Enhanced Biosorption and Recovery of Copper and Zinc from Acetic Acid-Extracted Livestock Wastewater Sludge Using Baker’s Yeast
Simple Summary
Abstract
1. Introduction
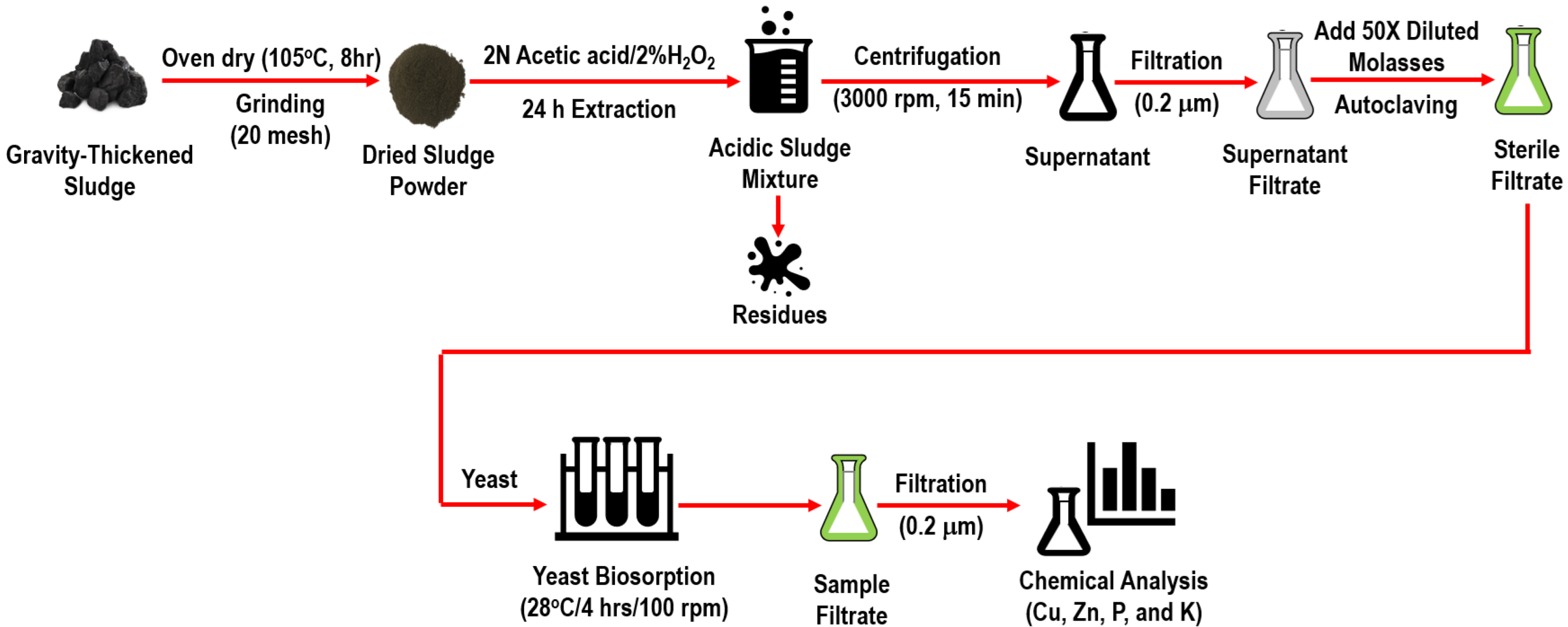
2. Materials and Methods
2.1. Collection and Preparation of the Livestock Sludge
2.2. Preparation of the Baker’s Yeast
2.3. Extraction of Copper and Zinc from the Sludge by Acetic Acid and Hydrogen Peroxide
2.4. Biosorption of Copper and Zinc from the Supernatant of Acidic Sludge Mixture
2.5. Quantitative Analysis of Heavy Metals
2.6. Analysis of Liquid Samples
2.7. Yeast Concentration (Dry Weight) Detection
2.8. Statistical Analysis
3. Results and Discussion
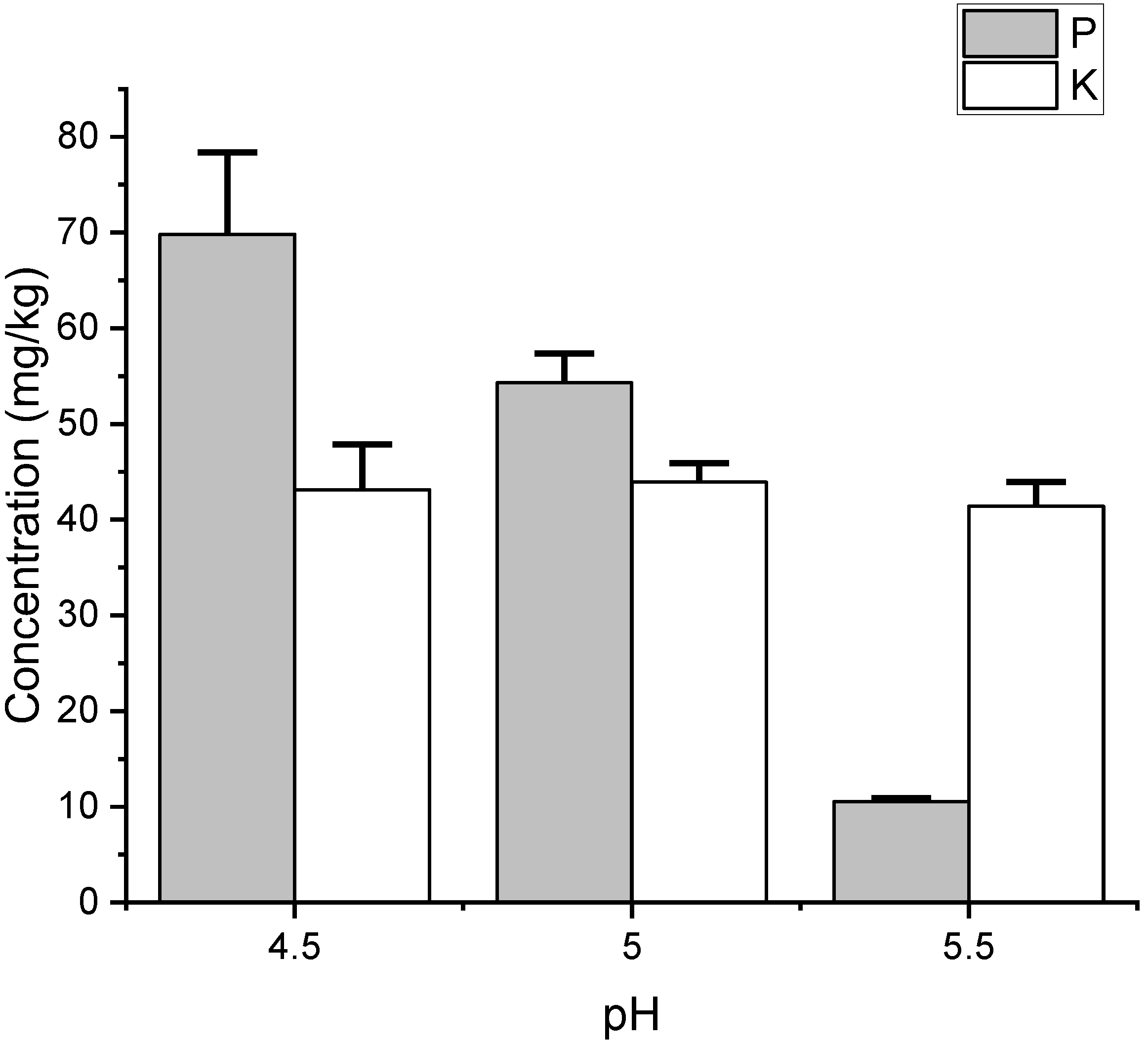
3.1. Impact of Various pH Values on Biosorption of Yeast
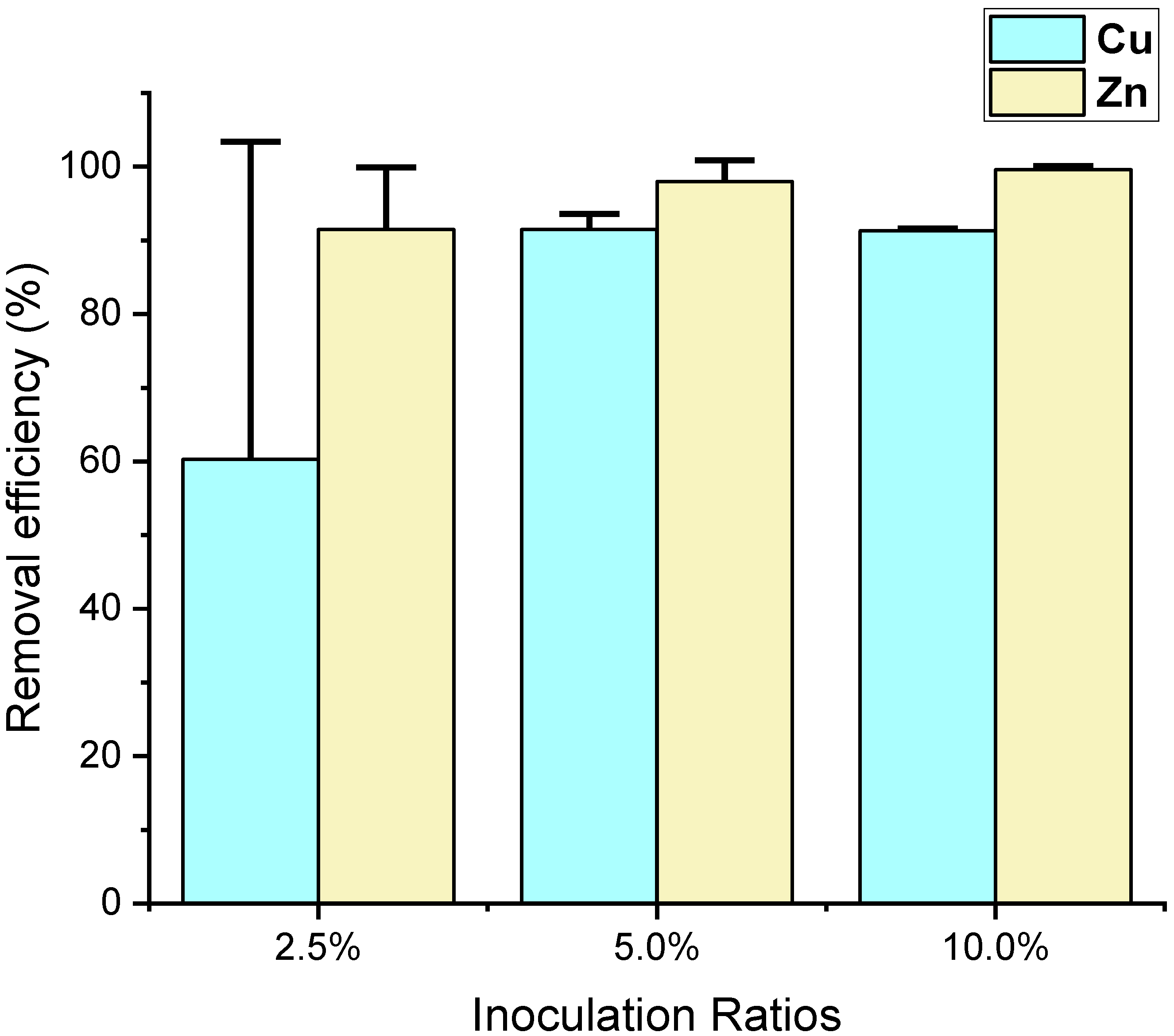
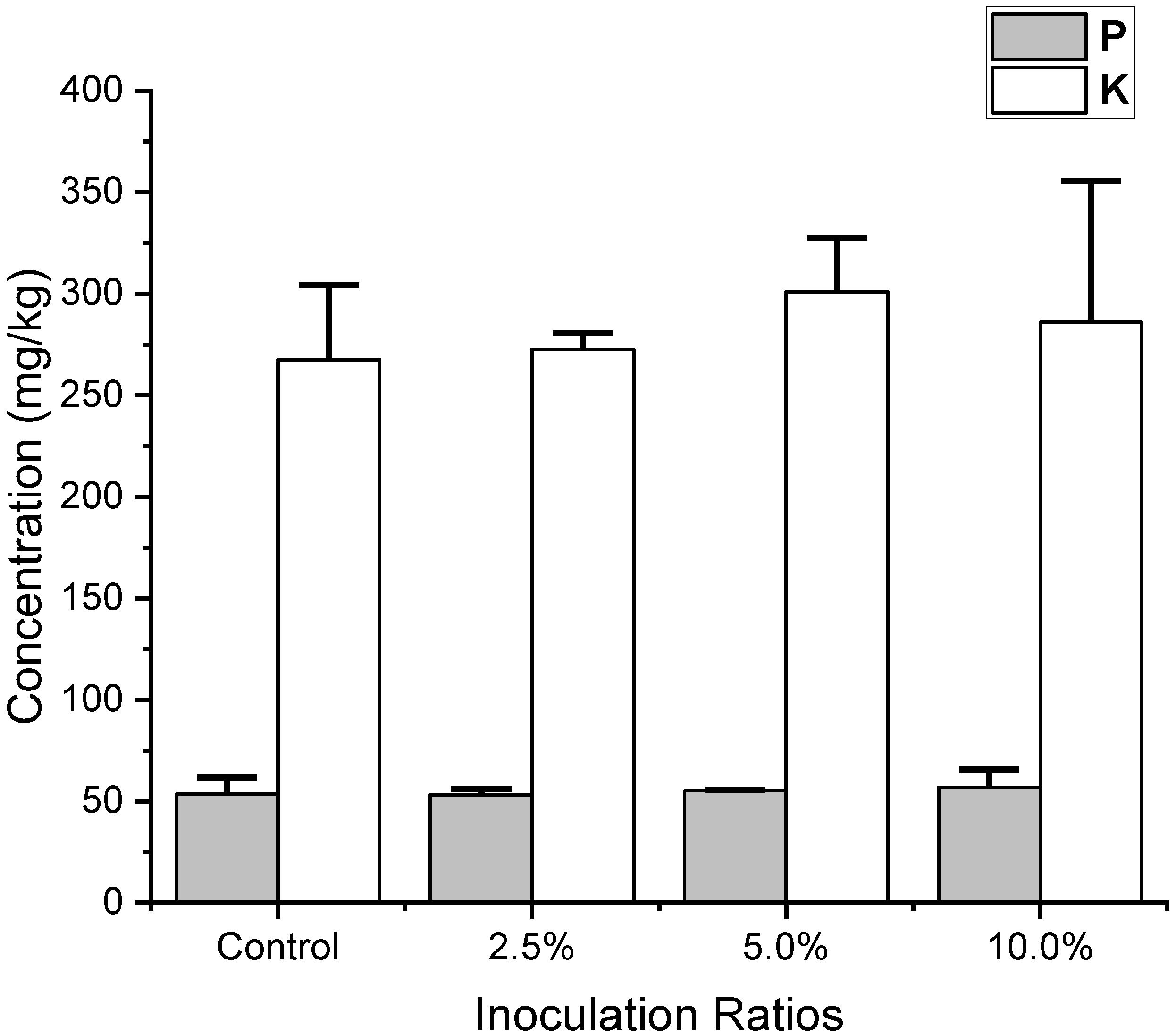
3.2. Effect of Different Inoculum Ratios of Yeast on the Efficiency of Biosorption
3.3. Factors Affecting Yeast Biosorption
3.3.1. Type of Biosorbent
3.3.2. Biosorption Time
3.3.3. pH Value
3.3.4. Incubation Temperature
3.3.5. Biosorbent Concentration
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhen, Y.; Wang, M.; Gu, Y.; Yu, X.; Shahzad, K.; Xu, J.; Gong, Y.; Li, P.; Loor, J.J. Biosorption of copper in swine manure using Aspergillus and Yeast: Characterization and its microbial diversity study. Front. Microbiol. 2021, 12, 687533. [Google Scholar] [CrossRef] [PubMed]
