Relationship Between Subclinical Mastitis Occurrence and Pathogen Prevalence in Two Different Automatic Milking Systems
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design, Dataset, and Farm Characteristics
2.2. Cows
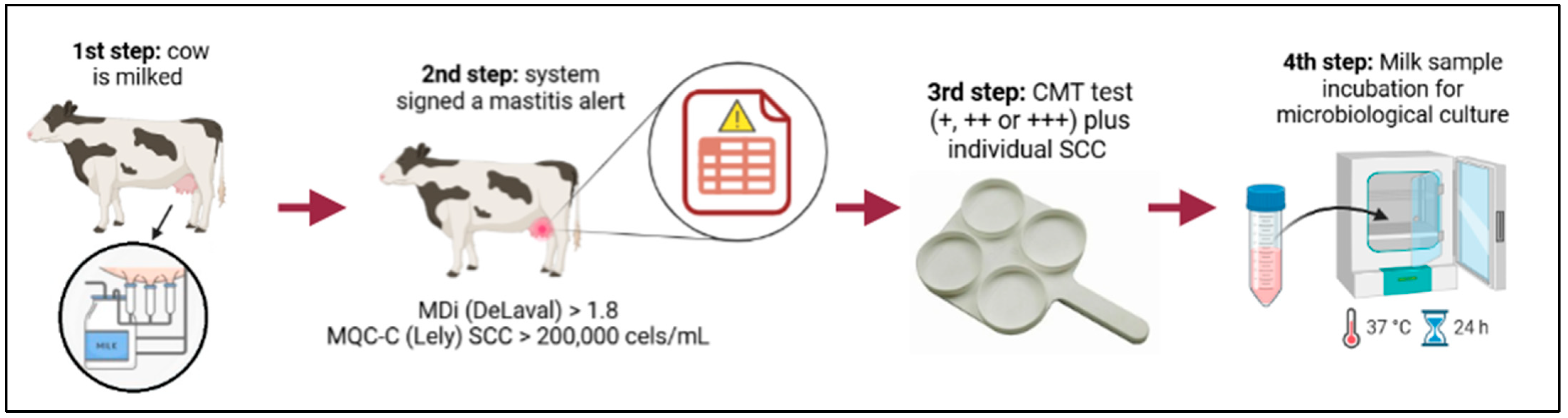
2.3. Epidemiological Indexes
2.4. Microbiological Culture
2.5. Statistical Analysis
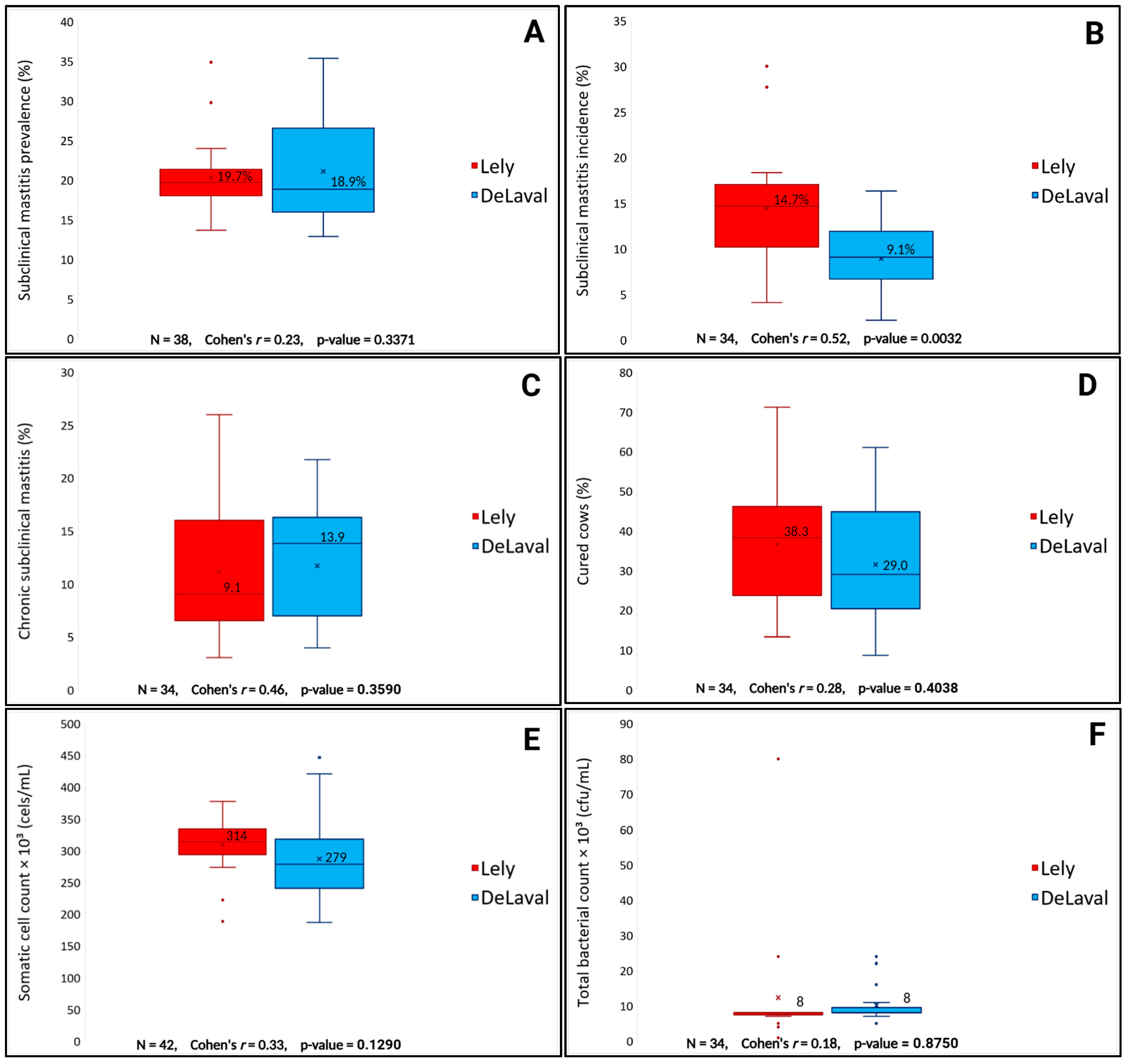
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| AMS | Automatic milking system |
| CBPB | Compost bedded pack barn |
| CM | Clinical mastitis |
| IMI | Intramammary infection |
| MDi | Mastitis detection index |
| SCC | Somatic cell counts |
| SCM | Subclinical mastitis |
| TBC | Total bacterial count |
References
- Emater (Empresa de Assistência Técnica e Extensão Rural). Relatório Socioeconômico da Cadeia Produtiva do Leite no RS 2023. Emater/RS, 19 p. 2023. Available online: https://todoelcampo.com.uy/wp-content/uploads/2024/10/PESQUISA_DO_LEITE_2023.pdf (accessed on 15 June 2024).
- Diavão, J.; Silva, A.S.; Silvi, R.R.; Teixeira, V.A.; Tomich, T.R.; Paiva, C.A.V.; Campos, M.M.; Machado, F.S.; Ferreira, R.E.P.; Dórea, J.R.R.; et al. Sistemas automatizados de ordenha no Brasil: Panorama e percepções. In Agricultura de Precisão: Um Novo Olhar na era Digital, 1st ed.; Bassoi, L.H., Bernardi, A.C.D.C., Vaz, C.M.P., Pires, J.L.F., Gebler, L., Jorge, L.A.D.C., Inamasu, R.Y., Eds.; São Carlos, SP: Cubo, Brazil, 2024; Cap. 78; pp. 716–723. [Google Scholar]
- Morales-Piñeyrúa, J.T.; Sant’Anna, A.C.; Banchero, G.; Damián, J.P. Dairy cows’ temperament and milking performance during the adaptation to an automatic milking system. Animals 2023, 13, 562. [Google Scholar] [CrossRef]
- Rodriguez, F.A.N.; Lopes, M.A.; Lima, A.L.R.; Almeida, G.A.D.; Novo, A.L.M.; Camargo, A.C.D.; Barbari, M.; Brito, S.C.; Reis, E.M.B.; Damasceno, F.A.; et al. Comparative Analysis of Milking and Behavior Characteristics of Multiparous and Primiparous Cows in Robotic Systems. An. Acad. Bras. Ciênc. 2024, 96, e20221078. [Google Scholar] [CrossRef]
- Rodriguez, F.A.N.; Lopes, M.A.; Lima, A.L.R.; De Almeida Júnior, G.A.; Novo, A.L.M.; Barbari, M.; Brito, S.C.; Bassotto, L.C.; De Camargo, A.C.; Nascimento, E.F.R. Correlations between milking characteristics and behavior of cows milked in robotic systems. Semin. Cienc. Agrar. 2023, 44, 1683–1696. [Google Scholar] [CrossRef]
