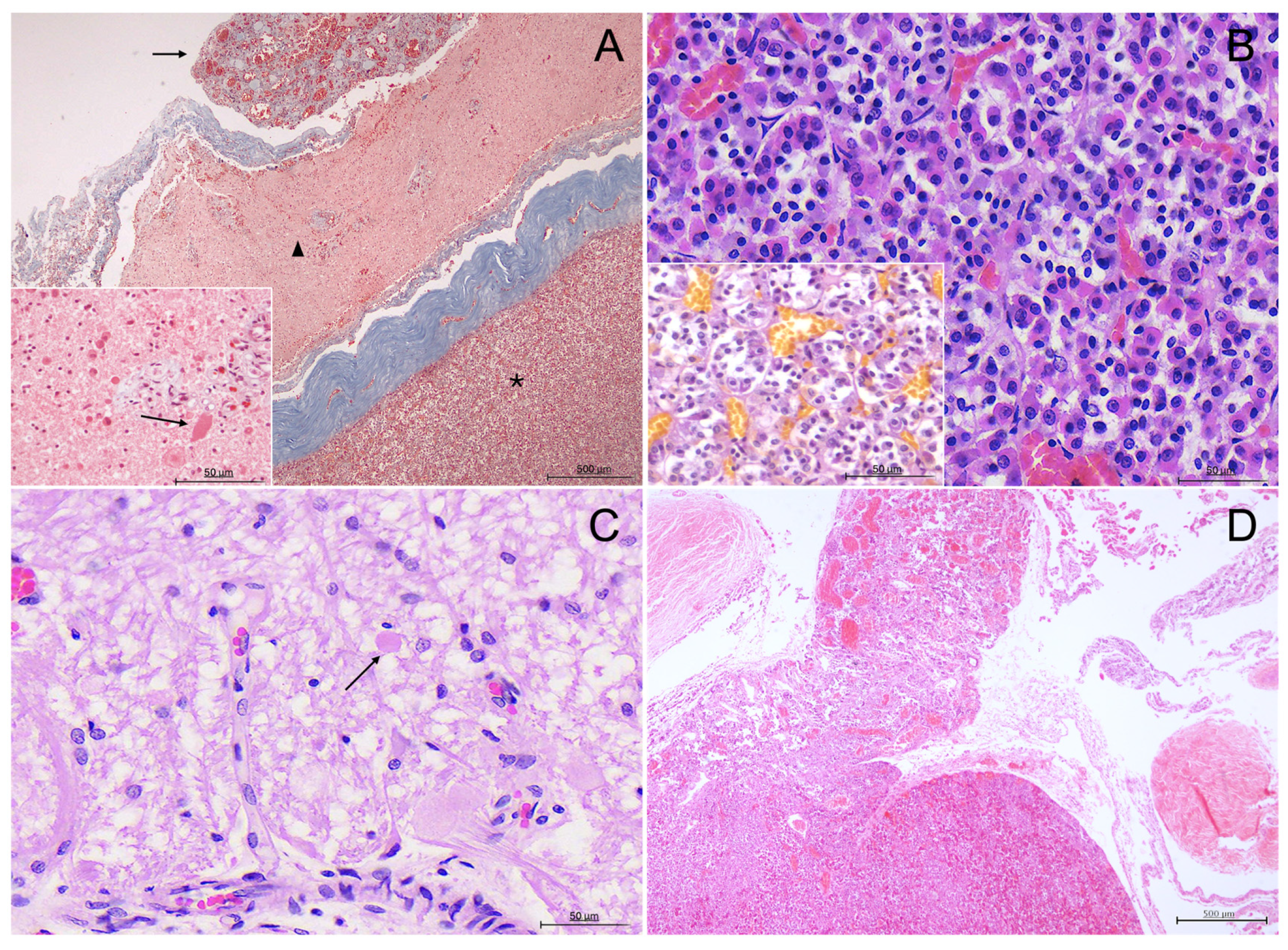
3.3. Histological Description
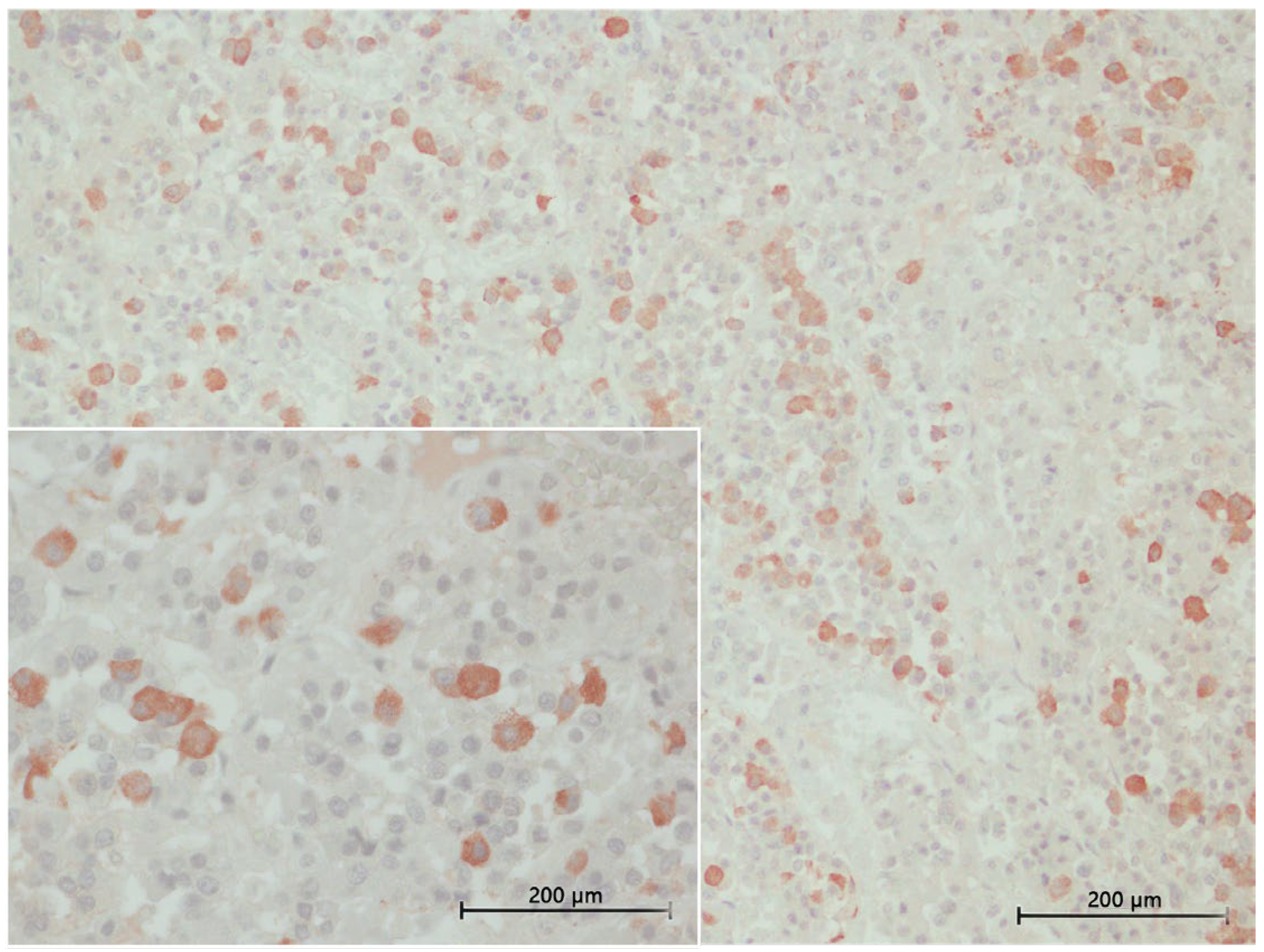
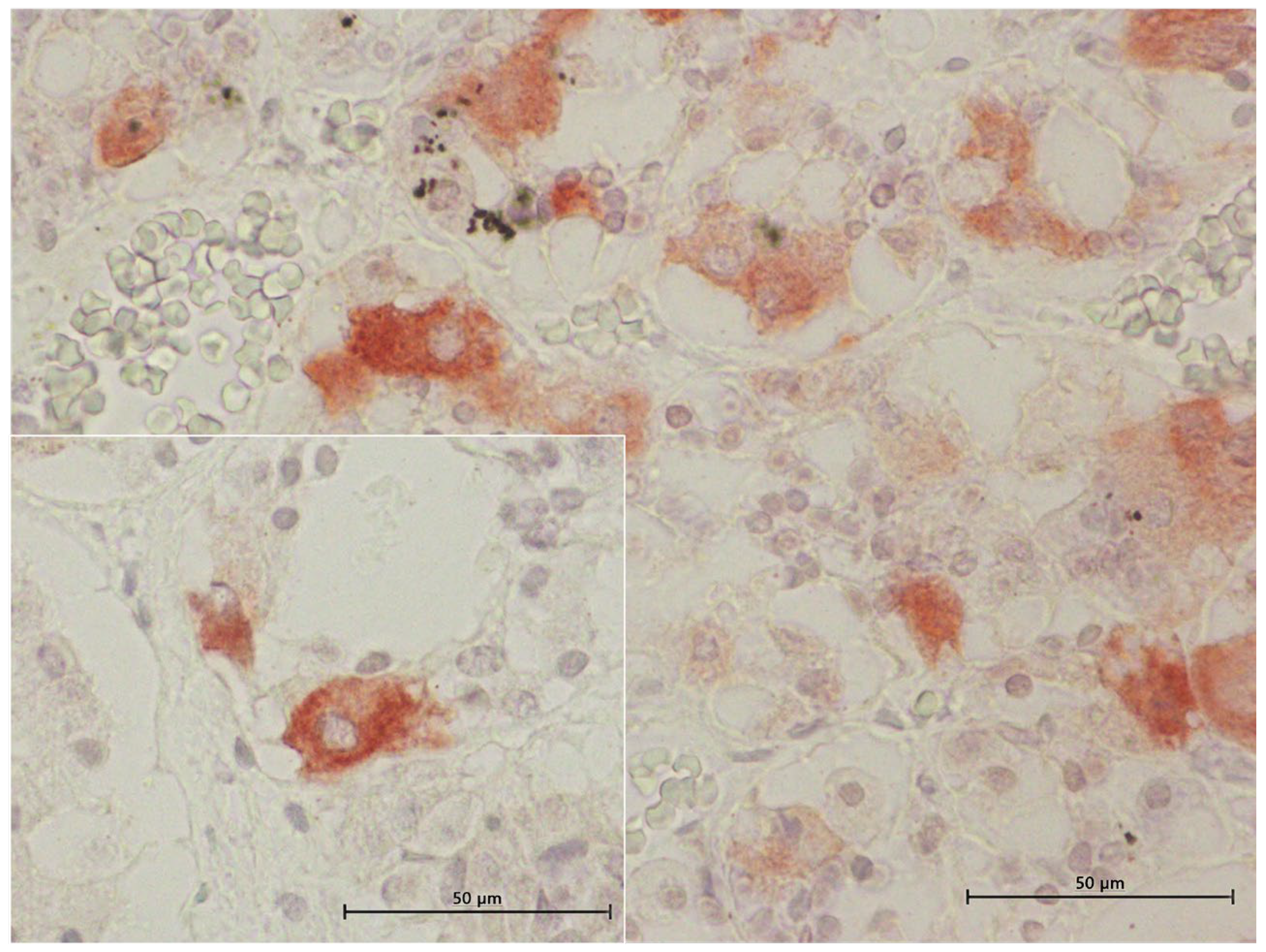
3.4. Inmunohistochemical Study
3.4.1. ACTH Labelling
3.4.2. MSH Labelling
3.4.3. TSH Labelling
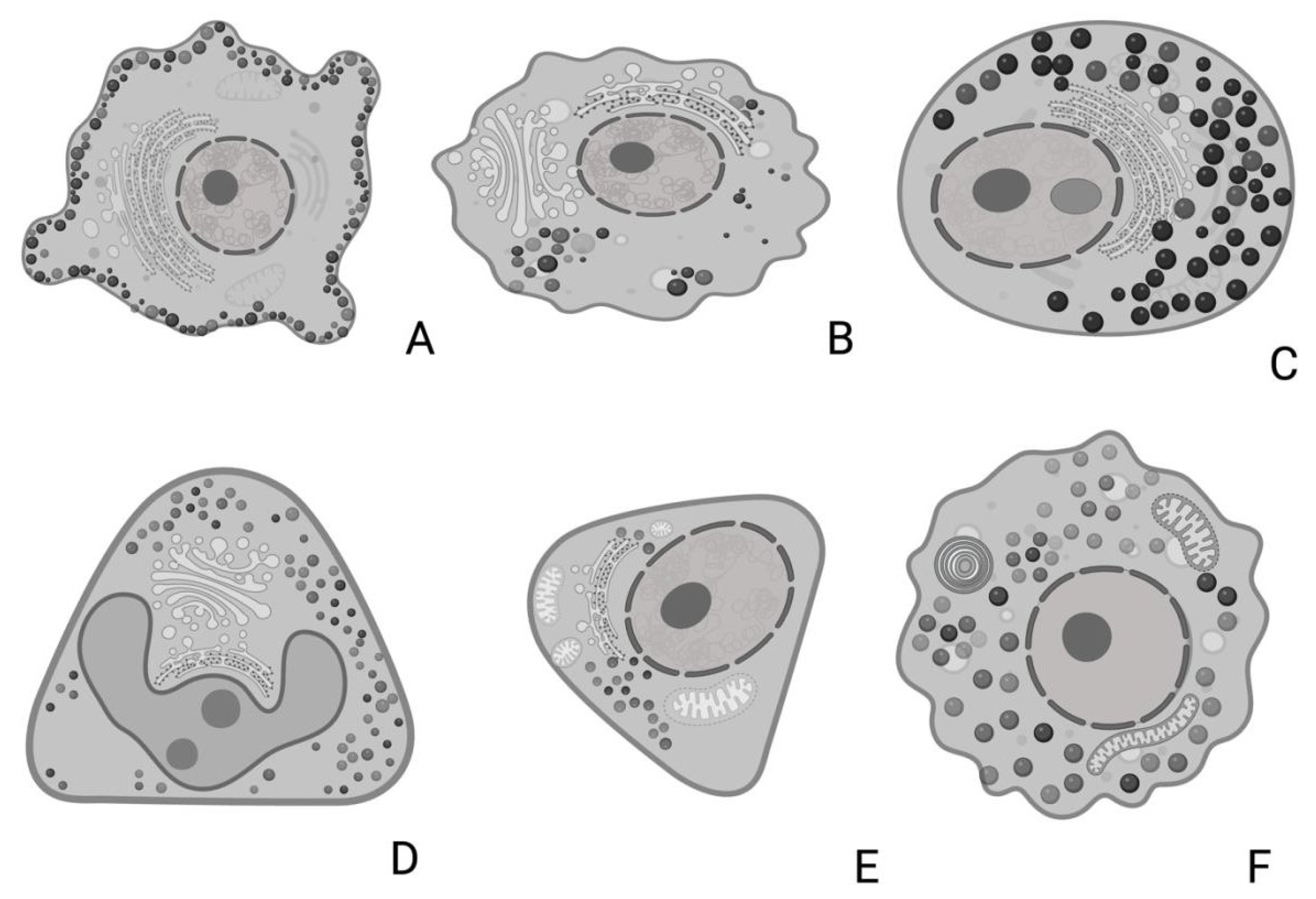
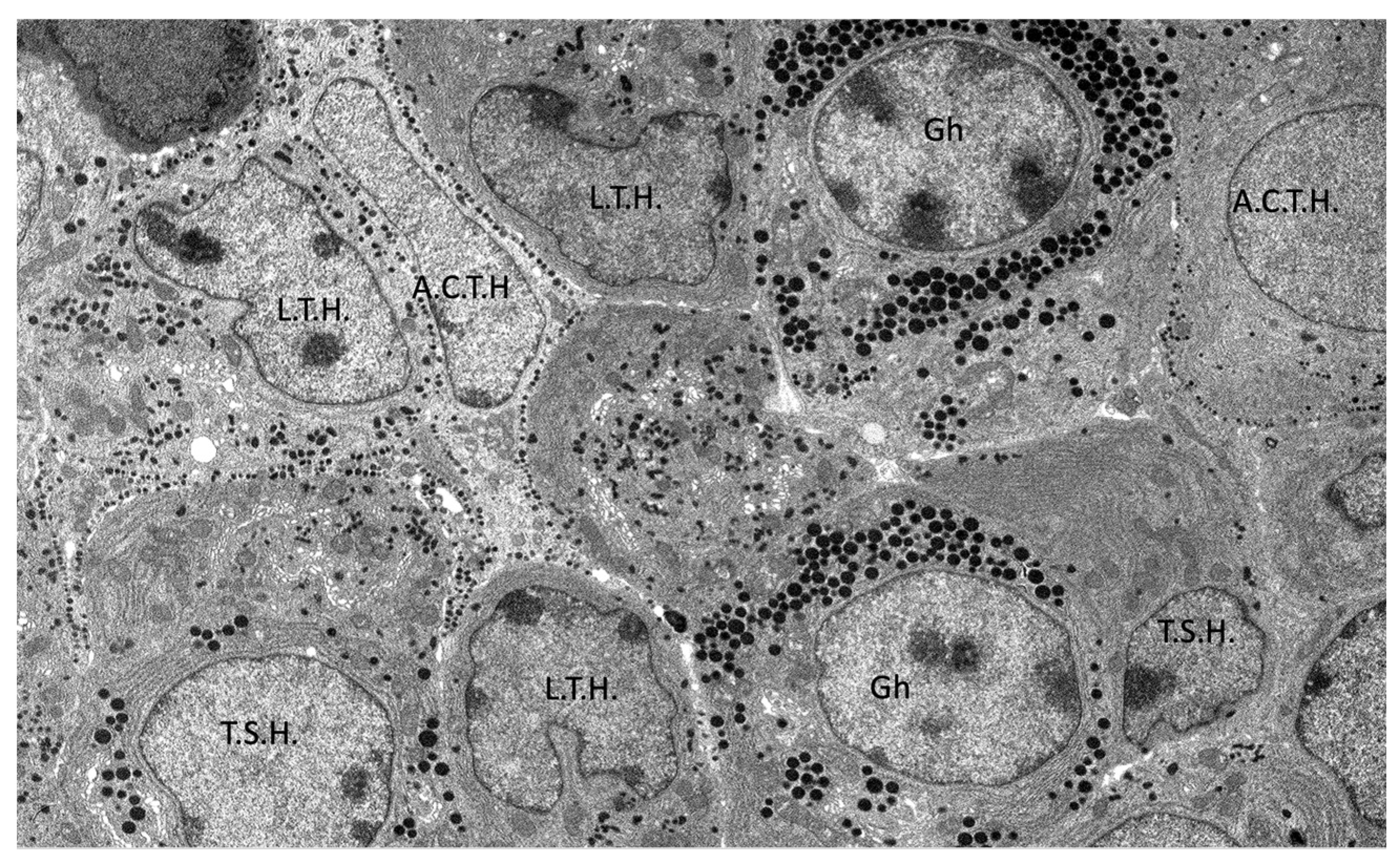
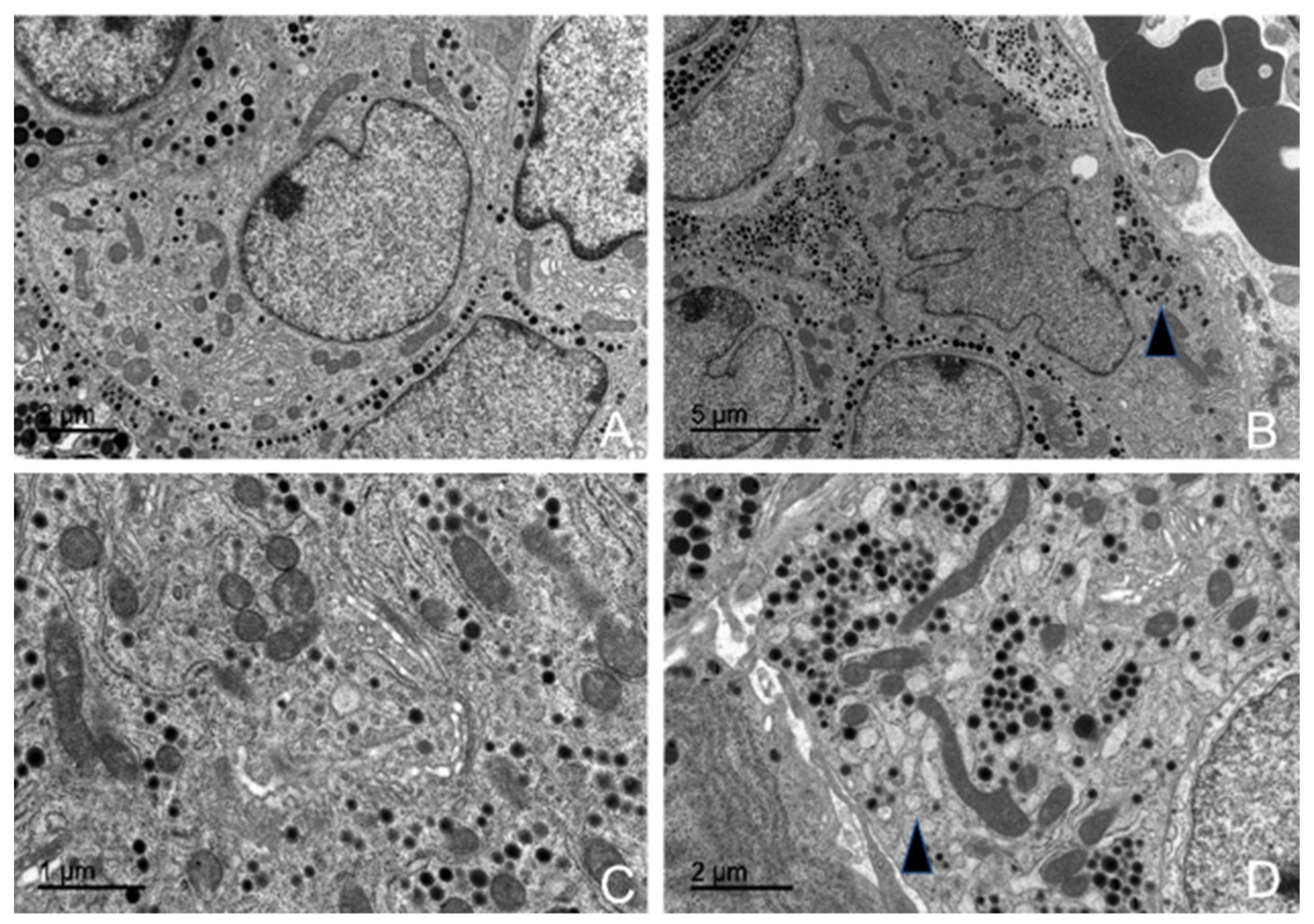
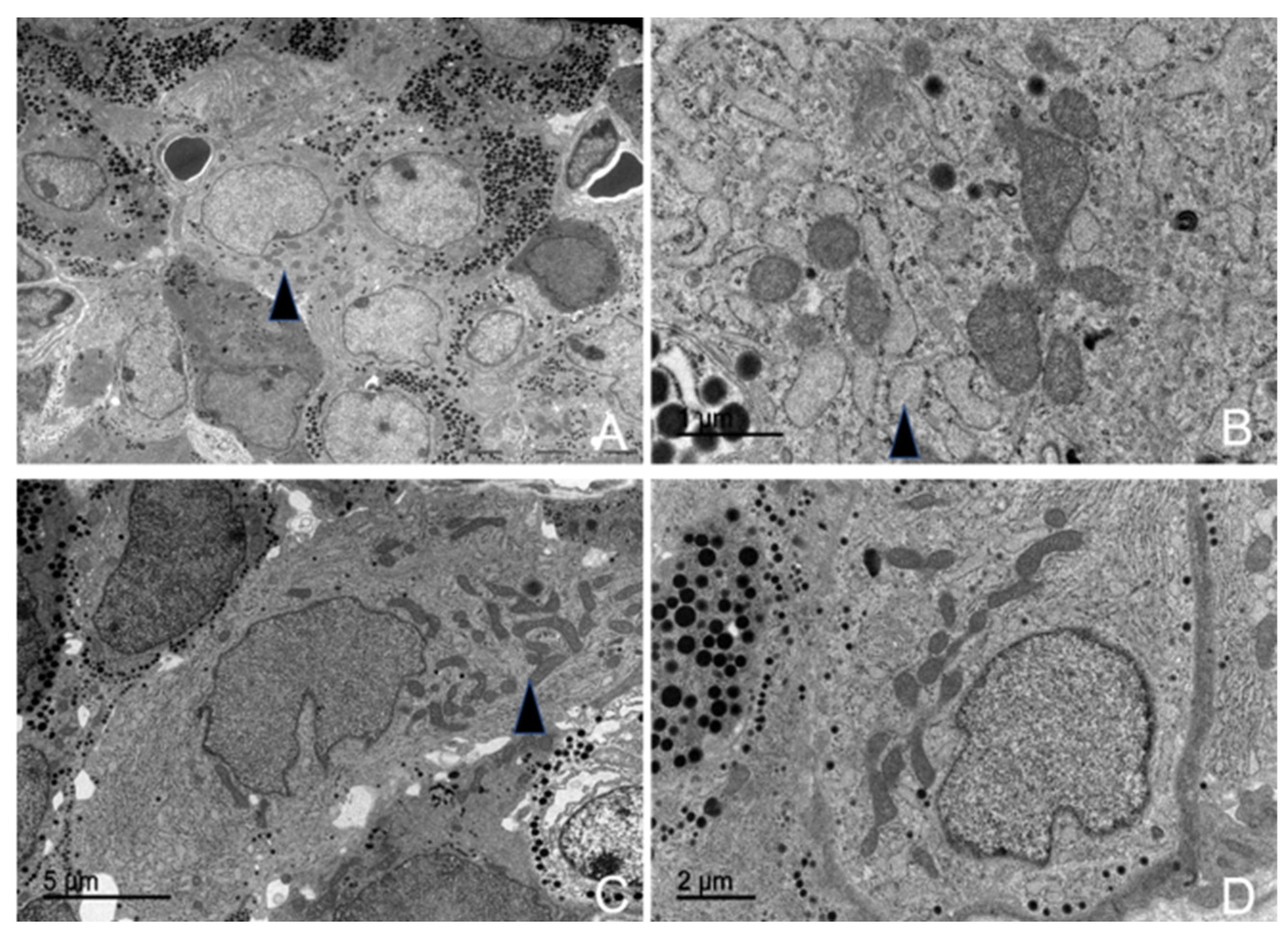
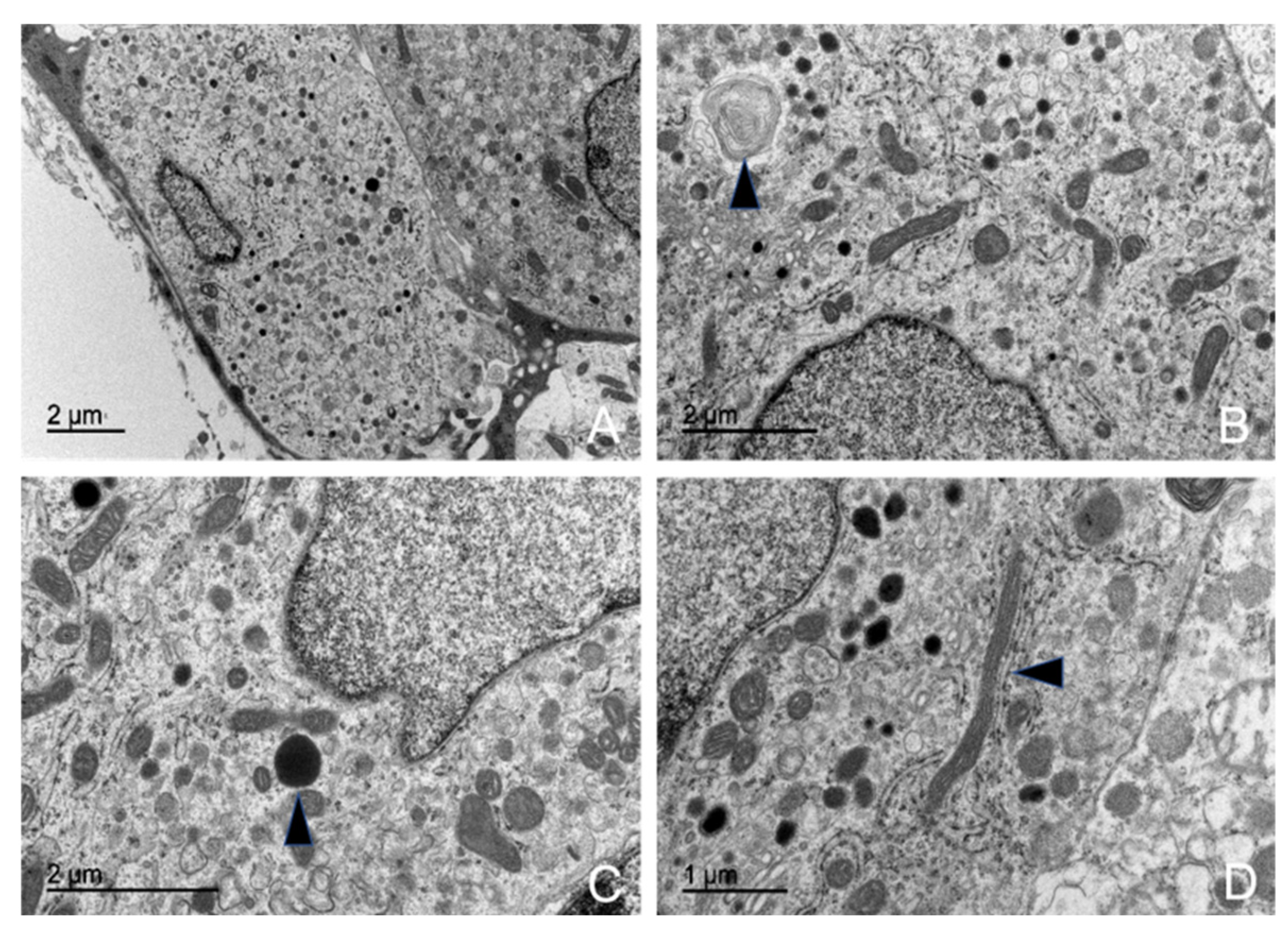
3.5. Ultrastructural Description of the Adenohypophysis Through Transmission Electron Microscopy (TEM)
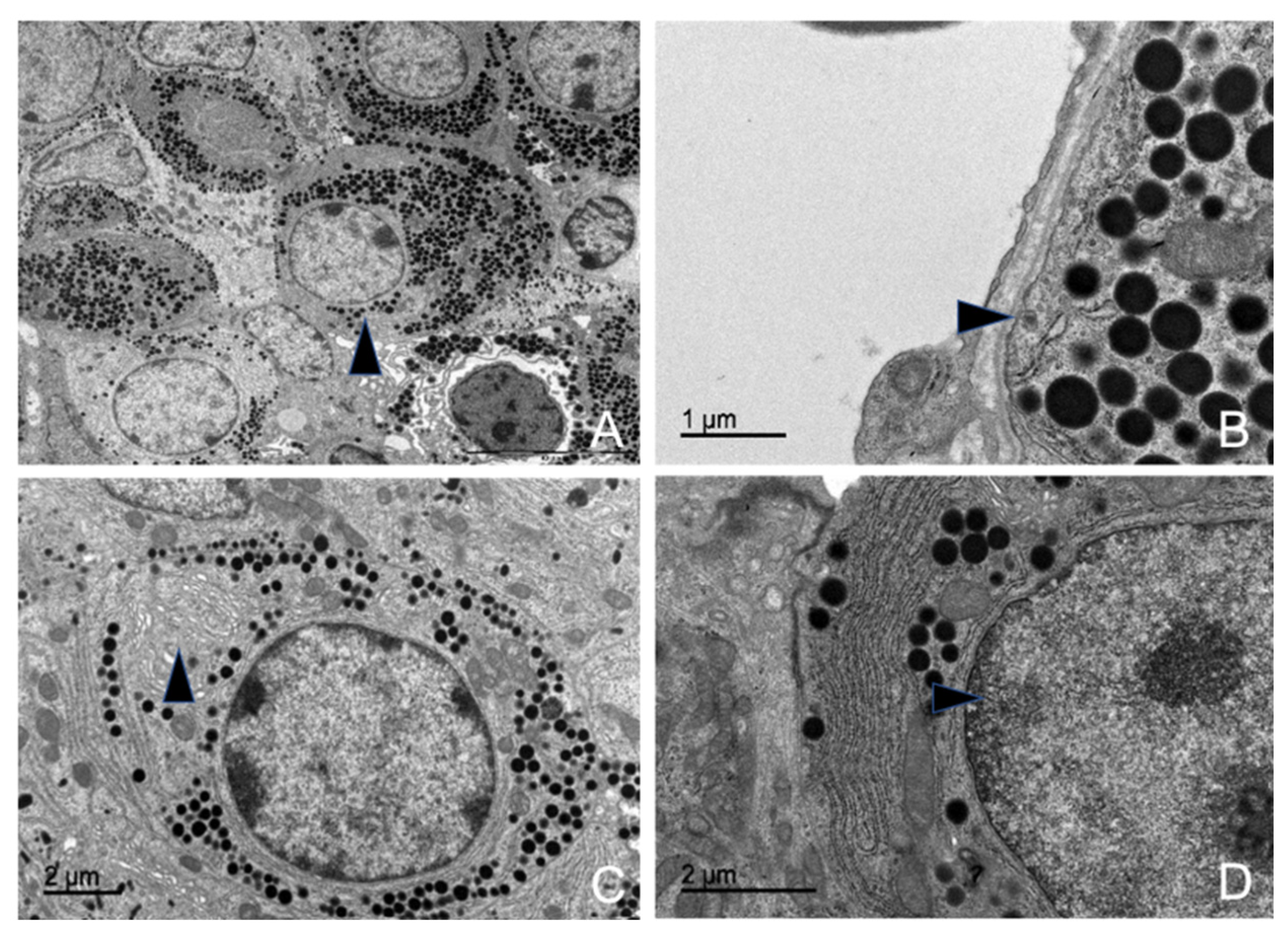
3.5.1. Corticotrophs (ACTH-Producing Cells)
3.5.2. Lactotrophs (LTH-Producing Cells)
3.5.3. Somatotrophs (GH-Producing Cells)
3.5.4. Gonadotrophs (GnRH -Producing Cells)
3.5.5. Thyrotrophs (TSH-Producing Cells)
3.5.6. Melanotrophs (MSH-Producing Cells)
3.5.7. Follicular Cells
3.5.8. Capsular Cells
4. Discussion
4.1. Methodological Advances in Dolphin Pituitary Extraction
4.2. Morphological and Inmunohistochemical Insights
4.3. Ultrastructural Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MRI | Magnetic Resonance Imaging |
| TEM | Transmission Electron Microscopy |
| ACTH | Adrenocorticotropic Hormone |
| GH | Growth Hormone |
| LTH | Luteotropic Hormone |
| PRL | Prolactin |
| GnRH | Gonadotropic Hormone |
| FSH | Follicle-Stimulating Hormone |
| LH | Luteinizing Hormone |
| STH | Somatotropic Hormone |
| MSH | Melanocyte-Stimulating Hormone |
| TSH | Thyroid-Stimulating Hormone |
| RER | Rough Endoplasmic Reticulum |
| H-E | Hematoxylin-Eosin |
| MT | Masson’s Trichrome |
| MT-OG | Masson’s Trichrome-Orange G |
| PAS | Periodic Acid Schiff |
| PAS-OG | Periodic Acid Schiff-Orange G |
| IHC | Immunohistochemistry |
References
- Barkhoudarian, G.; Kelly, D.F. The Pituitary Gland: Anatomy, Physiology, and Its Function as the Master Gland. In Cushing’s Disease: An Often Misdiagnosed and Not So Rare Disorder; Academic Press: Cambridge, MA, USA, 2017; pp. 1–41. [Google Scholar] [CrossRef]
- Cowan, D.F.; Haubold, E.M.; Tajima, Y. Histological, Immunohistochemical and Pathological Features of the Pituitary Gland of Odontocete Cetaceans from the Western Gulf of Mexico. J. Comp. Pathol. 2008, 139, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.J. Endocrine Organs: Hypophysis, Thyroid and Adrenal. In The Biology of Marine Mammals; Academic Press: New York, NY, USA, 1969; pp. 349–390. [Google Scholar]
- Hennings, H. The Whale Hypophysis with Special Reference to Its ACTH Content. Acta Endocrinol. 1950, 5, 376–386. [Google Scholar] [CrossRef]
