Advances in Timed Artificial Insemination: Integrating Omics Technologies for Enhanced Reproductive Efficiency in Dairy Cattle
Simple Summary
Abstract
1. Introduction
2. Estrous Synchronization Protocols of TAI in Dairy Cattle
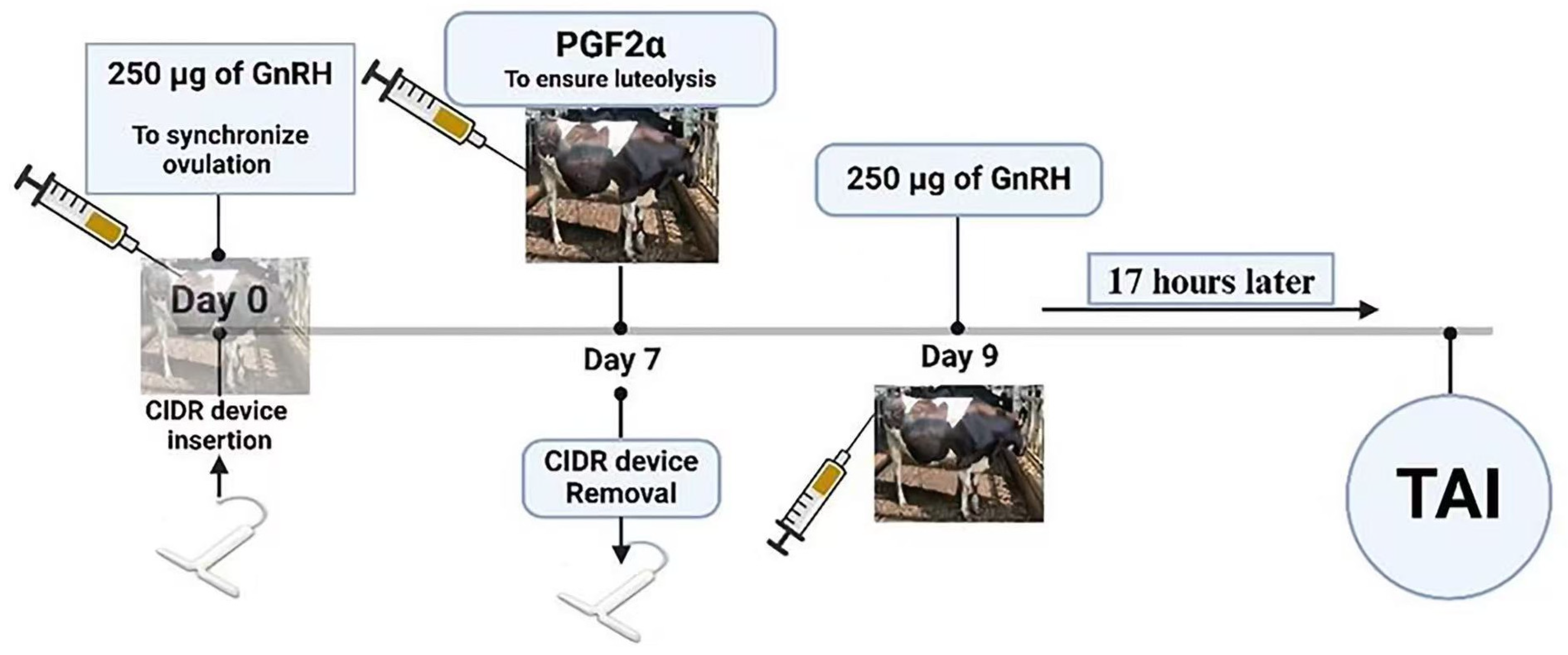
2.1. Gonadotropin-Releasing Hormone (GnRH)-Based Protocols
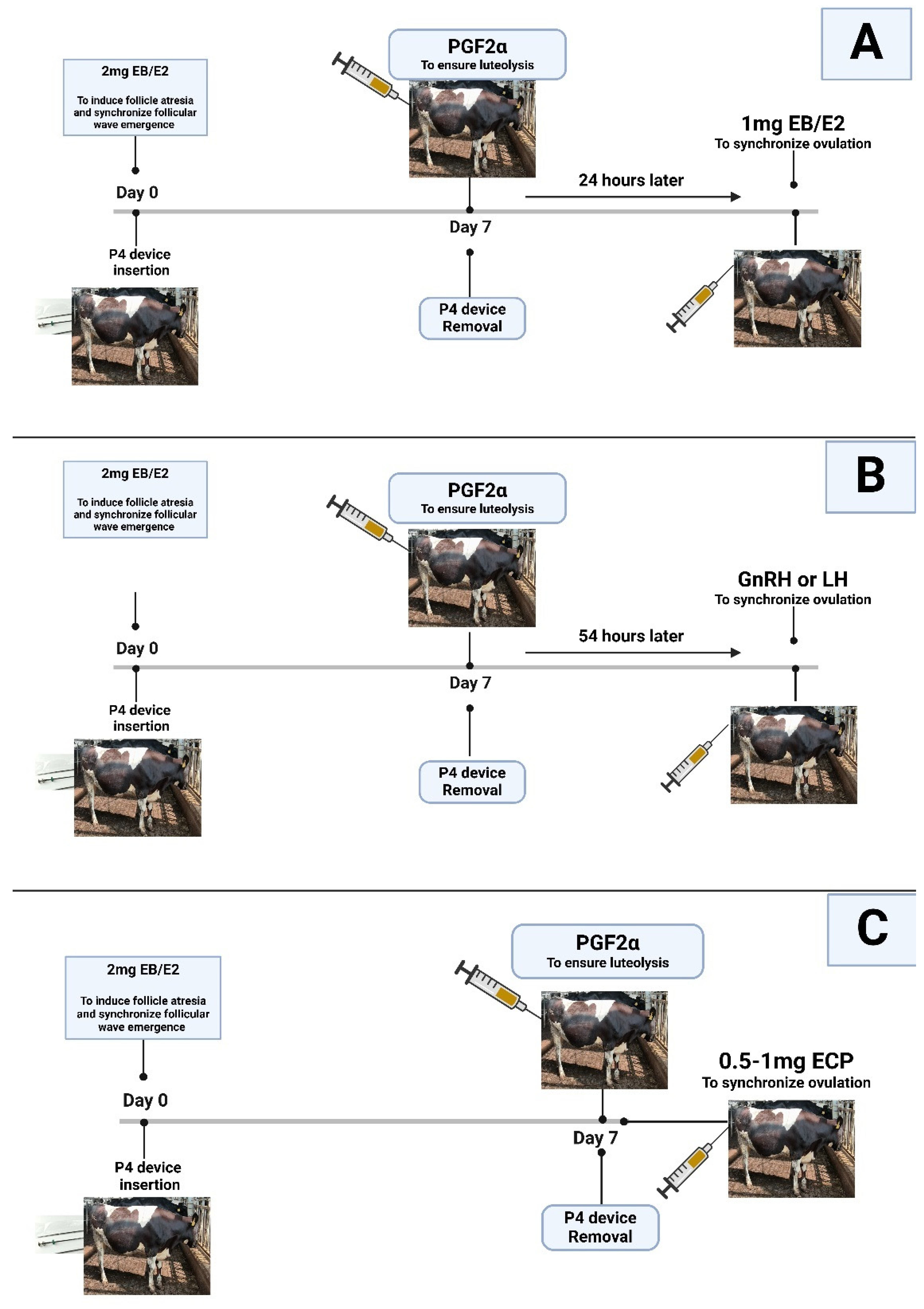
2.2. Estradiol/Progesterone (P4)-Based Protocols
2.3. Comparison of Protocols and Practical Considerations
| Protocol | P/TAI (%) | Reference |
|---|---|---|
| GnRH injection and CIDR Insertion Day 0 (Morning) + PGF2α injection, CIDR removal Day 5 (Morning) + Estrous detection Day 6 and 7 + GnRH injection and TAI Day 8 | 55.3 | [79] |
| Double-Ovsynch (Pre-Ovsynch followed by Ovsynch-56 protocol) | 56.3 | [80] |
| PGF2α injection + GnRH injection 3–4 days later + GnRH injection and CIDR insertion 7 days later + PGF2α, eCG injection and CIDR removal 7 days later + GnRH injection 56 h later + TAI 16 h later | 57.5 | [81] |
| GnRH injection and CIDR insertion Day 0 + PGF2α injection, CIDR removal Day 5 (AM) + PGF2α injection 6 h later + GnRH injection Day 7 (PM) + TAI 56 h after CIDR removal | 61.7 | [22] |
| GnRH and CIDR Day 0 + PGF2α, CIDR removal Day 5 (AM) + PGF2α injection 6 h later + GnRH injection Day 7 (PM) + TAI 56 h after CIDR removal | 63.6 | [82] |
| Double-Ovsynch = Calving date as Day 0, GnRH injection Day 36 + PGF2α injection Day 46 + GnRH injection Day 49 followed by Ovsynch-GnRH injection Day 56 + PGF2α injection Day 63 + GnRH injection 56 h later + TAI 16 h later | 69.5 | [83] |
| EB injection + PGF2α injection Day 7 + GnRH injection Day 9 (EPG) during winter, Pregnancy Diagnosis on Day 60 | 68.75 | [21] |
| PGF2α injection (Dinoprost) + EB injection 24 h later + TAI 24 h after EB | 64.5 | [73] |
| EB injection + GnRH injection Day 0 + PGF2α injection Day 7 + PGF2α injection Day 9 | 57.2 | [68] |
| EB injection and P4 Device insertion Day 0 + GnRH injection Day 2 + PGF2α injection Day 7 + PGF2α, ECP injection and P4 Removal Day 9 + TAI Day11 | 40.5 | [72] |
| EB, GnRH injection and P4 insertion Day 0 + PGF2α injection, P4 removal Day 7 + PGF2α, eCG and ECP injection Day 8 + Estrus and AI Day 10 + Pregnancy Diagnosis Day 30 | 57.4 | [66] |
| EB, GnRH injection and P4 insertion Day 10 + PGF2α injection and US Day 3 + ECP, PGF2α injection, US, and P4 removal Day 2 + FTAI and US Day 0 + Pregnancy Diagnosis Day 32 | 60.9 | [84] |
3. Omics Integration in TAI
3.1. Genomics
3.2. Transcriptomics and Proteomics
3.3. Metabolomics
4. Challenges of Implementing Omics Technologies in TAI in Dairy Cattle
- Establishing consistent protocols for data collection, analysis, and interpretation to improve the reproducibility of findings.
- Conducting large-scale studies to validate the reliability of identified biomarkers for predicting fertility and pregnancy outcomes.
- Translating omics insights into actionable strategies that farmers can easily adopt, accounting for the variability in herd management systems.
- Designing synchronization strategies tailored to individual cows’ genetic and physiological profiles to maximize TAI success.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Terefe, E.; Belay, G.; Admasu, E. Reproductive Performance of Crossbred Dairy Cattle under Semi-Intensive Management System in Arsi Highland, Ethiopia. AJSI Arsi J. Sci. Innov. 2021, 6, 1–19. [Google Scholar]
- Yusuf, M.; Sahiruddin. Reproductive Performance of Dairy Cows in a Smallholder Farm. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2020; Volume 492. [Google Scholar] [CrossRef]
- Chebel, R.C.; Mirzaei, A.; Peixoto, P.M.G.; Factor, L.; Montevecchio, A.B.; Bisinotto, R.S.; De Vries, A.; Galvão, K.N.; Bilby, T.R.; Jones, K. Targeted Reproductive Management for Lactating Holstein Cows: Reproductive and Economic Outcomes of Double-Ovsynch Compared with a Targeted Approach Based on Resumption of Estrus. J. Dairy Sci. 2025, in press. [Google Scholar] [CrossRef]
- Sitko, E.M.; Di Croce, F.A.; McNeel, A.K.; Weigel, D.J.; Giordano, J.O. Effect of Reproductive Management Programs That Prioritized Artificial Insemination at Detected Estrus or Timed Artificial Insemination on the Economic Performance of Primiparous Holstein Cows of Different Genetic Merit for Fertility. J. Dairy Sci. 2023, 106, 6495–6514. [Google Scholar] [CrossRef] [PubMed]