- Yen, K.W.; Chen, W.C.; Su, J.J. Recovery of copper and zinc from livestock bio–sludge with an environmentally friendly organic acid extraction. Animals 2024, 14, 342. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.; Gadd, G.M. Biosorption refers to a collection of physical, chemical, and metabolism–independent processes such as absorption, adsorption, ion exchange, surface complexation, and precipitation. Bioresour. Technol. 2014, 160, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K.; Witek-Krowiak, A. State of the art for the biosorption process—A review. Appl. Biochem. Biotechnol. 2013, 170, 1389–1416. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, X.; Wang, X.; Lu, X.; Shen, Y. Different biosorption mechanisms of uranium (VI) by live and heat–killed Saccharomyces cerevisiae under environmentally relevant conditions. J. Environ. Radioact. 2017, 167, 92–99. [Google Scholar] [CrossRef]
- Andreea, S. Brewer’s yeast: An alternative for heavy metal biosorption from wastewaters. ProEnviroment 2013, 6, 457–464. [Google Scholar]
- Liu, N.; Liao, J.; Yang, Y.; Luo, S.; Luo, Q.; An, A.; Duan, Y.; Liu, M.; Zhao, P. Biosorption of 241Am by Saccharomyces cerevisiae: Preliminary investigation on mechanism. J. Radioanal. Nucl. Chem. 2008, 275, 173–180. [Google Scholar] [CrossRef]
- Kapoor, A.; Viraraghavan, T. Fungi biosorption—An alternative treatment option for heavy metal bearing wastewaters: A review. Bioresour. Technol. 1995, 53, 195–206. [Google Scholar]
- Stehlik–Tomas, V.; Zetic, V.G.; Stanzer, D.; Grba, S.; Vacic, N. Zinc, copper, and manganese enrichment in yeast Saccharomyces cerevisiae. Food Technol. Biotechnol. 2004, 42, 115–120. [Google Scholar]