- Wislocki, G.B. The Hypophysis of the Porpoise (Tursiops Trunatus). Arch. Surg. 1929, 18, 1403. [Google Scholar] [CrossRef]
- Wislocki, G.B.; Geiling, E.M.K. The Anatomy of the Hypophysis of Whales. Anat. Rec. 1936, 66, 17–41. [Google Scholar] [CrossRef]
- Vuković, S.; Lucić, H.; Đuras Gomerčić, M.; Galov, A.; Gomerčić, T.; Ćurković, S.; Škrtić, D.; Domitran, G.; Gomerčić, H. Anatomical and Histological Characteristics of the Pituitary Gland in the Bottlenose Dolphin (Tursiops Truncatus) from the Adriatic Sea. Vet. Arhiv 2011, 81, 143–151. [Google Scholar]
- Panin, M.; Gabai, G.; Ballarin, C.; Peruffo, A.; Cozzi, B. Evidence of Melatonin Secretion in Cetaceans: Plasma Concentration and Extrapineal HIOMT-like Presence in the Bottlenose Dolphin Tursiops Truncatus. Gen. Comp. Endocrinol. 2012, 177, 238–245. [Google Scholar] [CrossRef]
- Arvy, L. Endocrine Glands and Hormonal Secretion in Cetaceans. Investig. Cetacea 1971, 3, 229–300. [Google Scholar]
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of Veterinary Anatomy-E-Book; Elsevier Health Sciences: Amsterdam, The Netherlands, 2009; ISBN 1437708757. [Google Scholar]
- Gorbman, A. Comparative Anatomy and Physiology of the Anterior Pituitary. Q. Rev. Biol. 1941, 16, 294–310. [Google Scholar] [CrossRef]
- Takeuchi, M. The Mammalian Pars Intermedia—Structure and Function—. Zool. Sci. 2001, 18, 133–144. [Google Scholar] [CrossRef]
- Flanigan, N.J. The Central Nervous System. In Mammals of the Sea: Biology and Medicine; Ridgway, S.H., Ed.; Charles C. Thomas: Springfield, IL, USA, 1972; pp. 215–245. [Google Scholar]
- Cowan, D.F.; Gatalica, Z. Immunohistochemistry in Cetaceans. In Molecular and Cell Biology of Marine Mammals; Pfeiffer, C.J., Ed.; Kreiger Publishing Co.: Malabar, FL, USA, 2002; pp. 280–288. [Google Scholar]
- Kumar, D.; Cowan, D.F. Cross-reactivity of Antibodies to Human Antigens with Tissues of the Bottlenose Dolphin, Tursiops Truncatus, Using Immunoperoxidase Techniques. Mar. Mamm. Sci. 1994, 10, 188–194. [Google Scholar] [CrossRef]
- Young, B.A.; Harrison, R.J. Ultrastructure of the Dolphin Adenohypophysis. Z. Zellforsch. Mikrosk. Anat. 1970, 103, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A.; Harrison, R.J. The Ultrastructure of the Thyroid and Pars Distalis of the Dolphin. Aquat. Mamm. 1977, 5, 1–8. [Google Scholar]
- Kuiken, T.; García-Hartmann, M. Proceedings of the First European Cetacean Society Workshop on Cetacean Pathology: Dissection Techniques and Tissue Sampling. In Proceedings of the ECS Workshop on Cetacean Pathology, Leiden, The Netherlands, 13–14 September 1991; p. 20. [Google Scholar]