- Wicaksono, A.; Edwardes, F.; Steeneveld, W.; van den Borne, B.H.P.; Pinho, P.; Randi, F.; Hogeveen, H. The Economic Effect of Cow-Based Reproductive Management Programs with a Systematic Use of Reproductive Hormones. J. Dairy Sci. 2024, 107, 11016–11035. [Google Scholar] [CrossRef]
- Gonzalez, T.D.; Factor, L.; Mirzaei, A.; Montevecchio, A.B.; Casaro, S.; Merenda, V.R.; Prim, J.G.; Galvão, K.N.; Bisinotto, R.S.; Chebel, R.C. Targeted Reproductive Management for Lactating Holstein Cows: Reducing the Reliance on Exogenous Reproductive Hormones. J. Dairy Sci. 2023, 106, 5788–5804. [Google Scholar] [CrossRef]
- Luz, G.B.; Maffi, A.S.; Farias, L.B.; Xavier, E.G.; Lima, M.E.; Corrêa, M.N.; Brauner, C.C. Effects of the Bull on Conception Rate of Dairy Cows in Different Seasons and According to AI Type. Acta Sci. Vet. 2018, 46, 6. [Google Scholar] [CrossRef]
- Dirandeh, E.; Roodbari, A.R.; Colazo, M.G. Double-Ovsynch, Compared with Presynch with or without GnRH, Improves Fertility in Heat-Stressed Lactating Dairy Cows. Theriogenology 2015, 83, 438–443. [Google Scholar] [CrossRef]
- Stangaferro, M.L.; Wijma, R.; Masello, M.; Giordano, J.O. Reproductive Performance and Herd Exit Dynamics of Lactating Dairy Cows Managed for First Service with the Presynch-Ovsynch or Double-Ovsynch Protocol and Different Duration of the Voluntary Waiting Period. J. Dairy Sci. 2018, 101, 1673–1686. [Google Scholar] [CrossRef]
- Brozos, C.; Kiossis, E.; Hatzieffraimidis, S.; Praxitelous, A.; Gouvias, I.; Kanoulas, V.; Tsousis, G. Comparison of 5 versus 7-Day Ovsynch + Progesterone Releasing Intravaginal Device Protocols (PRID) and a Modified G7G with an Option of Heat Detection Protocol for 1st Service in Lactating Dairy Cows. Animals 2021, 11, 2955. [Google Scholar] [CrossRef]
- Lynch, C.; Oliveira Junior, G.A.; Schenkel, F.S.; Baes, C.F. Effect of Synchronized Breeding on Genetic Evaluations of Fertility Traits in Dairy Cattle. J. Dairy Sci. 2021, 104, 11820–11831. [Google Scholar] [CrossRef]
- Marques, M.d.O.; Morotti, F.; Lorenzetti, E.; Bizarro-Silva, C.; Seneda, M.M. Intensified Use of TAI and Sexed Semen on Commercial Farms. Anim. Reprod. 2018, 15, 197–203. [Google Scholar] [CrossRef]
- Rial, C.; Laplacette, A.; Giordano, J.O. Effect of a Targeted Reproductive Management Program Designed to Prioritize Insemination at Detected Estrus and Optimize Time to Insemination on the Reproductive Performance of Lactating Dairy Cows. J. Dairy Sci. 2022, 105, 8411–8425. [Google Scholar] [CrossRef]
- Brito, L.F.; Bedere, N.; Douhard, F.; Oliveira, H.R.; Arnal, M.; Peñagaricano, F.; Schinckel, A.P.; Baes, C.F.; Miglior, F. Review: Genetic Selection of High-Yielding Dairy Cattle toward Sustainable Farming Systems in a Rapidly Changing World. Animal 2021, 15, 100292. [Google Scholar] [CrossRef]
- Walsh, D.P.; Fahey, A.G.; Lonergan, P.; Wallace, M. Economics of Timed Artificial Insemination with Unsorted or Sexed Semen in a High-Producing, Pasture-Based Dairy Production System. J. Dairy Sci. 2022, 105, 3192–3208. [Google Scholar] [CrossRef]
- Lima, F.S.; Silvestre, F.T.; Peñagaricano, F.; Thatcher, W.W. Early Genomic Prediction of Daughter Pregnancy Rate Is Associated with Improved Reproductive Performance in Holstein Dairy Cows. J. Dairy Sci. 2020, 103, 3312–3324. [Google Scholar] [CrossRef]
- Madureira, A.M.L.; Plenio, J.L.; Vasconcelos, J.L.M.; Guida, T.G.; Cerri, R.L.A.; Borchardt, S. Association between Genomic Daughter Pregnancy Rate and Expected Milk Production on the Resumption of Estrus Behavior in Holstein Cattle. J. Dairy Sci. 2024, 107, 1592–1602. [Google Scholar] [CrossRef]
- Cristian Campos, C.; Luiz do Prado, F.; Paulo Justo dos Reis, J.; Cunha Carneiro, L.; Regina Basso Silva, P.; Faria de Moraes, G.; Maria dos Santos, R. Effects of Clinical Mastitis and Puerperal Diseases on Reproductive Efficiency of Dairy Cows. Trop. Anim. Health Prod. 2020, 52, 3061–3068. [Google Scholar] [CrossRef]
- Molina-Coto, R.; Moore, S.G.; Mayo, L.M.; Lamberson, W.R.; Poock, S.E.; Lucy, M.C. Ovarian Function and the Establishment and Maintenance of Pregnancy in Dairy Cows with and without Evidence of Postpartum Uterine Disease. J. Dairy Sci. 2020, 103, 10715–10727. [Google Scholar] [CrossRef]
- Bogado Pascottini, O.; Hostens, M.; Sys, P.; Vercauteren, P.; Opsomer, G. Cytological Endometritis at Artificial Insemination in Dairy Cows: Prevalence and Effect on Pregnancy Outcome. J. Dairy Sci. 2017, 100, 588–597. [Google Scholar] [CrossRef]