- Córdova, H.D.A.; Alessio, D.R.M.; Cardozo, L.L.; Thaler, A. Impact of the factors of animal production and welfare on robotic milking frequency. Pesqui. Agropecu. Bras. 2018, 53, 238–246. [Google Scholar] [CrossRef]
- Miguel-Pacheco, G.G.; Kaler, J.; Remnant, J.; Cheyne, L.; Abbott, C.; French, A.P.; Pridmore, T.P.; Huxley, J.N. Behavioural changes in dairy cows with lameness in an automatic milking system. Appl. Anim. Behav. Sci. 2014, 150, 1–8. [Google Scholar] [CrossRef]
- Hovinen, M.; Aisla, A.M.; Pyörälä, S. Visual detection of technical success and effectiveness of teat cleaning in two automatic milking systems. J. Dairy Sci. 2005, 88, 3354–3362. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, W.; Neijenhuis, F.; Hogeveen, H. Relationship between udder health and hygiene on farms with an automatic milking system. J. Dairy Sci. 2010, 93, 4019–4033. [Google Scholar] [CrossRef]
- Penry, J.F. Mastitis control in automatic milking systems. Vet. Clin. N. Am. Food Anim. Pract. 2018, 34, 439–456. [Google Scholar] [CrossRef]
- Sharipov, D.R.; Yakimov, O.A.; Gainullina, M.K.; Kashaeva, A.R.; Kamaldinov, I.N. Development of automatic milking systems and their classification. IOP Conf. Ser. Earth Environ. Sci. 2021, 659, 012080. [Google Scholar] [CrossRef]
- Bull, C.R.; McFarlane, N.J.B.; Zwiggelaar, R.; Allen, C.J.; Mottram, T.T. Inspection of teats by colour image analysis for automatic milking systems. Comput. Electron. Agric. 1996, 15, 15–26. [Google Scholar] [CrossRef]
- Busanello, M.; Rossi, R.S.; Cassoli, L.D.; Pantoja, J.C.F.; Machado, P.F. Estimation of prevalence and incidence of subclinical mastitis in a large population of Brazilian dairy herds. J. Dairy Sci. 2017, 100, 6545–6553. [Google Scholar] [CrossRef]
- Cobirka, M.; Tancin, V.; Slama, P. Epidemiology and Classification of Mastitis. Animals 2020, 10, 2212. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, H.; Klaas, I.C.; Dalen, G.; Honig, H.; Zecconi, A.; Kelton, D.F.; Mainar, M.S. Novel ways to use sensor data to improve mastitis management. J. Dairy Sci. 2021, 104, 11317–11332. [Google Scholar] [CrossRef] [PubMed]
- Bausewein, M.; Mansfeld, R.; Doherr, M.G.; Harms, J.; Sorge, U.S. Sensitivity and specificity for the detection of clinical mastitis by automatic milking systems in Bavarian dairy herds. Animals 2022, 12, 2131. [Google Scholar] [CrossRef] [PubMed]