- Eide, D. Molecular biology of iron and zinc uptake in eukaryotes. Curr. Opin. Cell Biol. 1997, 9, 573–577. [Google Scholar] [CrossRef]
- Veglio, F.; Beolchini, F. Removal of metals by biosorption: A review. Hydrometallurgy 1997, 44, 301–316. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorption of heavy metals by Saccharomyces cerevisiae: A review. Biotechnol. Adv. 2006, 24, 427–451. [Google Scholar] [CrossRef] [PubMed]
- De Rossi, A.; Rigon, M.R.; Zaparoli, M.; Braido, R.D.; Colla, L.M.; Dotto, G.L.; Piccin, J.S. Chromium(VI) biosorption by Saccharomyces cerevisiae subjected to chemical and thermal treatments. Environ. Sci. Pollut. Res. 2018, 25, 19179–19186. [Google Scholar] [CrossRef] [PubMed]
- Göksungur, Y.; Üren, S.; Güvenc, U. Biosorption of copper ions by caustic treated waste baker’s yeast biomass. Turk. J. Biol. 2003, 27, 23–29. [Google Scholar]
- Kordialik-Bogacka, E. Surface properties of yeast cells during heavy metal biosorption. Cent. Eur. J. Chem. 2011, 9, 348–351. [Google Scholar] [CrossRef]
- Ali, I.H.; Alrafai, H.A. Kinetic, isotherm and thermodynamic studies on biosorption of chromium(VI) by using activated carbon from leaves of Ficus nitida. Chem. Cent. J. 2016, 10, 36. [Google Scholar] [CrossRef]
- Ojima, Y.; Kosako, S.; Kihara, M.; Miyoshi, N.; Igarashi, K.; Azuma, M. Recovering metals from aqueous solutions by biosorption onto phosphorylated dry baker’s yeast. Sci. Rep. 2019, 9, 225. [Google Scholar] [CrossRef]