- Allahyari, I.; Gharagozlou, F.; Vojgani, M.; Pooladzadeh, P.; Mobedi, E.; Akbarinejad, V. Replacement of the First GnRH by Estradiol in the Breeding Ovsynch of Double Ovsynch Protocol Could Improve Fertility in Holstein Dairy Cows. Anim. Reprod. Sci. 2023, 252, 107228. [Google Scholar] [CrossRef]
- Say, E.; Çoban, S.; Nak, Y.; Nak, D.; Kara, U.; White, S.; Kasimanickam, V.; Kasimanickam, R. Fertility of Holstein Heifers after Two Doses of PGF2α in 5-Day CO-Synch Progesterone-Based Synchronization Protocol. Theriogenology 2016, 86, 988–993. [Google Scholar] [CrossRef]
- Vazquez Belandria, R.; Denholm, K.; Pepler, P.T.; Cook, J.G.; Pinho, P.; Randi, F.; Viora, L. Comparison of Three Reproductive Management Strategies for Lactating Dairy Cows Using Combination of Estrus Detection or Ovulation Synchronization and Fixed-Timed Artificial Insemination. Anim. Reprod. Sci. 2023, 257, 107331. [Google Scholar] [CrossRef]
- Pereira, M.H.C.; Wiltbank, M.C.; Guida, T.G.; Lopes, F.R.; Vasconcelos, J.L.M. Comparison of 2 Protocols to Increase Circulating Progesterone Concentration before Timed Artificial Insemination in Lactating Dairy Cows with or without Elevated Body Temperature. J. Dairy Sci. 2017, 100, 8455–8470. [Google Scholar] [CrossRef]
- Marques, L.R.; de Almeida, J.V.N.; Oliveira, A.C.; Paim, T.D.P.; Marques, T.C.; Leão, K.M. Artificial Insemination Timing on Pregnancy Rate of Holstein Cows Using an Automated Activity Monitoring. Cienc. Rural 2024, 54, e20220557. [Google Scholar] [CrossRef]
- Laplacette, A.L.; Rial, C.; Sitko, E.; Perez, M.M.; Tompkins, S.; Stangaferro, M.L.; Thomas, M.J.; Giordano, J.O. Delaying Induction of Ovulation and Timed AI in a Double-Ovsynch Protocol Increased Expression of Estrus and Altered First Service Reproductive Outcomes of Lactating Dairy Cows. J. Dairy Sci. 2024, 108, 1103–1124. [Google Scholar] [CrossRef]
- Ataide Junior, G.A.; Kloster, A.; de Moraes, É.G.; Motta, I.G.; Claro Junior, I.; Vasconcelos, J.L.M.; Ferraz, P.A.; de Paula Nogueira, G.; Pugliesi, G. Early Resynchronization of Follicular Wave Emergence among Nelore Cattle Using Injectable and Intravaginal Progesterone for Three Timed Artificial Inseminations. Anim. Reprod. Sci. 2021, 229, 106759. [Google Scholar] [CrossRef]
- Fleming, A.; Abdalla, E.A.; Maltecca, C.; Baes, C.F. Invited Review: Reproductive and Genomic Technologies to Optimize Breeding Strategies for Genetic Progress in Dairy Cattle. Arch. Anim. Breed. 2018, 61, 43–57. [Google Scholar] [CrossRef]
- Sitko, E.M.; Laplacette, A.; Duhatschek, D.; Rial, C.; Perez, M.M.; Tompkins, S.; Kerwin, A.L.; Giordano, J.O. Reproductive Physiological Outcomes of Dairy Cows with Different Genomic Merit for Fertility: Biomarkers, Uterine Health, Endocrine Status, Estrus Features, and Response to Ovarian Synchronization. J. Dairy Sci. 2024, 107, 8670–8687. [Google Scholar] [CrossRef]
- Kafi, M.; Ghaemi, M.; Azari, M.; Mirzaei, A.; Azarkaman, S.; Torfi, Y. Effects of Pre-Ovulatory Follicular Fluid of Repeat Breeder Dairy Cows on Bovine Fertility Transcriptomic Markers and Oocytes Maturation and Fertilization Capacity. Front. Vet. Sci. 2021, 8, 670121. [Google Scholar] [CrossRef]
- Kibre, D.; Ashebir, G.; Gebrekidan, B.; Fesseha, H. Study on Factors Affecting Estrus Synchronization in Smallholder Dairy Farming Systems of Tigray, Northern Ethiopia. Vet. Med. Int. 2022, 2022, 2435696. [Google Scholar] [CrossRef]
- Xu, Z.Z. Control of Estrus Cycles: Synchronization of Estrus. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Masho, W.; Foato, A.; Begna, R. Breeding Practices and Evaluation of Hormonal Estrus Synchronization and Mass Artificial Insemination of Dairy Cattle in Southwest Ethiopia. J. Agric. Food Res. 2024, 16, 101167. [Google Scholar] [CrossRef]
- Haile, S.M.; Abebe, B.K.; Tesfa, T.W. Efficiency Evaluation of Two Estrus Synchronization Protocols in Estrus Response and Conception Rate of Dairy Cows in the Dalocha District, Ethiopia. Heliyon 2023, 9, e12781. [Google Scholar] [CrossRef]
- Munthe-Kaas, M.; Sveberg, G.; Holmøy, I.H.; Kommisrud, E.; Haadem, C.S.; Martin, A.D. Pilot Study Investigating Estrus Length and Estrus Behavior in Norwegian Red Cattle on a Commercial Dairy Farm. Front. Vet. Sci. 2023, 10, 1219001. [Google Scholar] [CrossRef]
- Hassanein, E.M.; Szelényi, Z.; Szenci, O. Gonadotropin-Releasing Hormone (GnRH) and Its Agonists in Bovine Reproduction I: Structure, Biosynthesis, Physiological Effects, and Its Role in Estrous Synchronization. Animals 2024, 14, 1473. [Google Scholar] [CrossRef]