- Hammer, J.F.; Morton, J.M.; Kerrisk, K.L. Quarter-milking-, quarter-, udder-and lactation-level risk factors and indicators for clinical mastitis during lactation in pasture-fed dairy cows managed in an automatic milking system. Aust. Vet. J. 2012, 90, 167–174. [Google Scholar] [CrossRef]
- Penry, J.F.; Crump, P.M.; Ruegg, P.L.; Reinemann, D.J. Short communication: Cow- and quarter-level milking indicators and their associations with clinical mastitis in an automatic milking system. J. Dairy Sci. 2017, 100, 9267–9272. [Google Scholar] [CrossRef]
- Hiitiö, H.; Vakkamäki, J.; Simojoki, H.; Autio, T.; Junnila, J.; Pelkonen, S.; Pyörälä, S. Prevalence of subclinical mastitis in Finnish dairy cows: Changes during recent decades and impact of cow and herd factors. Acta Vet. Scand. 2017, 59, 22. [Google Scholar] [CrossRef]
- Castro, A.; Pereira, J.M.; Amiama, C.; Bueno, J. Mastitis diagnosis in ten Galician dairy herds (NW Spain) with automatic milking systems. SJAR 2015, 13, e0504. [Google Scholar] [CrossRef]
- Mahmmod, Y.S.; Klaas, I.C.; Svennesen, L.; Pedersen, K.; Ingmer, H. Communications of Staphylococcus aureus and non-aureus Staphylococcus species from bovine intramammary infections and teat apex colonization. J. Dairy Sci. 2018, 101, 7322–7333. [Google Scholar] [CrossRef]
- Skarbye, A.P.; Krogh, M.A.; Denwood, M.; Bjerring, M.; Østergaard, S. Effect of enhanced hygiene on transmission of Staphylococcus aureus, Streptococcus agalactiae, and Streptococcus dysgalactiae in dairy herds with automatic milking systems. J. Dairy Sci. 2021, 104, 7195–7209. [Google Scholar] [CrossRef]
- Sargeant, J.M.; O’Connor, A.M. Issues of reporting in observational studies in veterinary medicine. Prev. Vet. Med. 2014, 15, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Gonçalves, J.L.M.; Sparovek, G. Köppen’s climate classification map for Brazil. Meteorol. Z. 2013, 22, 711–728. [Google Scholar] [CrossRef] [PubMed]
- DeLaval. DeLaval VMS™ 300: A System Approach. 23p. 2019. Available online: https://www.delaval.com/globalassets/inriverresources/pdfs/2/2-page-view-vms-series-brochure-online-uk12.pdf (accessed on 8 July 2024).
- Salovuo, H.; Ronkainen, P.; Heino, A. Introduction of automatic milking system in Finland effect on milk quality. Agric. Food Sci. 2005, 14, 346–353. [Google Scholar] [CrossRef]
