Evaluating Past Range Shifts and Niche Dynamics of Giant Pandas Since the Last Interglacial
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
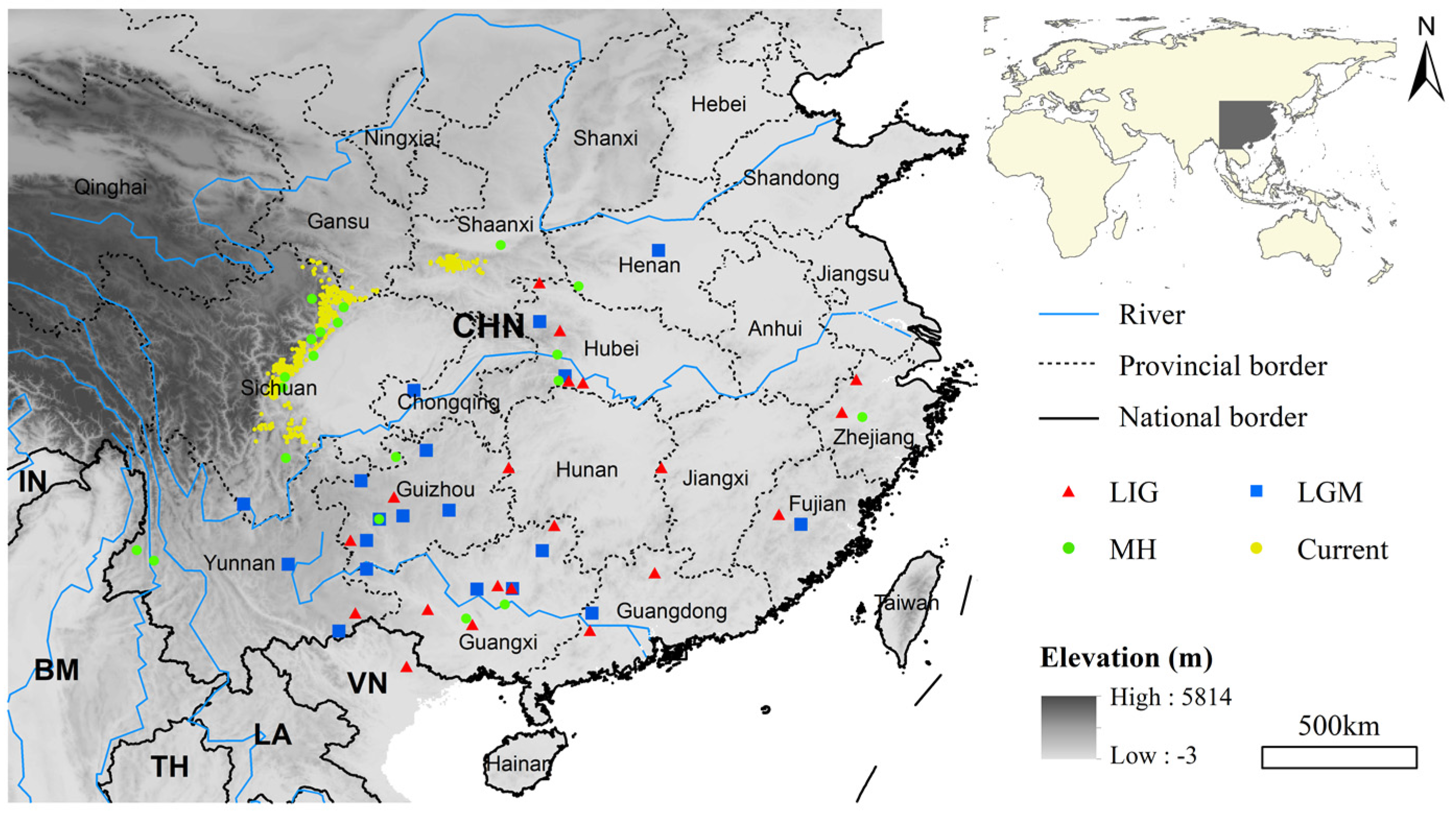
2.1. Study Area and Occurrence Data
2.2. Climate Variables
2.3. Model Construction and Optimization
2.4. Spatial Analyses
2.5. Comparison of Climatic Niches
3. Results
3.1. Model Optimization and Accuracy Evaluation
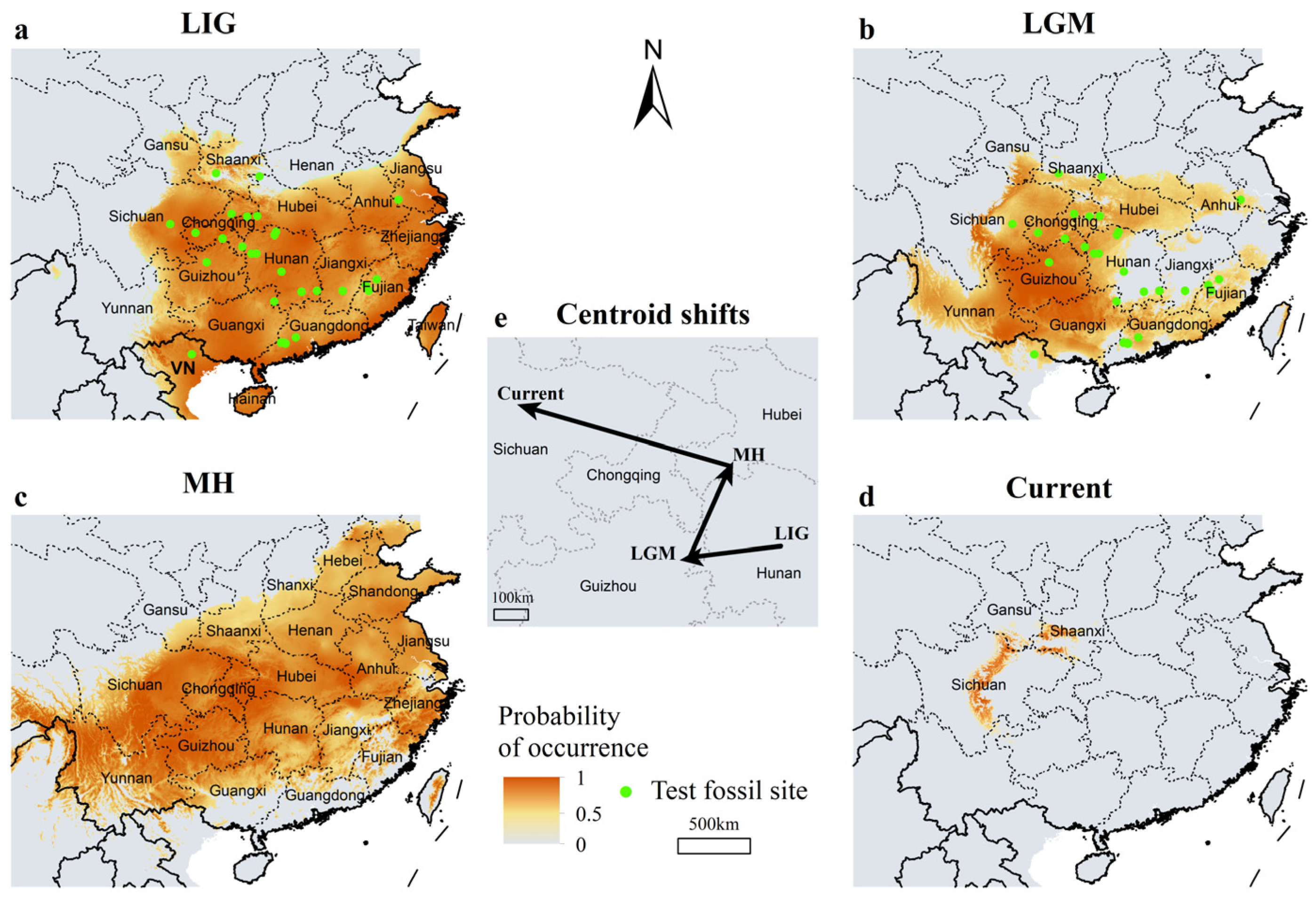
3.2. Range Shifts of Giant Pandas Since the Last Interglacial
3.3. Impacts of Climatic Variables
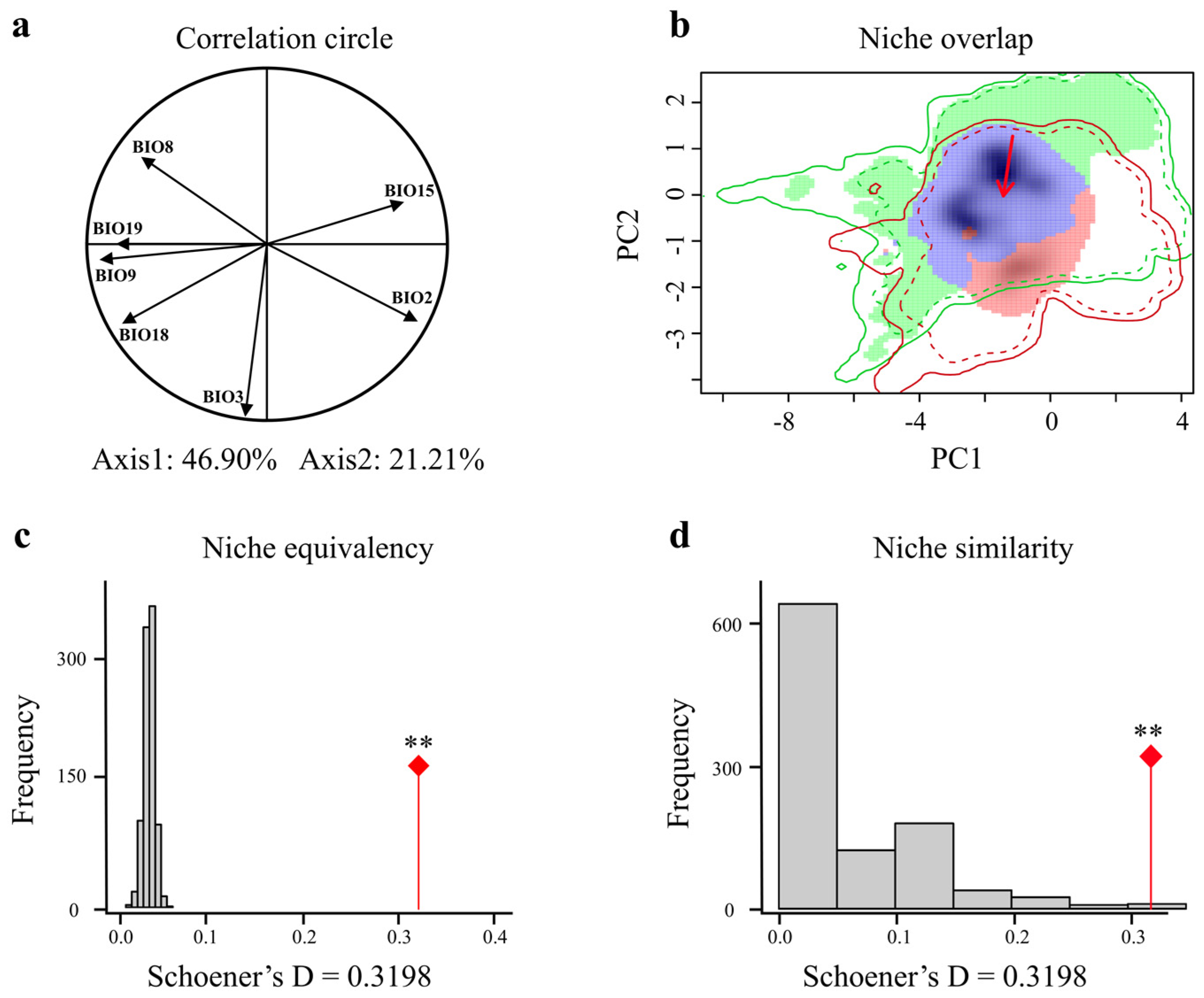
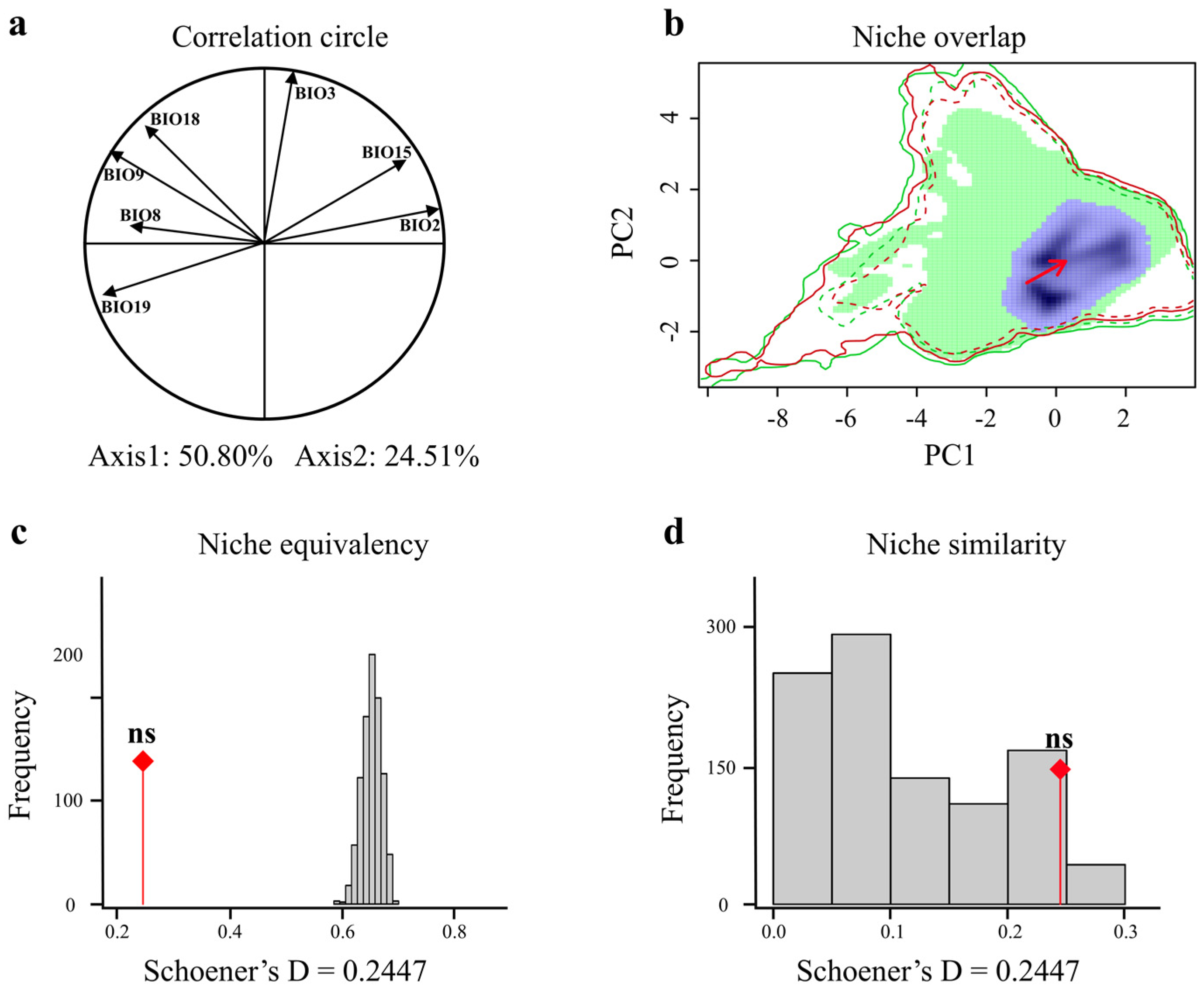
3.4. Comparison of Giant Pandas’ Climatic Niches
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holmes, J.; Lowe, J.; Wolff, E.; Srokosz, M. Rapid climate change: Lessons from the recent geological past. Glob. Planet. Change 2011, 79, 157–162. [Google Scholar] [CrossRef]
- Bellard, C.; Bertelsmeier, C.; Leadley, P.; Thuiller, W.; Courchamp, F. Impacts of climate change on the future of biodiversity. Ecol. Lett. 2012, 15, 365–377. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef] [PubMed]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A globally coherent fingerprint of climate change impacts across natural systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Walther, G.-R.; Post, E.; Convey, P.; Menzel, A.; Parmesan, C.; Beebee, T.J.C.; Fromentin, J.-M.; Hoegh-Guldberg, O.; Bairlein, F. Ecological responses to recent climate change. Nature 2002, 416, 389–395. [Google Scholar] [CrossRef]
- Li, J.; Liu, F.; Xue, Y.; Zhang, Y.; Li, D. Assessing vulnerability of giant pandas to climate change in the Qinling Mountains of China. Ecol. Evol. 2017, 7, 4003–4015. [Google Scholar] [CrossRef]
- Lunn, N.J.; Servanty, S.; Regehr, E.V.; Converse, S.J.; Richardson, E.; Stirling, I. Demography of an apex predator at the edge of its range: Impacts of changing sea ice on polar bears in Hudson Bay. Ecol. Appl. 2016, 26, 1302–1320. [Google Scholar] [CrossRef]
- Stirling, I.; Derocher, A.E. Effects of climate warming on polar bears: A review of the evidence. Glob. Change Biol. 2012, 18, 2694–2706. [Google Scholar] [CrossRef]
- Scranton, K.; Amarasekare, P. Predicting phenological shifts in a changing climate. Proc. Natl. Acad. Sci. USA 2017, 114, 13212–13217. [Google Scholar] [CrossRef]