- Wang, H.Q.; Zhang, W.D.; Yuan, B.; Zhang, J.B. Advances in the Regulation of Mammalian Follicle-Stimulating Hormone Secretion. Animals 2021, 11, 1134. [Google Scholar] [CrossRef]
- Tibary, A.; Patino, C.; Ciccarelli, M. Synchronization of Estrus and Ovulation in Dairy Cattle. Spermova 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Bihon, A.; Assefa, A. Prostaglandin Based Estrus Synchronization in Cattle: A Review. Cogent Food Agric. 2021, 7, 1932051. [Google Scholar] [CrossRef]
- López-Gatius, F. Ovarian Response to Prostaglandin F2α in Lactating Dairy Cows: A Clinical Update. J. Reprod. Dev. 2022, 68, 104–109. [Google Scholar] [CrossRef]
- Jonczyk, A.W.; Piotrowska-Tomala, K.K.; Skarzynski, D.J. Comparison of Intra-CL Injection and Peripheral Application of Prostaglandin F2α Analog on Luteal Blood Flow and Secretory Function of the Bovine Corpus Luteum. Front. Vet. Sci. 2022, 8, 811809. [Google Scholar] [CrossRef]
- Picard-Hagen, N.; Lhermie, G.; Florentin, S.; Merle, D.; Frein, P.; Gayrard, V. Effect of Gonadorelin, Lecirelin, and Buserelin on LH Surge, Ovulation, and Progesterone in Cattle. Theriogenology 2015, 84, 177–183. [Google Scholar] [CrossRef]
- Rantala, M.H.; Taponen, J. LH Secretion around Induced Ovulation during Early and Late Diestrus and Its Effect on the Appearance of Short Estrous Cycles in Cyclic Dairy Heifers. Theriogenology 2015, 83, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Bekuma, A.; Ketema, H. Estrus Synchronization in Ethiopian Dairy Cattle: Principle, Purpose and Influencing Factors. J. Anim. Husb. Sci. Technol. 2019, 1, 101. [Google Scholar]
- Hamid, M.; Abduraman, S.; Tadesse, B. Risk Factors for the Efficiency of Artificial Insemination in Dairy Cows and Economic Impact of Failure of First Service Insemination in and around Haramaya Town, Oromia Region, Eastern Ethiopia. Vet. Med. Int. 2021, 2021, 6622487. [Google Scholar] [CrossRef] [PubMed]
- Cardoso Consentini, C.E.; Wiltbank, M.C.; Sartori, R. Factors That Optimize Reproductive Efficiency in Dairy Herds with an Emphasis on Timed Artificial Insemination Programs. Animals 2021, 11, 301. [Google Scholar] [CrossRef]
- Barański, W.; Nowicki, A.; Crowe, M.A.; Tobolski, D.; Zduńczyk, S. Effect of Repeated Low Doses of Gonadotropin-Releasing Hormone on the Secretion of Luteinizing Hormone and Follicle-Stimulating Hormone, and Ovarian Function in Dairy Cows Suffering from Anovulation Type I. Anim. Reprod. Sci. 2024, 270, 107602. [Google Scholar] [CrossRef]
- Fricke, P.M.; Wiltbank, M.C. Symposium Review: The Implications of Spontaneous versus Synchronized Ovulations on the Reproductive Performance of Lactating Dairy Cows. J. Dairy Sci. 2022, 105, 4679–4689. [Google Scholar] [CrossRef]
- Udin, Z.; Hendri, H.; Masrizal, M. Increasing the Success of Artificial Insemination through Control of Local Cattle Estrus as a Genetic Resource. Int. J. Health Sci. 2022, 6, 2117–2132. [Google Scholar] [CrossRef]
- López-Gatius, F.; Garcia-Ispierto, I. Treatment with an Elevated Dose of the GnRH Analogue Dephereline in the Early Luteal Phase Improves Pregnancy Rates in Repeat-Breeder Dairy Cows. Theriogenology 2020, 155, 12–16. [Google Scholar] [CrossRef]
- Monteiro, P.L.J.; Borsato, M.; Silva, F.L.M.; Prata, A.B.; Wiltbank, M.C.; Sartori, R. Increasing Estradiol Benzoate, Pretreatment with Gonadotropin-Releasing Hormone, and Impediments for Successful Estradiol-Based Fixed-Time Artificial Insemination Protocols in Dairy Cattle. J. Dairy Sci. 2015, 98, 3826–3839. [Google Scholar] [CrossRef]
- Silva, L.O.e.; Folchini, N.P.; Alves, R.L.O.R.; Madureira, G.; Consentini, C.E.C.; Motta, J.C.L.; Wiltbank, M.C.; Sartori, R. Effect of Progesterone from Corpus Luteum, Intravaginal Implant, or Both on Luteinizing Hormone Release, Ovulatory Response, and Subsequent Luteal Development after Gonadotropin-Releasing Hormone Treatment in Cows. J. Dairy Sci. 2023, 106, 4413–4428. [Google Scholar] [CrossRef]