- Jago, J.G.; Davis, K.L.; Copeman, P.J.; Woolford, M.M. The effect of pre-milking teat-brushing on milk processing time in an automated milking system. J. Dairy Res. 2006, 73, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Monov, V.; Karastoyanov, D. Innovations in robotic cow milking systems. In Proceedings of the 20th International Conference on Advanced Robotics (ICAR), Ljubljana, Slovenia, 6–10 December 2021; pp. 58–63. [Google Scholar]
- LELY. Lely Astronaut A4—Sistema Robotizado de Ordenha. 26p. 2019. Available online: https://www.lely.com/media/lely-centers-files/brochures/published/lely-astronaut_A4-PT.pdf (accessed on 19 July 2024).
- Zagidullin, L.R.; Khisamov, R.R.; Kayumov, R.R.; Shaidullin, R.R.; Zinnatov, F.F.; Sadykov, N.F. Dairy robotic milking system. BIO Web Conf. 2023, 71, 01004. [Google Scholar] [CrossRef]
- Dohoo, I.R.; Leslie, K.E. Evaluation of changes in somatic cell counts as indicators of new intramammary infections. Prev. Vet. Med. 1991, 10, 225–237. [Google Scholar] [CrossRef]
- Fávero, S.; Portilho, F.V.R.; Oliveira, A.C.R.; Langoni, H.; Pantoja, J.C.F. Factors associated with mastitis epidemiologic indexes, animal hygiene, and bulk milk bacterial concentrations in dairy herds housed on compost bedding. Livest. Sci. 2015, 181, 220–230. [Google Scholar] [CrossRef]
- Garcia, B.L.N.; Martins, C.M.M.R.; Porto, L.F.; Nobrega, D.B.; Dos Santos, M.V. Accuracy of an AI-based automated plate reading mobile application for the identification of clinical mastitis-causing pathogens in chromogenic culture media. Sci. Rep. 2024, 14, 1208. [Google Scholar] [CrossRef]
- Suojala, L.; Kaartinen, L.; Pyörälä, S. Treatment for bovine Escherichia coli mastitis–an evidence-based approach. J. Vet. Pharmacol. Ther. 2013, 36, 521–531. [Google Scholar] [CrossRef]
- Ruegg, P.L. Making antibiotic treatment decisions for clinical mastitis. Vet. Clin. Food Anim. 2018, 34, 413–425. [Google Scholar] [CrossRef]
- Fritz, C.O.; Morris, P.E.; Richler, J.J. Effect size estimates: Current use, calculations, and interpretation. J. Exp. Psychol. Gen. 2011, 141, 1–18. [Google Scholar] [CrossRef]
- Sharpe, D. “Chi-Square Test is Statistically Significant: Now What?”. Pract. Assess. Res. Eval. 2015, 20, 8. [Google Scholar] [CrossRef]
- SAS Institute, Inc. SAS OnDemand for Academics. Release 9.04.01M5P09132017; SAS Institute Inc.: Cary, NC, USA, 2012; Available online: https://odamid.oda.sas.com/SASStudio/ (accessed on 10 May 2024).