- Yang, L.H.; Rudolf, V.H.W. Phenology, ontogeny and the effects of climate change on the timing of species interactions. Ecol. Lett. 2010, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.D.; Cameron, A.; Green, R.E.; Bakkenes, M.; Beaumont, L.J.; Collingham, Y.C.; Erasmus, B.F.N.; de Siqueira, M.F.; Grainger, A.; Hannah, L.; et al. Extinction risk from climate change. Nature 2004, 427, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Hill, J.K.; Ohlemüller, R.; Roy, D.B.; Thomas, C.D. Rapid Range Shifts of Species Associated with High Levels of Climate Warming. Science 2011, 333, 1024–1026. [Google Scholar] [CrossRef]
- Comte, L.; Grenouillet, G. Distribution shifts of freshwater fish under a variable climate: Comparing climatic, bioclimatic and biotic velocities. Divers. Distrib. 2015, 21, 1014–1026. [Google Scholar] [CrossRef]
- Tingley, M.W.; Monahan, W.B.; Beissinger, S.R.; Moritz, C. Birds track their Grinnellian niche through a century of climate change. Proc. Natl. Acad. Sci. USA 2009, 106, 19637–19643. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Sánchez-Cordero, V. Conservatism of Ecological Niches in Evolutionary Time. Science 1999, 285, 1265–1267. [Google Scholar] [CrossRef]
- Wiens, J.J.; Graham, C.H. Niche Conservatism: Integrating Evolution, Ecology, and Conservation Biology. Annu. Rev. Ecol. Evol. Syst. 2005, 36, 519–539. [Google Scholar] [CrossRef]
- Smith, F.A.; Betancourt, J.L. The effect of Holocene temperature fluctuations on the evolution and ecology of Neotoma (woodrats) in Idaho and northwestern Utah. Quat. Res. 2003, 59, 160–171. [Google Scholar] [CrossRef]
- Liu, B.; Liu, Z.; Li, C.; Yu, H.; Wang, H. Geographical distribution and ecological niche dynamics of Crassostrea sikamea (Amemiya, 1928) in China’s coastal regions under climate change. Sci. Total Environ. 2024, 920, 171061. [Google Scholar] [CrossRef]
- Rocha-Méndez, A.; Prieto-Torres, D.A.; Sánchez-González, L.A.; Navarro-Sigüenza, A.G. Climatic niche shifts and ecological sky-island dynamics in Mesoamerican montane birds. Ecol. Evol. 2024, 14, e70236. [Google Scholar] [CrossRef]
- Tomiolo, S.; Ward, D. Species migrations and range shifts: A synthesis of causes and consequences. Perspect. Plant Ecol. Evol. Syst. 2018, 33, 62–77. [Google Scholar] [CrossRef]
- Hannah, L.; Midgley, G.F.; Millar, D. Climate change-integrated conservation strategies. Glob. Ecol. Biogeogr. 2002, 11, 485–495. [Google Scholar] [CrossRef]