- Barletta, R.V.; Carvalho, P.D.; Santos, V.G.; Melo, L.F.; Consentini, C.E.; Netto, A.S.; Fricke, P.M. Effect of Dose and Timing of Prostaglandin F2α Treatments during a Resynch Protocol on Luteal Regression and Fertility to Timed Artificial Insemination in Lactating Holstein Cows. J. Dairy Sci. 2018, 101, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Burnett, T.A.; Madureira, A.M.L.; Bauer, J.W.; Cerri, R.L.A. Impact of Gonadotropin-Releasing Hormone Administration at the Time of Artificial Insemination on Conception Risk and Its Association with Estrous Expression. J. Dairy Sci. 2022, 105, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Leão, I.M.R.; El Azzi, M.S.; Anta-Galván, E.; Valdés-Arciniega, T.; Martins, J.P.N. Effect of 200 Μg of Gonadorelin at the First Gonadotropin-Releasing Hormone of the Resynch-25 on Ovarian Dynamics and Fertility in Lactating Holstein Cows. J. Dairy Sci. 2024, 107, 3319–3334. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, B.; Adamov, N.; Celeska, I.; Ilievska, K.; Angjelovski, B.; Trbogazov, Z.; Davkov, F.; Dovenski, T.; Opsomer, G.; Stevenson, J. Modification of the Standard 7-Day Ovsynch Protocol to Increase the Luteolytic and Synchronization Risks in Dairy Cows. Maced. Vet. Rev. 2020, 43, 161–167. [Google Scholar] [CrossRef]
- Luchterhand, M.; Gamarra, C.A.; Gennari, R.S.; Carvalho, P.D.; Barletta, R.V.; Souza, A.H. Ovulation and Fertility Response to Commercially Available GnRH Products in Lactating Cows Synchronized with the Double-Ovsynch Protocol. Anim. Reprod. Sci. 2019, 202, 42–48. [Google Scholar] [CrossRef]
- Nowicki, A.; Barański, W.; Baryczka, A.; Janowski, T. OvSynch Protocol and Its Modifications in the Reproduction Management of Dairy Cattle Herds -an Update. J. Vet. Res. 2017, 61, 329–336. [Google Scholar] [CrossRef]
- Câmara-de-Almeida, Í.; Albani-Oliveira, F.; Madureira, A.P.; Barioni, G.; Alves-Torres, C.A. Effect of Follicular Growth Promoters on Timed Artificial Insemination in Dairy Cows. Rev. MVZ Cordoba 2021, 26, 1–8. [Google Scholar] [CrossRef]
- Randi, F.; Sánchez, J.M.; Herlihy, M.M.; Valenza, A.; Kenny, D.A.; Butler, S.T.; Lonergan, P. Effect of Equine Chorionic Gonadotropin Treatment during a Progesterone-Based Timed Artificial Insemination Program on Reproductive Performance in Seasonal-Calving Lactating Dairy Cows. J. Dairy Sci. 2018, 101, 10526–10535. [Google Scholar] [CrossRef]
- Funakoshi, D.; Shiotani, H.; Seki, M. Equine Chorionic Gonadotropin Treatment and Timed Artificial Insemination for Dairy Cow Production under Heat Stress. J. Reprod. Dev. 2024, 70, 30. [Google Scholar] [CrossRef]
- Liu, T.C.; Chiang, C.F.; Ho, C.T.; Chan, J.P.W. Effect of GnRH on Ovulatory Response after Luteolysis Induced by Two Low Doses of PGF2α in Lactating Dairy Cows. Theriogenology 2018, 105, 45–50. [Google Scholar] [CrossRef]
- Bahrami, M.M.; Nava, H.G.; Akbarinejad, V.; Tabatabaee, S.A.A. Conception Rate of Pre-Synchronization and Two Short Term Heat-Synch Programs Using Two Doses of PGF2α in Lactating Holstein Dairy Cows. Iran. J. Vet. Med. 2021, 15, 423–431. [Google Scholar] [CrossRef]
- Stevenson, J.S.; Sauls, J.A.; Mendonça, L.G.D.; Voelz, B.E. Dose Frequency of Prostaglandin F2α Administration to Dairy Cows Exposed to Presynchronization and Either 5- or 7-Day Ovsynch Program Durations: Ovulatory and Luteolytic Risks. J. Dairy Sci. 2018, 101, 9575–9590. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, A.; Barański, W.; Tobolski, D.; Zduńczyk, S.; Janowski, T. Second Prostaglandin F 2α Treatment during Ovsynch Protocol Does Not Improve Fertility Outcomes in Dairy Cows. Pol. J. Vet. Sci. 2019, 22, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.C.; Menchaca, A.; Mapletoft, R.J.; Bó, G.A. Treatment Alternatives to Induce Follicular Wave Emergence for Timed-AI in Lactating Dairy Cows. Theriogenology 2024, 226, 343–349. [Google Scholar] [CrossRef]
- Bisinotto, R.S.; Ribeiro, E.S.; Santos, J.E.P. Synchronisation of Ovulation for Management of Reproduction in Dairy Cows. Animal 2014, 8 (Suppl. 1), 151–159. [Google Scholar] [CrossRef]
- Tschopp, J.C.; Bó, G.A. Success of Artificial Insemination Based on Expression of Estrus and the Addition of GnRH to an Estradiol/Progesterone-Based Protocol on Pregnancy Rates in Lactating Dairy Cows. Anim. Reprod. Sci. 2022, 238, 106954. [Google Scholar] [CrossRef] [PubMed]
- Madureira, A.M.L.; Polsky, L.B.; Burnett, T.A.; Silper, B.F.; Soriano, S.; Sica, A.F.; Pohler, K.G.; Vasconcelos, J.L.M.; Cerri, R.L.A. Intensity of Estrus Following an Estradiol-Progesterone-Based Ovulation Synchronization Protocol Influences Fertility Outcomes. J. Dairy Sci. 2019, 102, 3598–3608. [Google Scholar] [CrossRef]
- Rocha, C.C.; Martins, T.; Mello, B.P.; Dalmaso de Mello, G.; Motta, I.G.; Lemes, K.M.; Binelli, M.; Madureira, E.H.; Pugliesi, G. Comparing the Effect of Estradiol Benzoate and 17β-Estradiol plus Progesterone on Follicular Turnover and Development, and Pregnancy Outcomes in a Timed Artificial Insemination Protocol. Theriogenology 2022, 192, 73–80. [Google Scholar] [CrossRef]