- Arbelo, M.; de Los Monteros, A.E.; Herráez, P.; Andrada, M.; Sierra, E.; Rodríguez, F.; Jepson, P.D.; Fernández, A. Pathology and Causes of Death of Stranded Cetaceans in the Canary Islands (1999−2005). Dis. Aquat. Organ. 2013, 103, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Delgado, J.; Fernández, A.; Sierra, E.; Sacchini, S.; Andrada, M.; Vela, A.I.; Quesada-Canales, Ó.; Paz, Y.; Zucca, D.; Groch, K. Pathologic Findings and Causes of Death of Stranded Cetaceans in the Canary Islands (2006–2012). PLoS ONE 2018, 13, e0204444. [Google Scholar] [CrossRef]
- Sacchini, S.; Herráez, P.; Arbelo, M.; Espinosa de los Monteros, A.; Sierra, E.; Rivero, M.; Bombardi, C.; Fernández, A. Methodology and Neuromarkers for Cetaceans’ Brains. Vet. Sci. 2022, 9, 38. [Google Scholar] [CrossRef]
- Fumero-Hernández, M.; Encinoso, M.; Melian, A.; Nuez, H.A.; Salman, D.; Jaber, J.R. Cross Sectional Anatomy and Magnetic Resonance Imaging of the Juvenile Atlantic Puffin Head (Aves, Alcidae, Fratercula Arctica). Animals 2023, 13, 3434. [Google Scholar] [CrossRef]
- Cozzi, B.; Huggenberger, S.; Oelschläger, H.A. Anatomy of Dolphins: Insights into Body Structure and Function; Academic Press: Cambridge, MA, USA, 2016; ISBN 0124076556. [Google Scholar]
- Elsberry, W.R.; Cranford, T.W.; Ridgway, S.H.; Carder, D.A.; VanBonn, W.G.; Blackwood, D.J.; Carr, J.A.; Evans, W.E. Interrelationships between Intranarial Pressure and Biosonar Clicks in the Bottlenose Dolphin (Tursiops Truncatus). J. Acoust. Soc. Am. 2002, 111, 2343. [Google Scholar] [CrossRef]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008; ISBN 0443102791. [Google Scholar]
- Larkin, S.; Ansorge, O. Development and Microscopic Anatomy of the Pituitary Gland. In Endotext; MDText.com, Inc.: South Dartmouth, MA, USA, 2017. [Google Scholar]
- Taylor, C.R.; Shi, S.-R.; Barr, N.J.; Wu, N. Techniques of Immunohistochemistry: Principles, Pitfalls, and Standardization. Diagn. Immunohistochem. 2013, 2, 1–42. [Google Scholar]
- Paquet, P.C.; Darimont, C.T. Wildlife Conservation and Animal Welfare: Two Sides of the Same Coin? Anim. Welf. 2010, 19, 177–190. [Google Scholar] [CrossRef]
- Stephen, C. Toward a Modernized Definition of Wildlife Health. J. Wildl. Dis. 2014, 50, 427–430. [Google Scholar] [CrossRef]
- Clegg, I.L.K.; Butterworth, A. Assessing the Welfare of Cetacea. In Marine Mammal Welfare. Animal Welfare; Butterworth, A., Ed.; Springer: Cham, Switzerland, 2017; Volume 17. [Google Scholar] [CrossRef]