- Pollock, L.J.; O’Connor, L.M.J.; Mokany, K.; Rosauer, D.F.; Talluto, M.V.; Thuiller, W. Protecting Biodiversity (in All Its Complexity): New Models and Methods. Trends Ecol. Evol. 2020, 35, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Bronk Ramsey, C. Bayesian Analysis of Radiocarbon Dates. Radiocarbon 2009, 51, 337–360. [Google Scholar] [CrossRef]
- Cheng, H.; Edwards, R.L.; Hoff, J.; Gallup, C.D.; Richards, D.A.; Asmerom, Y. The half-lives of uranium-234 and thorium-230. Chem. Geol. 2000, 169, 17–33. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species distribution models: Ecological explanation and prediction across space and time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Ishihama, F.; Takenaka, A.; Yokomizo, H.; Kadoya, T. Evaluation of the ecological niche model approach in spatial conservation prioritization. PLoS ONE 2019, 14, e0226971. [Google Scholar] [CrossRef]
- Cooper, D.M.; Dugmore, A.J.; Gittings, B.M.; Scharf, A.K.; Wilting, A.; Kitchener, A.C. Predicted Pleistocene–Holocene range shifts of the tiger (Panthera tigris). Divers. Distrib. 2016, 22, 1199–1211. [Google Scholar] [CrossRef]
- Leonardi, M.; Boschin, F.; Boscato, P.; Manica, A. Following the niche: The differential impact of the last glacial maximum on four European ungulates. Commun. Biol. 2022, 5, 1038. [Google Scholar] [CrossRef]
- Xu, D.; Zhuo, Z.; Wang, R.; Ye, M.; Pu, B. Modelling the distribution of Zanthoxylum armatum in China with MaxEnt modelling. Glob. Ecol. Conserv. 2019, 19, e00691. [Google Scholar] [CrossRef]
- Shrestha, U.B.; Sharma, K.P.; Devkota, A.; Siwakoti, M.; Shrestha, B.B. Potential impact of climate change on the distribution of six invasive alien plants in Nepal. Ecol. Indictors 2018, 95, 99–107. [Google Scholar] [CrossRef]
- Hof, C.; Levinsky, I.; AraÚJo, M.B.; Rahbek, C. Rethinking species’ ability to cope with rapid climate change. Glob. Change Biol. 2011, 17, 2987–2990. [Google Scholar] [CrossRef]
- Ferreiro, A.M.; Soibelzon, E.; Pinotti, J.D.; Poljak, S.; Chiappero, M.B. Reconstructing the distribution of Chacoan biota from current and past evidence: The case of the southern three-banded armadillo Tolypeutes matacus (Desmarest, 1804). J. Mamm. Evol. 2022, 29, 783–795. [Google Scholar] [CrossRef]
- Tang, C.Q.; Dong, Y.F.; Herrando-Moraira, S.; Matsui, T.; Ohashi, H.; He, L.Y.; Nakao, K.; Tanaka, N.; Tomita, M.; Li, X.S.; et al. Potential effects of climate change on geographic distribution of the Tertiary relict tree species Davidia involucrata in China. Sci. Rep. 2017, 7, 43822. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zheng, P.; Dong, S.; Zhan, X.; Wu, Q.; Guo, X.; Hu, Y.; He, W.; Zhang, S.; Fan, W.; et al. Whole-genome sequencing of giant pandas provides insights into demographic history and local adaptation. Nat. Genet. 2013, 45, 67–71. [Google Scholar] [CrossRef]