- Wang, J.L. Biosorption of copper (II) by chemically modified biomass of Saccharomyces cerevisiae. Process Biochem. 2002, 37, 847–850. [Google Scholar]
- Özer, A.; Özer, D. Comparative study of the biosorption of lead (II), nickel (II), and chromium (VI) ions onto Saccharomyces cerevisiae: Determination of biosorption heats. J. Hazard. Mater. 2003, 100, 219–229. [Google Scholar] [CrossRef]
- Singleton, I.; Simmons, P. Factors affecting silver biosorption by an industrial strain of Saccharomyces cerevisiae. J. Chem. Tech. Biotechnol. 1996, 65, 21–28. [Google Scholar] [CrossRef]
- Farhan, S.N.; Khadom, A.A. Biosorption of heavy metals from aqueous solutions by Saccharomyces cerevisiae. Int. J. Ind. Chem. 2015, 6, 119–130. [Google Scholar] [CrossRef]
- Hadiani, M.R.; Darani, K.K.; Rahimifard, N.; Younesi, H. Biosorption of low concentration levels of lead (II) and cadmium (II) from aqueous solution by Saccharomyces cerevisiae: Response surface methodology. Biocatal. Agric. Biotechnol. 2018, 15, 25–34. [Google Scholar] [CrossRef]
- Ghorbani, F.; Younesi, H.; Ghasempouri, S.M.; Zinatizadeh, A.A.; Amini, M.; Daneshia, A. Application of response surface methodology for optimization of cadmium biosorption in an aqueous solution by Saccharomyces cerevisiae. Chem. Eng. J. 2008, 145, 267–275. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media, 4th ed.; CRC Press: Washington, DC, USA, 2010. [Google Scholar]