- Boys, R.M.; Beausoleil, N.J.; Pawley, M.D.M.; Littlewood, K.E.; Betty, E.L.; Stockin, K.A. Fundamental Concepts, Knowledge Gaps and Key Concerns Relating to Welfare and Survival of Stranded Cetaceans. Diversity 2022, 14, 338. [Google Scholar] [CrossRef]
- Nicol, C.; Bejder, L.; Green, L.; Johnson, C.; Keeling, L.; Noren, D.; Van der Hoop, J.; Simmonds, M. Anthropogenic Threats to Wild Cetacean Welfare and a Tool to Inform Policy in This Area. Front. Vet. Sci. 2020, 7, 517869. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.B.; O’Callaghan, J.P. Neuroendocrine Aspects of the Response to Stress. Metab.-Clin. Exp. 2002, 51, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Denver, R.J. Structural and Functional Evolution of Vertebrate Neuroendocrine Stress Systems. Ann. N. Y. Acad. Sci. 2009, 1163, 1–16. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603. [Google Scholar]
- Tsigos, C.; Chrousos, G.P. Hypothalamic–Pituitary–Adrenal Axis, Neuroendocrine Factors and Stress. J. Psychosom. Res. 2002, 53, 865–871. [Google Scholar] [CrossRef]
- Helmreich, D.L.; Parfitt, D.B.; Lu, X.-Y.; Akil, H.; Watson, S.J. Relation between the Hypothalamic-Pituitary-Thyroid (HPT) Axis and the Hypothalamic-Pituitary-Adrenal (HPA) Axis during Repeated Stress. Neuroendocrinology 2005, 81, 183–192. [Google Scholar] [CrossRef]
- Edinger, T. The Pituitary Body in Giant Animals Fossil and Living: A Survey and a Suggestion. Q. Rev. Biol. 1942, 17, 31–45. [Google Scholar] [CrossRef]
- Harris, G.W. Hypothalamo-Hypophysial Connexions in the Cetacea. J. Physiol. 1950, 111, 361. [Google Scholar] [CrossRef]
- Geiling, E.; Vos, B.; Oldham, F.K.O.F.K. The Pharmacology and Anatomy of the Hypophysis of the Porpoise. Endocrinology 1940, 27, 309–316. [Google Scholar] [CrossRef]
- Tsui, H.C.L.; Kot, B.C.W.; Chung, T.Y.T.; Chan, D.K.P. Virtopsy as a Revolutionary Tool for Cetacean Stranding Programs: Implementation and Management. Front. Mar. Sci. 2020, 7, 542015. [Google Scholar] [CrossRef]
- Dellmann, H.-D.; Rodriguez, E.M. Herring Bodies; an Electron Microscopic Study of Local Degeneration and Regeneration of Neurosecretory Axons. Z. Zellforsch. Mikrosk. Anat. 1970, 111, 293–315. [Google Scholar] [CrossRef]
- Perks, A.M. The Neurohypophysis. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 1969; Volume 2, pp. 111–205. ISBN 1546-5098. [Google Scholar]
- Orekhova, K.; Centelleghe, C.; Di Guardo, G.; Graïc, J.M.; Cozzi, B.; Trez, D.; Verin, R.; Mazzariol, S. Systematic Validation and Assessment of Immunohistochemical Markers for Central Nervous System Pathology in Cetaceans, with Emphasis on Auditory Pathways. PLoS ONE 2022, 17, e0269090. [Google Scholar] [CrossRef] [PubMed]