- Dutton, A.; Carlson, A.E.; Long, A.J.; Milne, G.A.; Clark, P.U.; DeConto, R.; Horton, B.P.; Rahmstorf, S.; Raymo, M.E. Sea-level rise due to polar ice-sheet mass loss during past warm periods. Science 2015, 349, aaa4019. [Google Scholar] [CrossRef]
- Clark Peter, U.; Dyke Arthur, S.; Shakun Jeremy, D.; Carlson Anders, E.; Clark, J.; Wohlfarth, B.; Mitrovica Jerry, X.; Hostetler Steven, W.; McCabe, A.M. The Last Glacial Maximum. Science 2009, 325, 710–714. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Lambeck, K.; Deckker, P.D.; Johnston, P.; Fifield, L.K. Timing of the Last Glacial Maximum from observed sea-level minima. Nature 2000, 406, 713–716. [Google Scholar] [CrossRef]
- Liu, Z.; Harrison, S.P.; Kutzbach, J.; Otto-Bliesner, B. Global monsoons in the mid-Holocene and oceanic feedback. Clim. Dyn. 2004, 22, 157–182. [Google Scholar] [CrossRef]
- Shi, Y.; Kong, Z.; Wang, S.; Tang, L.; Wang, F.; Yao, T.; Zhao, X.; Zhang, P.; Shi, S. Mid-Holocene climates and environments in China. Glob. Planet. Change 1993, 7, 219–233. [Google Scholar] [CrossRef]
- Steig Eric, J. Mid-Holocene Climate Change. Science 1999, 286, 1485–1487. [Google Scholar] [CrossRef]
- Kang, D.; Li, J. Giant panda protection: Challenges and hopes. Environ. Sci. Pollut. Res. 2019, 26, 18001–18002. [Google Scholar] [CrossRef]
- Pan, W.; Lv, Z.; Zhu, X.; Wang, D.; Wang, H.; Long, Y.; Fu, D.; Zhou, X. A Chance for Lasting Survival: Ecology and Behavior of Wild Giant Pandas; Smithsonian Institution Scholarly Press: Washington, DC, USA, 2014. [Google Scholar]
- Zhang, J.; Pimm, S.L.; Xu, W.; Shi, X.; Xiao, Y.; Kong, L.; Fan, X.; Ouyang, Z. Relationship between giant panda populations and selected ecosystem services. Ecosyst. Serv. 2020, 44, 101130. [Google Scholar] [CrossRef]
- Hu, J. Research on the Giant Panda; Shanghai Science and Technology Education Press: Shanghai, China, 2001; pp. 64–84. [Google Scholar]
- Jin, C.; Ciochon, R.L.; Dong, W.; Hunt, R.M.; Liu, J.; Jaeger, M.; Zhu, Q. The first skull of the earliest giant panda. Proc. Natl. Acad. Sci. USA 2007, 104, 10932–10937. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.-K. On the taxonomic status of species, geological distribution and evolutionary history of Ailuropoda. Acta Zool. Sin. 1974, 20, 197–2012. [Google Scholar]