- Hamza, S.M.; Ahmed, H.F.; Ehab, A.M.; Mohammad, F.M. Optimization of cadmium, zinc, and copper biosorption in an aqueous solution by Saccharomyces cerevisiae. J. Am. Sci. 2010, 6, 597–604. [Google Scholar]
- Ririhena, S.A.J.; Astuti, A.D.; Fachrul, M.F.; Silalahi, M.D.S.; Hadisoebroto, R.; Rinanti, A. Biosorption of heavy metal copper (Cu2+) by Saccharomyces cerevisiae. IOP Conf. Ser. Earth Environ. Sci. 2018, 106, 012090. [Google Scholar] [CrossRef]
- Pohl, A. Removal of heavy metal ions from water and wastewaters by sulfur–containing precipitation agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Junghans, K.; Straube, G. Biosorption of copper by yeasts. Biol. Met. 1991, 4, 233–237. [Google Scholar] [CrossRef]
- Danial, A.W.; Dardir, F.M. Copper biosorption by Bacillus pumilus OQ931870 and Bacillus subtilis OQ931871 isolated from Wadi Nakheil, Red Sea, Egypt. Microb. Cell Fact. 2023, 22, 152. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef]
- Machado, M.D.; Janssens, S.; Soares, H.M.; Soares, E.V. Removal of heavy metals using a brewer’s yeast strain of Saccharomyces cerevisiae: Advantages of using dead biomass. J. Appl. Microbiol. 2009, 106, 1792–1804. [Google Scholar] [CrossRef]
- Garanin, R.; Lykov, I. Biosorption of zinc and copper ions by immobilized yeast under aerobic and anaerobic conditions. E3S Web Conf. 2024, 548, 02004. [Google Scholar] [CrossRef]

| Variables | Supernatant/ Diluted Molasses Vol. (mL) | Yeast Concentrations (mg/L) | Inoculation Ratios (%) | pH Values | Incubation Temp./Time/Shaker Speed (°C/h/rpm) |
|---|---|---|---|---|---|