- Costa, J.C.M.; Espeschit, I.D.F.; Pieri, F.A.; Benjamin, L.A.; Moreira, M.A.S. Increase in biofilm formation by Escherichia coli under conditions that mimic the mastitic mammary gland. Cienc. Rural. 2014, 44, 666–671. [Google Scholar] [CrossRef]
- Fidelis, C.E.; Orsi, A.M.; Freu, G.; Gonçalves, J.L.; Santos, M.V.D. Biofilm Formation and Antimicrobial Resistance of Staphylococcus aureus and Streptococcus uberis Isolates from Bovine Mastitis. Vet. Sci. 2024, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Petermann, M.; Wolter, W.; Rittershaus, C.; Kloppert, B.; Seufert, H.; Zschock, M. Automatic milking systems: Udder health and milk flow profiles. In Proceedings of the 1st North American Conference on Robotic Milking, Toronto, ON, Canada, 20–22 March 2002; Wageningen Pers. IV. pp. 67–70. [Google Scholar]
- Rotz, C.A.; Coiner, C.U.; Soder, K.J. Automatic milking systems, farm size, and milk production. J. Dairy Sci. 2003, 86, 4167–4177. [Google Scholar] [CrossRef]
- Campbell, J.R.; Marshall, R.T. Dairy Production and Processing: The Science of Milk and Milk Products; Waveland Press: Long Grove, IL, USA, 2016. [Google Scholar]
- Edmondson, P. Mastitis control in robotic milking systems. In Pract. 2012, 34, 260–268. [Google Scholar] [CrossRef]
- Pankey, J.W. Premilking udder hygiene. J. Dairy Sci. 1989, 72, 1308–1312. [Google Scholar] [CrossRef]
- Wieland, M.; Geary, C.M.; Nydam, D.V.; Virkler, P.D.; Zurakowski, M.; Watters, R.D.; Lynch, R. A randomized controlled trial to study the effects of an automated premilking stimulation system on milking performance, teat tissue condition, and udder health in Holstein dairy cows. J. Dairy Sci. 2023, 106, 6551–6566. [Google Scholar] [CrossRef]
- Hogenboom, J.A.; Pellegrino, L.; Sandrucci, A.; Rosi, V.; D’Incecco, P. Invited review: Hygienic quality, composition, and technological performance of raw milk obtained by robotic milking of cows. J. Dairy Sci. 2019, 102, 7640–7654. [Google Scholar] [CrossRef]
- Córdova, H.A.; Cardozo, L.L.; Alessio, D.R.M.; Thaler Neto, A. Influence of udder depth on cleaning teats and health of the mammary gland in robotic milking. Arq. Bras. Med. Vet. Zootec. 2018, 70, 1443–1452. [Google Scholar] [CrossRef]
- Cardozo, L.L.; Thaler Neto, A.; Souza, G.N.; Picinin, L.C.A.; Felipus, N.C.; Reche, N.L.M.; Schmidt, F.A.; Werncke, D.; Simon, E.E. Risk factors for the occurrence of new and chronic cases of subclinical mastitis in dairy herds in southern Brazil. J. Dairy Sci. 2015, 98, 7675–7685. [Google Scholar] [CrossRef]
- Hovinen, M.; Pyörälä, S. Invited review: Udder health of dairy cows in automatic milking. J. Dairy Sci. 2011, 94, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Hogeveen, H.; Ouweltjes, W.; de Koning, C.J.A.M.; Stelwagen, K. Milking interval, milk production and milk flow-rate in an automatic milking system. Livest. Prod. Sci. 2001, 72, 157–167. [Google Scholar] [CrossRef]
- Edmondson, P. Raised herd somatic cell count due to Staphylococcus aureus following the failure of an automatic teat spraying system. Vet. Rec. 2012, 170, 287. [Google Scholar] [CrossRef] [PubMed]
- Mehrzad, J.; Duchateau, L.; Burvenich, C. Viability of milk neutrophils and severity of bovine coliform mastitis. J. Dairy Sci. 2004, 87, 4150–4162. [Google Scholar] [CrossRef]