- Wu, B. New Exploration for the Development of Giant Pandas in China. J. Sichuan Teach. Coll. (Nat. Sci.) 2002, 1–10. [Google Scholar]
- Zhao, X. Giant Pandas: Natural Heritage of the Humanity; China Forestry Publishing House: Beijing, China, 2006; pp. 42–43. [Google Scholar]
- Pan, W. The Opportunity for the Giant Panda to Exist; Peking University Press: Beijing, China, 2001. [Google Scholar]
- Fan, J.; Li, J.; Xia, R.; Hu, L.; Wu, X.; Li, G. Assessing the impact of climate change on the habitat distribution of the giant panda in the Qinling Mountains of China. Ecol. Model. 2014, 274, 12–20. [Google Scholar] [CrossRef]
- Li, R.; Xu, M.; Wong, M.H.G.; Qiu, S.; Li, X.; Ehrenfeld, D.; Li, D. Climate change threatens giant panda protection in the 21st century. Biol. Conserv. 2015, 182, 93–101. [Google Scholar] [CrossRef]
- Songer, M.; Delion, M.; Biggs, A.; Huang, Q. Modelling Impacts of Climate Change on Giant Panda Habitat. Int. J. Ecol. 2012, 2012, 108752. [Google Scholar] [CrossRef]
- Han, H.; Wei, W.; Hu, Y.; Nie, Y.; Ji, X.; Yan, L.; Zhang, Z.; Shi, X.; Zhu, L.; Luo, Y.; et al. Diet Evolution and Habitat Contraction of Giant Pandas via Stable Isotope Analysis. Curr. Biol. 2019, 29, 664–669.e2. [Google Scholar] [CrossRef]
- State Forestry Administration of China. The Third National Survey Report on Giant Panda in China; Science Press: Beijing, China, 2006. [Google Scholar]
- Li, T.; Lai, X.; Wang, W.; Zhou, X. Taxonomy and evolution of giant panda. Geol. Sci. Technol. Inf. 2004, 40–46. [Google Scholar]
- Min-Shan Ko, A.; Zhang, Y.; Yang, M.A.; Hu, Y.; Cao, P.; Feng, X.; Zhang, L.; Wei, F.; Fu, Q. Mitochondrial genome of a 22,000-year-old giant panda from southern China reveals a new panda lineage. Curr. Biol. 2018, 28, R693–R694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yuan, S.-B.; Zhang, Z.-J. A Preliminary Study on Geohistoric Distributions of Giant Pandas (Ailuropoda melanoleuca). J. China West Norm. Univ. (Nat. Sci.) 2013, 34, 323–330. [Google Scholar]
- Pol, D.; Norell, M.A. Uncertainty in the Age of Fossils and the Stratigraphic Fit to Phylogenies. Syst. Biol. 2006, 55, 512–521. [Google Scholar] [CrossRef]
- Gent, P.R.; Danabasoglu, G.; Donner, L.J.; Holland, M.M.; Hunke, E.C.; Jayne, S.R.; Lawrence, D.M.; Neale, R.B.; Rasch, P.J.; Vertenstein, M.; et al. The Community Climate System Model Version 4. J. Clim. 2011, 24, 4973–4991. [Google Scholar] [CrossRef]