- Dhami, A.J.; Nakrani, B.B.; Hadiya, K.K.; Patel, J.A.; Shah, R.G. Comparative Efficacy of Different Estrus Synchronization Protocols on Estrus Induction Response, Fertility and Plasma Progesterone and Biochemical Profile in Crossbred Anestrus Cows. Vet. World 2015, 8, 1310–1316. [Google Scholar] [CrossRef]
- Consentini, C.E.C.; Carneiro, T.O.; Neri, H.; Batista, E.O.S.; e Silva, L.O.; Souza, A.H.; Sartori, R. Improved Fertility Following a Gonadotropin-Releasing Hormone Treatment on Day 2 of an Estradiol and Progesterone-Based Timed-Artificial Insemination Protocol in Lactating Dairy Cows. JDS Commun. 2022, 3, 212–216. [Google Scholar] [CrossRef]
- Bandai, K.; Kusaka, H.; Miura, H.; Kikuchi, M.; Sakaguchi, M. A Simple and Practical Short-Term Timed Artificial Insemination Protocol Using Estradiol Benzoate with Prostaglandin F2α in Lactating Dairy Cows. Theriogenology 2020, 141, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Borchardt, S.; Pohl, A.; Heuwieser, W. Luteal Presence and Ovarian Response at the Beginning of a Timed Artificial Insemination Protocol for Lactating Dairy Cows Affect Fertility: A Meta-Analysis. Animals 2020, 10, 1551. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Santos, V.G.; Giordano, J.O.; Wiltbank, M.C.; Fricke, P.M. Development of Fertility Programs to Achieve High 21-Day Pregnancy Rates in High-Producing Dairy Cows. Theriogenology 2018, 114, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Consentini, C.E.C.; Melo, L.F.; Abadia, T.; Gonzales, B.; Motta, J.C.L.; Alves, R.L.O.R.; e Silva, L.O.; Wiltbank, M.C.; Sartori, R. Comparison of Gonadotropin-Releasing Hormone and Estradiol Benzoate plus Gonadotropin-Releasing Hormone to Initiate a Progesterone-Based Timed Artificial Insemination Resynchronization Protocol in Lactating Dairy Cows. J. Dairy Sci. 2024, 107, 5122–5131. [Google Scholar] [CrossRef]
- Zwiefelhofer, E.M.; Lillico, W.; Adams, G.P. Development of a Letrozole-Based Synchronization Protocol for Fixed-Time Artificial Insemination in Beef Cattle. Anim. Reprod. Sci. 2022, 240, 106975. [Google Scholar] [CrossRef] [PubMed]
- Yapura, M.J.; Zwiefelhofer, E.M.; Pierson, R.A.; Adams, G.P. Aromatase Inhibitors: A New Approach for Controlling Ovarian Function in Cattle. Theriogenology 2018, 112, 18–25. [Google Scholar] [CrossRef]
- Macmillan, K.; Loree, K.; Mapletoft, R.J.; Colazo, M.G. Short Communication: Optimization of a Timed Artificial Insemination Program for Reproductive Management of Heifers in Canadian Dairy Herds. J. Dairy Sci. 2017, 100, 4134–4138. [Google Scholar] [CrossRef]
- Carvalho, P.D.; Wiltbank, M.C.; Fricke, P.M. Manipulation of Progesterone to Increase Ovulatory Response to the First GnRH Treatment of an Ovsynch Protocol in Lactating Dairy Cows Receiving First Timed Artificial Insemination. J. Dairy Sci. 2015, 98, 8800–8813. [Google Scholar] [CrossRef]
- Yotov, S.; Fasulkov, I.; Atanasov, A.; Kistanova, E.; Sinapov, B.; Ivanova, B.; Yarkov, D.; Zaimova, D. Influence of Ovarian Status and Steroid Hormone Concentration on Day of Timed Artificial Insemination (TAI) on the Reproductive Performance of Dairy Cows Inseminated with Sexed Semen. Animals 2023, 13, 896. [Google Scholar] [CrossRef]
- White, S.S.; Kasimanickam, R.K.; Kasimanickam, V.R. Fertility after Two Doses of PGF2α Concurrently or at 6-Hour Interval on the Day of CIDR Removal in 5-Day CO-Synch Progesterone-Based Synchronization Protocols in Beef Heifers. Theriogenology 2016, 86, 785–790. [Google Scholar] [CrossRef]
- Jeong, J.K.; Kim, U.H.; Kim, I.H. Efficacy of a Modified Double-Ovsynch Protocol for the Enhancement of Reproductive Performance in Hanwoo Cattle. Anim. Biosci. 2023, 36, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Melo, L.F.; Monteiro, P.L.J.; Nascimento, A.B.; Drum, J.N.; Spies, C.; Prata, A.B.; Wiltbank, M.C.; Sartori, R. Follicular Dynamics, Circulating Progesterone, and Fertility in Holstein Cows Synchronized with Reused Intravaginal Progesterone Implants That Were Sanitized by Autoclave or Chemical Disinfection. J. Dairy Sci. 2018, 101, 3554–3567. [Google Scholar] [CrossRef]
- Maru, D.; Kumar, A. Applications of Omics Technologies in Livestock Production, Improvement and Sustainability. In Sustainable Agriculture Reviews: Animal Biotechnology for Livestock Production 4; Kumar Yata, V., Mohanty, A.K., Lichtfouse, E., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 1–54. [Google Scholar] [CrossRef]