- McGrath, P. Observations on the Intracranial Carotid Rete and the Hypophysis in the Mature Female Pig and Sheep. J. Anat. 1977, 124, 689. [Google Scholar]
- Mitchell, G.; Lust, A. The Carotid Rete and Artiodactyl Success. Biol. Lett. 2008, 4, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Fukuta, K.; Kudo, H.; Sasaki, M.; Kimura, J.; Ismail, D.B.; Endo, H. Absence of Carotid Rete Mirabile in Small Tropical Ruminants: Implications for the Evolution of the Arterial System in Artiodactyls. J. Anat. 2007, 210, 112. [Google Scholar] [CrossRef] [PubMed]
- McConnell, E.M. The Arterial Blood Supply of the Human Hypophysis Cerebri. Anat. Rec. 1953, 115, 175–203. [Google Scholar] [CrossRef]
- Feldt-Rasmussen, U.; Effraimidis, G.; Klose, M. The Hypothalamus-Pituitary-Thyroid (HPT)-Axis and Its Role in Physiology and Pathophysiology of Other Hypothalamus-Pituitary Functions. Mol. Cell Endocrinol. 2021, 525, 111173. [Google Scholar] [CrossRef]
- Nabi, G.; Robeck, T.R.; Yujiang, H.; Tang, B.; Zheng, J.; Wang, K.; Wang, D. Circulating Concentrations of Thyroid Hormones and Cortisol in Wild and Semi-Natural Yangtze Finless Porpoise (Neophocaena Asiaeorientalis). Conserv. Physiol. 2021, 9, coab034. [Google Scholar] [CrossRef]
- Whittow, G.C. Thermoregulatory Adaptations in Marine Mammals: Interacting Effects of Exercise and Body Mass. A Review. Mar. Mamm. Sci. 1987, 3, 220–241. [Google Scholar] [CrossRef]
- Besson, M.; Feeney, W.E.; Moniz, I.; François, L.; Brooker, R.M.; Holzer, G.; Metian, M.; Roux, N.; Laudet, V.; Lecchini, D. Anthropogenic Stressors Impact Fish Sensory Development and Survival via Thyroid Disruption. Nat. Commun. 2020, 11, 3614. [Google Scholar] [CrossRef] [PubMed]
- Rolland, R.M. A Review of Chemically-Induced Alterations in Thyroid and Vitamin A Status from Field Studies of Wildlife and Fish. J. Wildl. Dis. 2000, 36, 615–635. [Google Scholar] [CrossRef] [PubMed]
- Coppock, R.W.; Dziwenka, M.M. Endocrine Disruption in Wildlife Species. In Reproductive and Developmental Toxicology; Academic Press: Cambridge, MA, USA, 2022; pp. 1423–1442. [Google Scholar] [CrossRef]
- Allen, M.J.; Sharma, S. Physiology, Adrenocorticotropic Hormone (ACTH). In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Lauderdale, L.K.; Mellen, J.D.; Walsh, M.T.; Granger, D.A.; Miller, L.J. Towards Understanding the Welfare of Cetaceans in Accredited Zoos and Aquariums. PLoS ONE 2021, 16, e0255506. [Google Scholar] [CrossRef] [PubMed]