- Munoz, M.A.; Bennett, G.J.; Ahlstrom, C.; Griffiths, H.M.; Schukken, Y.H.; Zadoks, R.N. Cleanliness scores as indicator of Klebsiella exposure in dairy cows. J. Dairy Sci. 2008, 91, 3908–3916. [Google Scholar] [CrossRef]
- Salfer, J.A.; Siewert, J.M.; Endres, M.I. Housing, management characteristics, and factors associated with lameness, hock lesion, and hygiene of lactating dairy cattle on Upper Midwest United States dairy farms using automatic milking systems. J. Dairy Sci. 2018, 101, 8586–8594. [Google Scholar] [CrossRef]
- Souza, F.N.; Cunha, A.F.; Rosa, D.L.; Brito, M.A.V.; Guimarães, A.S.; Mendonça, L.C.; Souza, G.N.; Lage, A.P.; Blagitz, M.G.; Della Libera, A.M.M.P.; et al. Somatic cell count and mastitis pathogen detection in composite and single or duplicate quarter milk samples. Pesq. Vet. Bras. 2016, 36, 811–818. [Google Scholar] [CrossRef]
- Svennersten-Sjaunja, K.M.; Pettersson, G. Pros and cons of automatic milking in Europe. J. Anim. Sci. 2008, 86, 37–46. [Google Scholar] [CrossRef]
- Mottram, T. Requirements for teat inspection and cleaning in automatic milking systems. Comput. Electron. Agric. 1997, 17, 63–77. [Google Scholar] [CrossRef]
- Taponen, S.; Liski, E.; Heikkilä, A.M.; Pyörälä, S. Factors associated with intramammary infection in dairy cows caused by coagulase-negative staphylococci, Staphylococcus aureus, Streptococcus uberis, Streptococcus dysgalactiae, Corynebacterium bovis, or Escherichia coli. J. Dairy Sci. 2017, 100, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Janni, K.A.; Endres, M.I.; Reneau, J.K.; Schoper, W. Compost Dairy Barn Layout and Management Recommendations. Appl. Eng. Agric. 2007, 23, 97–102. [Google Scholar] [CrossRef]
- Nogara, K.F.; Busanello, M.; Tavares, Q.G.; De Assis, J.A.; Freu, G.; Dos Santos, M.V.; Vieira, F.M.C.; Zopollatto, M. Factors influencing milk quality and subclinical mastitis in dairy herds housed in compost-bedded pack barn system. Animals 2023, 13, 3638. [Google Scholar] [CrossRef] [PubMed]
- Nogara, K.F.; Busanello, M.; Haygert-Velho, I.M.P.; Zopollatto, M.; Frigeri, K.D.; Almeida, P.S.G. Characterization and relationship between bulk tank milk composition and compost bedded variables from dairy barns in Rio Grande do Sul state, Brazil. Turk. J. Vet. Anim. Sci. 2021, 45, 890–900. [Google Scholar] [CrossRef]
- Giambra, I.J.; Jahan, Y.; Yin, T.; Engel, P.; Weimann, C.; Brügemann, K.; König, S. Identification of thermophilic aerobic sporeformers in bedding material of compost-bedded dairy cows using microbial and molecular methods. Animals 2021, 11, 2890. [Google Scholar] [CrossRef]
- De Koning, K.; Slaghuis, B.; Van Der Vorst, Y. Robotic milking and milk quality: Effects on bacterial counts, somatic cell counts, freezing point and free fatty acids. Ital. J. Anim. Sci. 2003, 2, 291–299. [Google Scholar] [CrossRef]
- Dufour, S.; Fréchette, A.; Barkema, H.W.; Mussell, A.; Scholl, D.T. Invited review: Effect of udder health management practices on herd somatic cell count. J. Dairy Sci. 2011, 94, 563–579. [Google Scholar] [CrossRef]
- Vissio, C.; Mella, A.; Amestica, L.; Pol, M. Noninferiority study evaluating the efficacy of a teat disinfectant containing copper and zinc for prevention of naturally occurring intramammary infections in an automatic milking system. J. Dairy Sci. 2020, 103, 1776–1784. [Google Scholar] [CrossRef]