- Jungclaus, J.H.; Fischer, N.; Haak, H.; Lohmann, K.; Marotzke, J.; Matei, D.; Mikolajewicz, U.; Notz, D.; von Storch, J.S. Characteristics of the ocean simulations in the Max Planck Institute Ocean Model (MPIOM) the ocean component of the MPI-Earth system model. J. Adv. Model. Earth Syst. 2013, 5, 422–446. [Google Scholar] [CrossRef]
- Watanabe, S.; Hajima, T.; Sudo, K.; Nagashima, T.; Takemura, T.; Okajima, H.; Nozawa, T.; Kawase, H.; Abe, M.; Yokohata, T.; et al. MIROC-ESM: Model description and basic results of CMIP5-20c3m experiments. Geosci. Model Dev. Discuss. 2011, 4, 1063–1128. [Google Scholar] [CrossRef]
- Otto-Bliesner, B.L.; Marshall, S.J.; Overpeck, J.T.; Miller, G.H.; Hu, A.; null, n. Simulating Arctic Climate Warmth and Icefield Retreat in the Last Interglaciation. Science 2006, 311, 1751–1753. [Google Scholar] [CrossRef]
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Mansfield, E.R.; Helms, B.P. Detecting Multicollinearity. Am. Stat. 1982, 36, 158–160. [Google Scholar] [CrossRef]
- Marquaridt, D.W. Generalized Inverses, Ridge Regression, Biased Linear Estimation, and Nonlinear Estimation. Technometrics 1970, 12, 591–612. [Google Scholar] [CrossRef]
- Barbosa, A.M. FuzzySim: Applying fuzzy logic to binary similarity indices in ecology. Methods Ecol. Evol. 2015, 6, 853–858. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2020. [Google Scholar]
- Phillips, S.J.; Anderson, R.P.; Schapire, R.E. Maximum entropy modelling of species geographic distributions. Ecol. Model. 2006, 190, 231–259. [Google Scholar] [CrossRef]
- Støa, B.; Halvorsen, R.; Stokland, J.N.; Gusarov, V.I. How much is enough? Influence of number of presence observations on the performance of species distribution models. Sommerfeltia 2019, 39, 1–28. [Google Scholar] [CrossRef]
- Wisz, M.S.; Hijmans, R.J.; Li, J.; Peterson, A.T.; Graham, C.H.; Guisan, A. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008, 14, 763–773. [Google Scholar] [CrossRef]
- Zhao, X.-J.; Gong, J.-X.; Zhao, S.-S.; Meng, H.-X.; Yan, B.-Q.; Qi, X.-Y. Impact of sample size and spatial distribution on species distribution model. J. Lanzhou Univ. Nat. Sci. 2018, 54, 208–215. [Google Scholar] [CrossRef]
- Phillips, S.J. A Brief Tutorial on Maxent. Available online: http://biodiversityinformatics.amnh.org/open_source/maxent/ (accessed on 9 June 2021).