- Tallo-Parra, O.; Salas, M.; Manteca, X. Zoo Animal Welfare Assessment: Where Do We Stand? Animals 2023, 13, 1966. [Google Scholar] [CrossRef]
- Broom, D.M.; Johnson, K.G. Stress and Animal Welfare; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar] [CrossRef]
- Veissier, I.; Boissy, A. Stress and Welfare: Two Complementary Concepts That Are Intrinsically Related to the Animal’s Point of View. Physiol. Behav. 2007, 92, 429–433. [Google Scholar] [CrossRef]
- Quiring, D.P.; Harlan, C.F. On the Anatomy of the Manatee. J. Mammal. 1953, 34, 192. [Google Scholar] [CrossRef]
- Forbes, S.; Bui, S.; Robinson, B.R.; Hochgeschwender, U.; Brennan, M.B. Integrated Control of Appetite and Fat Metabolism by the Leptin-Proopiomelanocortin Pathway. Proc. Natl. Acad. Sci. USA 2001, 98, 4233–4237. [Google Scholar] [CrossRef]
- Gorbman, A.; Gorbman, C. Pituitary Gland, Evolution of. In Encyclopedia of Endocrine Diseases; Academic Press: Cambridge, MA, USA, 2004; pp. 644–646. [Google Scholar] [CrossRef]
- Balaskó, M.; Garami, A.; Soós, S.; Koncsecskó-Gáspár, M.; Székely, M.; Pétervári, E. Central alpha-MSH, energy balance, thermal balance, and antipyresis. J. Therm. Biol. 2010, 35, 211–217. [Google Scholar] [CrossRef]
- Hadley, M.E.; Levine, J.E. Endocrinology, 6th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2007; pp. 157–181. [Google Scholar]
- Moriarty, G. Adenohypophysis: Ultrastructural Cytochemistry a Review. J. Histochem. Cytochem. 1973, 21, 855–894. [Google Scholar] [CrossRef]
- Kurusomi, K.; Kobayashi, Y. Corticotrophs in the Anterior Pituitary Glands of Normal and Adrenalectomized Rats as Revealed by Electron Microscopy. Endocrinology 1966, 78, 745–758. [Google Scholar] [CrossRef]
- Shiino, M.; Yamauchi, K. en Secretory Granules of Prolactin Cells of Neonatally Thyroidectomized Female Rats. Cell Tissue Res. 1985, 242, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Christian, H.C.; Imirtziadis, L.; Tortonese, D. Ultrastructural Changes in Lactotrophs and Folliculo-Stellate Cells in the Ovine Pituitary during the Annual Reproductive Cycle. J. Neuroendocrinol. 2015, 27, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Voigt, K.H.; Martin, R. Pituitary Somatotrophs Contain [Met] Enkephalin-like Immunoreactivity. Proc. Natl. Acad. Sci. USA 1978, 75, 6134–6138. [Google Scholar] [CrossRef] [PubMed]
- Singh, U.P.; Krishna, A. Pituitary Adrenocorticotrophic (ACTH) Cells during Reproductive Cycle in a Vespertilionid Bat, Scotophilus Heathi. Acta Biol. Hung. 1997, 48, 409–420. [Google Scholar] [CrossRef]