- Koivula, M.; Pitkälä, A.; Pyörälä, S.; Mäntysaari, E.A. Distribution of bacteria and seasonal and regional effects in a new database for mastitis pathogens in Finland. Acta Agric. Scand. 2007, 5, 89–96. [Google Scholar] [CrossRef]
- Barkema, H.W.; Schukken, Y.H.; Zadoks, R.N. Invited review: The role of cow, pathogen, and treatment regimen in the therapeutic success of bovine Staphylococcus aureus mastitis. J. Dairy Sci. 2006, 89, 1877–1895. [Google Scholar] [CrossRef]
| Ingredients 1 | Multiparous | Primiparous |
|---|---|---|
| Corn silage | 40.00 kg | 30.00 kg |
| Soybean meal | 4.70 kg | 3.50 kg |
| Oat silage | 5.00 kg | 5.00 kg |
| Commercial concentrated feed | 3.50 kg | 3.00 kg |
| Pathogens Group 1 | AMS | Total of Cases | % | |||
|---|---|---|---|---|---|---|
| Lely | % | DeLaval | % | |||
| Major contagious | 126 | 23.1 a | 34 | 10.5 b | 160 | 18.4 |
| Minor contagious | 300 | 55.0 b | 221 | 68.0 a | 521 | 59.9 |
| Major environmental | 96 | 17.6 | 46 | 14.2 | 142 | 16.3 |
| Minor environmental | 23 | 4.2 b | 24 | 7.4 a | 47 | 5.4 |
| Total of cases | 545 | 100.0 | 325 | 100 | 870 | 100.0 |
| Mastitis-Causing Pathogen | AMS | Total of Cases | Relative Frequency % | |||
|---|---|---|---|---|---|---|
| Lely | % | DeLaval | % | |||
| Staphylococcus chromogenes | 121 | 22.2 | 73 | 22.5 | 194 | 22.3 |
| Staphylococcus aureus | 106 | 19.4 a | 25 | 7.7 b | 131 | 15.1 |
| Staphylococcus haemolyticus | 58 | 10.6 | 48 | 14.8 | 106 | 12.2 |
| Staphylococcus warneri | 49 | 9.0 | 36 | 11.1 | 85 | 9.8 |
| Klebsiella spp. | 46 | 8.4 | 32 | 9.8 | 78 | 9.0 |
| Escherichia coli | 45 | 8.3 a | 12 | 3.7 b | 57 | 6.6 |
| Corynebacterium bovis | 39 | 7.2 a | 8 | 2.5 b | 47 | 5.4 |
| Streptococcus agalactiae | 11 | 2.0 | 7 | 2.2 | 18 | 2.1 |
| Serratia spp. | 11 | 2.0 | 3 | 0.9 | 14 | 1.6 |
| Streptococcus dysgalactiae | 9 | 1.7 | 2 | 0.6 | 11 | 1.3 |
| Streptococcus uberis | 5 | 0.9 | 2 | 0.6 | 7 | 0.8 |
| Other non-aureus Staphylococci 1 | 33 | 6.1 b | 56 | 17.2 a | 89 | 10.2 |
| Other pathogens 2 | 12 | 2.2 b | 21 | 6.5 a | 33 | 3.8 |
| Total | 545 | 100.0 | 325 | 100.0 | 870 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogara, K.F.; Busanello, M.; Zopollatto, M. Relationship Between Subclinical Mastitis Occurrence and Pathogen Prevalence in Two Different Automatic Milking Systems. Animals 2025, 15, 776. https://doi.org/10.3390/ani15060776
Nogara KF, Busanello M, Zopollatto M. Relationship Between Subclinical Mastitis Occurrence and Pathogen Prevalence in Two Different Automatic Milking Systems. Animals. 2025; 15(6):776. https://doi.org/10.3390/ani15060776
Chicago/Turabian StyleNogara, Karise Fernanda, Marcos Busanello, and Maity Zopollatto. 2025. "Relationship Between Subclinical Mastitis Occurrence and Pathogen Prevalence in Two Different Automatic Milking Systems" Animals 15, no. 6: 776. https://doi.org/10.3390/ani15060776
APA StyleNogara, K. F., Busanello, M., & Zopollatto, M. (2025). Relationship Between Subclinical Mastitis Occurrence and Pathogen Prevalence in Two Different Automatic Milking Systems. Animals, 15(6), 776. https://doi.org/10.3390/ani15060776