- Valour, D.; Michot, P.; Eozenou, C.; Lefebvre, R.; Bonnet, A.; Capitan, A.; Uzbekova, S.; Sellem, E.; Ponsart, C.; Schibler, L. Dairy Cattle Reproduction Is a Tightly Regulated Genetic Process: Highlights on Genes, Pathways, and Biological Processes. Anim. Front. 2015, 5, 32–41. [Google Scholar] [CrossRef]
- Nayak, S.S.; Panigrahi, M.; Rajawat, D.; Ghildiyal, K.; Sharma, A.; Parida, S.; Bhushan, B.; Mishra, B.P.; Dutt, T. Comprehensive Selection Signature Analyses in Dairy Cattle Exploiting Purebred and Crossbred Genomic Data. Mamm. Genome 2023, 34, 615–631. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Camargo, L.S.A.; da Silva, M.V.G.B.; Saraiva, N.Z.; Quintão, C.C.; Machado, M.A. Embryo Biopsies for Genomic Selection in Tropical Dairy Cattle. Anim. Reprod. 2023, 20, e20230064. [Google Scholar] [CrossRef]
- Strandén, I.; Kantanen, J.; Russo, I.R.M.; Orozco-terWengel, P.; Bruford, M.W. Genomic Selection Strategies for Breeding Adaptation and Production in Dairy Cattle under Climate Change. Heredity 2019, 123, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Salilew-Wondim, D.; Tholen, E.; Held-Hoelker, E.; Shellander, K.; Blaschka, C.; Drillich, M.; Iwersen, M.; Suess, D.; Gebremedhn, S.; Tesfaye, D.; et al. Endometrial DNA Methylation Signatures during the Time of Breeding in Relation to the Pregnancy Outcome in Postpartum Dairy Cows Fed a Control Diet or Supplemented with Rumen-Protected Methionine. Front. Genet. 2023, 14, 1267053. [Google Scholar] [CrossRef]
- Sitko, E.M.; Laplacette, A.; Duhatschek, D.; Rial, C.; Perez, M.M.; Tompkins, S.; Kerwin, A.L.; Domingues, R.R.; Wiltbank, M.C.; Giordano, J.O. Ovarian Function and Endocrine Phenotypes of Lactating Dairy Cows during the Estrous Cycle Are Associated with Genomic-Enhanced Predictions of Fertility Potential. J. Dairy Sci. 2024, 107, 7352–7370. [Google Scholar] [CrossRef]
- Zolini, A.M.; Ortiz, W.G.; Estrada-Cortes, E.; Ortega, M.S.; Dikmen, S.; Sosa, F.; Giordano, J.O.; Hansen, P.J. Interactions of Human Chorionic Gonadotropin with Genotype and Parity on Fertility Responses of Lactating Dairy Cows. J. Dairy Sci. 2019, 102, 846–856. [Google Scholar] [CrossRef]
- Ponce-Barajas, P.; Colazo, M.G.; Behrouzi, A.; Ree, T.O.; Kastelic, J.P.; Ambrose, D.J. Morphologic, Steroidogenic, and Transcriptomic Assessment of the Corpus Luteum in Holstein Cows after Spontaneous or Hormone-Induced Ovulation. Animals 2023, 13, 2283. [Google Scholar] [CrossRef]
- Panda, B.S.K.; Mohapatra, S.K.; Chaudhary, D.; Alhussien, M.N.; Kapila, R.; Dang, A.K. Proteomics and Transcriptomics Study Reveals the Utility of ISGs as Novel Molecules for Early Pregnancy Diagnosis in Dairy Cows. J. Reprod. Immunol. 2020, 140, 103148. [Google Scholar] [CrossRef]
- Mazzoni, G.; Pedersen, H.S.; Rabaglino, M.B.; Hyttel, P.; Callesen, H.; Kadarmideen, H.N. Characterization of the Endometrial Transcriptome in Early Diestrus Influencing Pregnancy Status in Dairy Cattle after Transfer of in Vitro-Produced Embryos. Physiol Genom. 2020, 52, 269–279. [Google Scholar] [CrossRef]
- Morales Dalanezi, F.; Mogollon Garcia, H.D.; de Andrade Ferrazza, R.; Fagali Franchi, F.; Kubo Fontes, P.; de Souza Castilho, A.C.; Gouveia Nogueira, M.F.; dos Santos Schmidt, E.M.; Sartori, R.; Pinheiro Ferreira, J.C. Extracellular Vesicles of Follicular Fluid from Heat-Stressed Cows Modify the Gene Expression of in Vitro-Matured Oocytes. Anim. Reprod. Sci. 2019, 205, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, P.A.; Poit, D.A.S.; Ferreira Pinto, L.M.; Guerra, A.C.; Laurindo Neto, A.; do Prado, F.L.; Azrak, A.J.; Çakmakçı, C.; Baruselli, P.S.; Pugliesi, G. Accuracy of Early Pregnancy Diagnosis and Determining Pregnancy Loss Using Different Biomarkers and Machine Learning Applications in Dairy Cattle. Theriogenology 2024, 224, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, J.C.; Peñagaricano, F.; Baez, G.M.; Melo, L.F.; Motta, J.C.L.; Garcia-Guerra, A.; Meidan, R.; Pinheiro Ferreira, J.C.; Sartori, R.; Wiltbank, M.C. Mechanisms for Rescue of Corpus Luteum during Pregnancy: Gene Expression in Bovine Corpus Luteum Following Intrauterine Pulses of Prostaglandins E1 and F2α†. Biol. Reprod. 2018, 98, 465–479. [Google Scholar] [CrossRef]
- Sharawy, H.A.; Hegab, A.R.O.; Mostagir, A.; Adlan, F.; Bazer, F.W.; Elmetwally, M.A. Expression of Genes for Transport of Water and Angiogenesis, as Well as Biochemical Biomarkers in Holstein Dairy Cows during the Ovsynch Program. Theriogenology 2023, 208, 52–59. [Google Scholar] [CrossRef]