- Singh, U.P.; Krishna, A. Identification, Localization and Distribution of Pituitary Cell Types in Female Vespertilionid Bat, Scotophilus Heathi: A Combined Histochemical, Immunocytochemical and Electron Microscopic Study. Proc. Indian Natl. Sci. Acad. USA 1994, 60, 115–127. [Google Scholar]
- Chan, V.; Clayton, R.N.; Knox, G.; Catt, K.J. Ontogeny of Pituitary GnRH Receptors in the Rat. Endocrinology 1981, 108, 2086–2092. [Google Scholar] [CrossRef]
- Devnath, S.; Inoue, K. An Insight to Pituitary Folliculo-Stellate Cells. J. Neuroendocrinol. 2008, 20, 687–691. [Google Scholar] [CrossRef]
- Marin, F.; Stefaneanu, L.; Kovacs, K. Folliculostellate Cells of the Pituitary. Endocr. Pathol. 1991, 2, 180–192. [Google Scholar] [CrossRef]
- FernáNdez, A.J.; Sierra, M.A.; MéNdez, A.; Mozos, E.; Carrasco, L.; Moyano, M.C. Ultrastructural Modifications of the Cavities Formed by Folliculo-Stellate Cells in Chicken Adenohypophysis under Septic Shock Conditions. Cell Struct. Funct. 1986, 11, 379–382. [Google Scholar] [CrossRef]
- Leatherland, J.F.; Percy, R. Structure of the Nongranulated Cells in the Hypophyseal Rostral Pars Distalis of Cyclostomes and Actinopterygians. Cell Tissue Res. 1976, 166, 185–200. [Google Scholar] [CrossRef]
- Dingemans, K.P.; Feltkamp, C.A. Nongranulated Cells in the Mouse Adenohypophysis. Z. Zellforsch. Mikrosk. Anat. 1972, 124, 387–405. [Google Scholar] [CrossRef]
- Pérez Millán, M.I.; Cheung, L.Y.M.; Mercogliano, F.; Camilletti, M.A.; Chirino Felker, G.T.; Moro, L.N.; Miriuka, S.; Brinkmeier, M.L.; Camper, S.A. Pituitary Stem Cells: Past, Present and Future Perspectives. Nat. Rev. Endocrinol. 2023, 20, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Willis, T.L.; Lodge, E.J.; Andoniadou, C.L.; Yianni, V. Cellular Interactions in the Pituitary Stem Cell Niche. Cell. Mol. Life Sci. 2022, 79, 612. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.J.; Squires, E.L.; Imel, K.J.; Nett, T.M. Seasonal Variation in Hypothalamic Content of Gonadotropin-Releasing Hormone (GnRH), Pituitary Receptors for GnRH, and Pituitary Content of Luteinizing Hormone and Follicle-Stimulating Hormone in the Mare. Biol. Reprod. 1984, 30, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Schulte, B.A.; Seal, U.S.; Plotka, E.D.; Verme, L.J.; Ozoga, J.J.; Parsons, J.A. Seasonal Changes in Prolactin and Growth Hormone Cells in the Hypophyses of White-tailed Deer (Odocoileus Virginianus Borealis) Studied by Light Microscopic Immunocytochemistry and Radioimmunoassay. Am. J. Anat. 1980, 159, 369–377. [Google Scholar] [CrossRef]
- Bechtold, D.A.; Loudon, A.S.I. Hypothalamic Thyroid Hormones: Mediators of Seasonal Physiology. Endocrinology 2007, 148, 3605–3607. [Google Scholar] [CrossRef]