- Tesfamariam, B.G.; Gessesse, B.; Melgani, F. MaxEnt-based modelling of suitable habitat for rehabilitation of Podocarpus forest at landscape-scale. Environ. Syst. Res. 2022, 11, 4. [Google Scholar] [CrossRef]
- Muscarella, R.; Galante, P.J.; Soley-Guardia, M.; Boria, R.A.; Kass, J.M.; Uriarte, M.; Anderson, R.P. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods Ecol. Evol. 2014, 5, 1198–1205. [Google Scholar] [CrossRef]
- Merow, C.; Smith, M.J.; Silander, J.A., Jr. A practical guide to MaxEnt for modelling species’ distributions: What it does, and why inputs and settings matter. Ecography 2013, 36, 1058–1069. [Google Scholar] [CrossRef]
- Morales, N.S.; Fernández, I.C.; Baca-González, V. MaxEnt’s parameter configuration and small samples: Are we paying attention to recommendations? A systematic review. PeerJ 2017, 5, e3093. [Google Scholar] [CrossRef] [PubMed]
- Shcheglovitova, M.; Anderson, R.P. Estimating optimal complexity for ecological niche models: A jackknife approach for species with small sample sizes. Ecol. Model. 2013, 269, 9–17. [Google Scholar] [CrossRef]
- Syfert, M.M.; Smith, M.J.; Coomes, D.A. The Effects of Sampling Bias and Model Complexity on the Predictive Performance of MaxEnt Species Distribution Models. PLoS ONE 2013, 8, e55158. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef]
- Peterson, A.T.; Soberón, J.; Pearson, R.G.; Anderson, R.P.; Martínez-Meyer, E.; Nakamura, M.; Araújo, M.B. Ecological Niches and Geographic Distributions; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Warren, D.L.; Seifert, S.N. Ecological niche modelling in Maxent: The importance of model complexity and the performance of model selection criteria. Ecol. Appl. 2011, 21, 335–342. [Google Scholar] [CrossRef]
- Liu, C.; Berry, P.M.; Dawson, T.P.; Pearson, R.G. Selecting thresholds of occurrence in the prediction of species distributions. Ecography 2005, 28, 385–393. [Google Scholar] [CrossRef]
- Liu, C.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Brown, J.L.; Anderson, B. SDMtoolbox: A python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol. Evol. 2014, 5, 694–700. [Google Scholar] [CrossRef]
- Di Cola, V.; Broennimann, O.; Petitpierre, B.; Breiner, F.T.; D’Amen, M.; Randin, C.; Engler, R.; Pottier, J.; Pio, D.; Dubuis, A.; et al. Ecospat: An R package to support spatial analyses and modelling of species niches and distributions. Ecography 2017, 40, 774–787. [Google Scholar] [CrossRef]
- Broennimann, O.; Fitzpatrick, M.C.; Pearman, P.B.; Petitpierre, B.; Pellissier, L.; Yoccoz, N.G.; Thuiller, W.; Fortin, M.-J.; Randin, C.; Zimmermann, N.E.; et al. Measuring ecological niche overlap from occurrence and spatial environmental data. Glob. Ecol. Biogeogr. 2012, 21, 481–497. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. Environmental niche equivalency versus conservatism: Quantitative approaches to niche evolution. Evolution 2008, 62, 2868–2883. [Google Scholar] [CrossRef] [PubMed]
- Luna-Arangure, C.; Vazquez-Dominguez, E. Of pandas, fossils, and bamboo forests: Ecological niche modelling of the giant panda (Ailuropoda melanoleuca) during the Last Glacial Maximum. J. Mammal. 2021, 102, 718–730. [Google Scholar] [CrossRef]
- Pineda-Munoz, S.; Wang, Y.; Lyons, S.K.; Tóth, A.B.; McGuire, J.L. Mammal species occupy different climates following the expansion of human impacts. Proc. Natl. Acad. Sci. USA 2021, 118, e1922859118. [Google Scholar] [CrossRef]
- Davis, M.B.; Shaw, R.G. Range Shifts and Adaptive Responses to Quaternary Climate Change. Science 2001, 292, 673–679. [Google Scholar] [CrossRef]
- Shen, J.; Fan, S.; Xu, Q.; Liu, G. Research Progress on Factors Influencing Bamboo Growth. World Bamboo Ratt. 2024, 22, 96–102. [Google Scholar]
- Zhou, C.; Moon, J.R.; Peacock, S. Rotary Forging of Sintered Iron Based Composites. Powder Metall. 1991, 34, 33–38. [Google Scholar] [CrossRef]
- Li, Y.; Rao, T.; Gai, L.; Price, M.L.; Yuxin, L.; Jianghong, R. Giant pandas are losing their edge: Population trend and distribution dynamic drivers of the giant panda. Glob. Change Biol. 2023, 29, 4480–4495. [Google Scholar] [CrossRef]
- Gong, M.; Guan, T.; Hou, M.; Liu, G.; Zhou, T. Hopes and challenges for giant panda conservation under climate change in the Qinling Mountains of China. Ecol. Evol. 2017, 7, 596–605. [Google Scholar] [CrossRef]