| pH Values | 45/2.5 | 3.59 | 5 | 4.5, 5.0, 5.5 | 28/4/100 |
| Inoculation Ratios | 45/2.5 | 2.05 | 2.5, 5, 10 | 5.5 | 28/4/100 |
| Elements | pH Values | p-Value | |||
|---|---|---|---|---|---|
| 4.5 | 5.0 | 5.5 | |||
| Removal efficiency (%) | |||||
| Cu | 48.6 ± 4.8 | 54.9 ± 1.6 | 48.8 ± 25.8 | NS | |
| Zn | 32.9 ± 8.1 c | 46.7 ± 3.4 b | 97.3 ± 2.9 a | <0.05 | |
| Concentration (mg/kg) | |||||
| P | Initial | 72.3 | 55.6 | 10.9 | |
| Final | 69.8 ± 8.6 | 54.3 ± 3.1 | 10.5 ± 0.4 | <0.05 | |
| K | Initial | 44.7 | 46.5 | 40.8 | |
| Final | 43.1 ± 4.7 | 43.9 ± 2.0 | 41.4 ± 2.6 | NS | |
| Elements | Control (No Inoculation) | Inoculum Ratio (%) | p-Value | ||
|---|---|---|---|---|---|
| 2.5 | 5.0 | 10.0 | |||
| Removal efficiency (%) | |||||
| Cu | 60.3 ± 43.1 | 91.5 ± 2.1 | 91.3 ± 0.3 | NS | |
| Zn | 91.5 ± 8.4 | 98.0 ± 2.9 | 99.6 ± 0.5 | NS | |
| Concentration (mg/kg) | |||||
| P | 53.5 ± 8.1 | 53.3 ± 2.6 | 55.2 ± 0.6 | 56.9 ± 8.7 | NS |
| K | 267.5 ± 36.8 | 272.6 ± 8.0 | 301.0 ± 26.4 | 286.0 ± 69.6 | NS |
| Metals | pH Values | Yeast Condition | Yeast Source/ Biosorption Conditions | Initial Metal Concentrations/ Sources of Metals | Removal (%) | References |
|---|---|---|---|---|---|---|
| Cr (VI) | 5, 7, 9 | Dead (dry at 60 °C, 24 h) | Dried cell (25 °C, 3 h) | 100 mg/L (Potassium dichromate solution) | Cr: 99.66 (pH = 5) | [13] |
| Cu (II) | 2, 3, 4, 5, 6 | Dead (dry at 80 °C, 28 h) | Beer waste (28 °C, 2 h) | 33,746 mg/L (Electroplating industry waste) | Cu: 61.40 (pH = 4) | [26] |