- Atkinson, S.; Crocker, D.; Houser, D.; Mashburn, K. Stress Physiology in Marine Mammals: How Well Do They Fit the Terrestrial Model? J. Comp. Physiol. B 2015, 185, 463–486. [Google Scholar] [CrossRef]
- Noushad, S.; Ahmed, S.; Ansari, B.; Mustafa, U.-H.; Saleem, Y.; Hazrat, H. Physiological Biomarkers of Chronic Stress: A Systematic Review. Int. J. Health Sci. 2021, 15, 46. [Google Scholar]
- Edes, A.N.; Wolfe, B.A.; Crews, D.E. Evaluating Allostatic Load: A New Approach to Measuring Long-Term Stress in Wildlife. J. Zoo Wildl. Med. 2018, 49, 272–282. [Google Scholar] [CrossRef]
- Karaer, M.C.; Čebulj-Kadunc, N.; Snoj, T. Stress in Wildlife: Comparison of the Stress Response among Domestic, Captive, and Free-Ranging Animals. Front. Vet. Sci. 2023, 10, 1167016. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Almorox, P.; Blanco, A.; Fiorito, C.; Sierra, E.; Suárez-Santana, C.; Consolli, F.; Arbelo, M.; Guzmán, R.G.; Molpeceres-Diego, I.; Fernández Gómez, A.; et al. Dolphin Pituitary Gland: Immunohistochemistry and Ultrastructural Cell Characterization Following a Novel Anatomical Dissection Protocol and Non-Invasive Imaging (MRI). Animals 2025, 15, 735. https://doi.org/10.3390/ani15050735
Alonso-Almorox P, Blanco A, Fiorito C, Sierra E, Suárez-Santana C, Consolli F, Arbelo M, Guzmán RG, Molpeceres-Diego I, Fernández Gómez A, et al. Dolphin Pituitary Gland: Immunohistochemistry and Ultrastructural Cell Characterization Following a Novel Anatomical Dissection Protocol and Non-Invasive Imaging (MRI). Animals. 2025; 15(5):735. https://doi.org/10.3390/ani15050735
Chicago/Turabian StyleAlonso-Almorox, Paula, Alfonso Blanco, Carla Fiorito, Eva Sierra, Cristian Suárez-Santana, Francesco Consolli, Manuel Arbelo, Raiden Grandía Guzmán, Ignacio Molpeceres-Diego, Antonio Fernández Gómez, and et al. 2025. "Dolphin Pituitary Gland: Immunohistochemistry and Ultrastructural Cell Characterization Following a Novel Anatomical Dissection Protocol and Non-Invasive Imaging (MRI)" Animals 15, no. 5: 735. https://doi.org/10.3390/ani15050735
APA StyleAlonso-Almorox, P., Blanco, A., Fiorito, C., Sierra, E., Suárez-Santana, C., Consolli, F., Arbelo, M., Guzmán, R. G., Molpeceres-Diego, I., Fernández Gómez, A., Almunia, J., Castro-Alonso, A., & Fernández, A. (2025). Dolphin Pituitary Gland: Immunohistochemistry and Ultrastructural Cell Characterization Following a Novel Anatomical Dissection Protocol and Non-Invasive Imaging (MRI). Animals, 15(5), 735. https://doi.org/10.3390/ani15050735