- Li, X.; Mao, F.; Du, H.; Zhou, G.; Xing, L.; Liu, T.; Han, N.; Liu, Y.; Zhu, D.; Zheng, J.; et al. Spatiotemporal evolution and impacts of climate change on bamboo distribution in China. J. Environ. Manag. 2019, 248, 109265. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, X.; Wei, W.; Hong, M.; Zhou, H.; Tang, J.; Zhang, Z. Predicting range shifts of the giant pandas under future climate and land use scenarios. Ecol. Evol. 2022, 12, e9298. [Google Scholar] [CrossRef] [PubMed]
- Coulleri, J.P.; Simelane, D.O.; Mawela, K.; Ferrucci, M.S. Climatic Niche Dynamics of Three Widespread Cardiospermum (Paullinieae, Sapindaceae) Species Revealed Possible Dispersal Pathways. Syst. Bot. 2020, 45, 879–890. [Google Scholar] [CrossRef]
- Liu, H.; Ye, Q.; Wiens, J.J. Climatic-niche evolution follows similar rules in plants and animals. Nat. Ecol. Evol. 2020, 4, 753–763. [Google Scholar] [CrossRef]
- Cunze, S.; Kochmann, J.; Koch, L.K.; Klimpel, S. Niche conservatism of Aedes albopictus and Aedes aegypti—Two mosquito species with different invasion histories. Sci. Rep. 2018, 8, 7733. [Google Scholar] [CrossRef]
- Yan, H.; Feng, L.; Zhao, Y.; Feng, L.; Wu, D.; Zhu, C. Prediction of the spatial distribution of Alternanthera philoxeroides in China based on ArcGIS and MaxEnt. Glob. Ecol. Conserv. 2020, 21, e00856. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modelling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total. Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef]


| Code | Description | Unit |
|---|---|---|
| Bio1 | Annual mean temperature | °C |
| Bio2 * | Mean diurnal temperature range | °C |
| Bio3 * | Isothermality | − |
| Bio4 | Temperature seasonality | − |
| Bio5 | Max temperature of warmest month | °C |
| Bio6 | Min temperature of coldest month | °C |
| Bio7 | Temperature annual range | °C |
| Bio8 * | Mean temperature of wettest quarter | °C |
| Bio9 * | Mean temperature of driest quarter | °C |
| Bio10 | Mean temperature of warmest quarter | °C |
| Bio11 | Mean temperature of coldest quarter | °C |
| Bio12 | Annual precipitation | mm |
| Bio13 | Precipitation of wettest month | mm |
| Bio14 | Precipitation of driest month | mm |
| Bio15 * | Precipitation seasonality | − |
| Bio16 | Precipitation of wettest quarter | mm |
| Bio17 | Precipitation of driest quarter | mm |
| Bio18 * | Precipitation of warmest quarter | mm |
| Bio19 * | Precipitation of coldest quarter | mm |
| Target Period | Model | FC | RM | AUCTEST | AUCDIFF | OR10 | ΔAICc | MaxSSS |
|---|---|---|---|---|---|---|---|---|
| LIG | Default | LQHPT | 1 | 0.928 | 0.026 | 0.330 | 27.107 | − |
| Optimized | LQHP | 3.5 | 0.917 | 0.010 | 0.077 | 0 | 0.4758 | |
| LGM | Default | LQHPT | 1 | 0.957 | 0.018 | 0.205 | 82.820 | − |
| Optimized | LQH | 2 | 0.961 | 0.017 | 0.170 | 0 | 0.5194 | |
| MH | Default | LQHPT | 1 | 0.893 | 0.062 | 0.4 | NA | − |
| Optimized | LQH | 4 | 0.890 | 0.044 | 0.3 | 0 | 0.5240 | |
| Current | Default | LQHPT | 1 | 0.998 | 0.001 | 0.122 | 74.249 | − |
| Optimized | LQH | 0.5 | 0.998 | 0.001 | 0.129 | 0 | 0.1249 |
| Variables | Description | LIG (%) | LGM (%) | MH (%) | Current (%) |
|---|---|---|---|---|---|
| Bio2 | Mean diurnal temperature range | 89.8 | 22.6 | 1.7 | 0.9 |
| Bio3 | Isothermality | 1.3 | 6.6 | 0 | 6.6 |
| Bio8 | Mean temperature of wettest quarter | 8.7 | 2.5 | 0 | 24.1 |
| Bio9 | Mean temperature of driest quarter | 4 | 21.3 | 58.1 | 27.5 |
| Bio15 | Precipitation seasonality | 0 | 1 | 0 | 9.2 |
| Bio18 | Precipitation of warmest quarter | 0 | 20 | 40 | 22.8 |
| Bio19 | Precipitation of coldest quarter | 4.8 | 26 | 0.2 | 8.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Liu, X.; Yang, A.; Hao, Z.; Li, X.; Li, D.; Yu, X.; Ye, X. Evaluating Past Range Shifts and Niche Dynamics of Giant Pandas Since the Last Interglacial. Animals 2025, 15, 801. https://doi.org/10.3390/ani15060801
Xu Y, Liu X, Yang A, Hao Z, Li X, Li D, Yu X, Ye X. Evaluating Past Range Shifts and Niche Dynamics of Giant Pandas Since the Last Interglacial. Animals. 2025; 15(6):801. https://doi.org/10.3390/ani15060801
Chicago/Turabian StyleXu, Yadong, Xiaoan Liu, Aimei Yang, Ziyi Hao, Xuening Li, Dan Li, Xiaoping Yu, and Xinping Ye. 2025. "Evaluating Past Range Shifts and Niche Dynamics of Giant Pandas Since the Last Interglacial" Animals 15, no. 6: 801. https://doi.org/10.3390/ani15060801
APA StyleXu, Y., Liu, X., Yang, A., Hao, Z., Li, X., Li, D., Yu, X., & Ye, X. (2025). Evaluating Past Range Shifts and Niche Dynamics of Giant Pandas Since the Last Interglacial. Animals, 15(6), 801. https://doi.org/10.3390/ani15060801