| Pb, Zn, Cr, Co, Cd, Cu | 2, 3, 4, 5.5, 6, 8 | Dead (dry at 60 °C, 6 h) | Raw yeast (25 °C, 0.5 h) | 100 mg/L (Metal solutions) | Pb > Zn > Cr > Co > Cd > Cu (pH = 5.5) | [21] |
| Cd (II), Zn (II), Cu (II) | 3, 4.5, 6, 7, 8.5, 9.5, 11 | Living | Baker’s yeast (25 °C, 0.5 h) | 5.0 mg/L (Metal solutions) | Cu: 55.0 (pH = 6) Zn: 69.8 (pH = 6) Cd: 95.6 (pH = 8.5) | [25] |
| Cu (II), Zn (II) | 4.5, 5.0, 5.5 | Living | Baker’s yeast (28 °C, 4 h) | Cu: 4.01 mg/kg Zn: 43.7 mg/kg (Supernatant after acidic sludge extraction) | Cu: 48.8 (pH = 5.5) Zn: 97.3 (pH = 5.5) | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.-J.; Yen, K.-W.; Chen, W.-C. Enhanced Biosorption and Recovery of Copper and Zinc from Acetic Acid-Extracted Livestock Wastewater Sludge Using Baker’s Yeast. Animals 2025, 15, 794. https://doi.org/10.3390/ani15060794
Su J-J, Yen K-W, Chen W-C. Enhanced Biosorption and Recovery of Copper and Zinc from Acetic Acid-Extracted Livestock Wastewater Sludge Using Baker’s Yeast. Animals. 2025; 15(6):794. https://doi.org/10.3390/ani15060794
Chicago/Turabian StyleSu, Jung-Jeng, Kuang-Wei Yen, and Wei-Chen Chen. 2025. "Enhanced Biosorption and Recovery of Copper and Zinc from Acetic Acid-Extracted Livestock Wastewater Sludge Using Baker’s Yeast" Animals 15, no. 6: 794. https://doi.org/10.3390/ani15060794
APA StyleSu, J.-J., Yen, K.-W., & Chen, W.-C. (2025). Enhanced Biosorption and Recovery of Copper and Zinc from Acetic Acid-Extracted Livestock Wastewater Sludge Using Baker’s Yeast. Animals, 15(6), 794. https://doi.org/10.3390/ani15060794