- Sousa, L.M.M.d.C.; Mendes, G.P.; Campos, D.B.; Baruselli, P.S.; Papa, P.d.C. Equine Chorionic Gonadotropin Modulates the Expression of Genes Related to the Structure and Function of the Bovine Corpus Luteum. PLoS ONE 2016, 11, e0164089. [Google Scholar] [CrossRef]
- Berisha, B.; Thaqi, G.; Schams, D.; Rodler, D.; Sinowatz, F.; Pfaffl, M.W. Effect of the Gonadotropin Surge on Steroid Receptor Regulation in Preovulatory Follicles and Newly Formed Corpora Lutea in the Cow. Domest. Anim. Endocrinol. 2024, 89, 106876. [Google Scholar] [CrossRef]
- Ben Meir, Y.A.; Daddam, J.R.; Kra, G.; Kamer, H.; Portnick, Y.; Levin, Y.; Zachut, M. Proteomic Analysis of Adipose Tissue Revealing Differentially Abundant Proteins in Highly Efficient Mid-Lactating Dairy Cows. Sci. Rep. 2022, 12, 9721. [Google Scholar] [CrossRef]
- Zhang, J.; Gaowa, N.; Wang, Y.; Li, H.; Cao, Z.; Yang, H.; Zhang, X.; Li, S. Complementary Hepatic Metabolomics and Proteomics Reveal the Adaptive Mechanisms of Dairy Cows to the Transition Period. J. Dairy Sci. 2023, 106, 2071–2088. [Google Scholar] [CrossRef]
- Zachut, M.; Sood, P.; Levin, Y.; Moallem, U. Proteomic Analysis of Preovulatory Follicular Fluid Reveals Differentially Abundant Proteins in Less Fertile Dairy Cows. J. Proteom. 2016, 139, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Kim, K.W.; Han, D.W.; Lee, H.C.; Yang, B.C.; Chung, H.K.; Shim, M.R.; Choi, M.S.; Jo, E.B.; Jo, Y.M.; et al. Protein Profile in Corpus Luteum during Pregnancy in Korean Native Cows. Asian-Australas. J. Anim. Sci. 2012, 25, 1540–1545. [Google Scholar] [CrossRef]
- Kadarmideen, H.N.; Mazzoni, G. Transcriptomics-Genomics Data Integration and Expression Quantitative Trait Loci Analyses in Oocyte Donors and Embryo Recipients for Improving in Vitro Production of Dairy Cattle Embryos. Reprod. Fertil. Dev. 2019, 31, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Pate, J.L.; Hughes, C.K. Applications of Large-Scale Molecular Profiling Techniques to the Study of the Corpus Luteum. Anim. Reprod. 2018, 15, 791–804. [Google Scholar] [CrossRef]
- Phillips, K.M.; Read, C.C.; Kriese-Anderson, L.A.; Rodning, S.P.; Brandebourg, T.D.; Biase, F.H.; Marks, M.L.; Elmore, J.B.; Stanford, M.K.; Dyce, P.W. Plasma Metabolomic Profiles Differ at the Time of Artificial Insemination Based on Pregnancy Outcome, in Bos Taurus Beef Heifers. Sci. Rep. 2018, 8, 13196. [Google Scholar] [CrossRef]
- Gimeno, I.; Salvetti, P.; Carrocera, S.; Gatien, J.; García-Manrique, P.; López-Hidalgo, C.; Valledor, L.; Gómez, E. Biomarker Metabolite Mating of Viable Frozen-Thawed in Vitro-Produced Bovine Embryos with Pregnancy-Competent Recipients Leads to Improved Birth Rates. J. Dairy Sci. 2023, 106, 6515–6538. [Google Scholar] [CrossRef]
- Hessock, E.A.; Edwards, J.L.; Schrick, F.N.; Payton, R.R.; Campagna, S.R.; Pollock, A.B.; Clark, H.M.; Stokes, A.E.; Klabnik, J.L.; Hill, K.S.; et al. Metabolite Abundance in Bovine Preovulatory Follicular Fluid Is Influenced by Follicle Developmental Progression Post Estrous Onset in Cattle. Front. Cell Dev. Biol. 2023, 11, 1156060. [Google Scholar] [CrossRef]
- Chebel, R.C.; Veronese, A. Associations between Genomic Merit for Daughter Pregnancy Rate of Holstein Cows and Metabolites Postpartum and Estrus Characteristics. J. Dairy Sci. 2020, 103, 10754–10768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, Y.; Shu, S.; Wang, G.; Fu, C.; Huang, R.; Zhang, J.; Su, H.; He, Y.; Lei, C.; et al. Transcriptomics and Metabolomics of Blood, Urine and Ovarian Follicular Fluid of Yak at Induced Estrus Stage. BMC Genom. 2024, 25, 201. [Google Scholar] [CrossRef]
- Hoffmann, D.A.C.; Furtado, M.; Bragança, L.F.; Araujo, G.d.M.; Moreira, F.; Rabassa, V.R.; Feijó, J.O.; Corrêa, M.N.; Peripolli, V.; Schwegler, E. Metabolic Profile of Prepartum Dairy Cows and Its Influence on the Immediate Postpartum Period, Colostrum Quality and Passive Immunity Transference. Vet. J. 2024, 308, 106260. [Google Scholar] [CrossRef]
- Reed, C.B.; Kuhn-Sherlock, B.; Burke, C.R.; Meier, S. Estrous Activity in Nulliparous Heifers with Divergent Genetic Merit for Fertility Traits. J. Dairy Sci. 2024, 107, 9875–9887. [Google Scholar] [CrossRef] [PubMed]
- Akbarinejad, V.; Gharagozlou, F.; Vojgani, M.; Bagheri Amirabadi, M.M. Nulliparous and Primiparous Cows Produce Less Fertile Female Offspring with Lesser Concentration of Anti-Müllerian Hormone (AMH) as Compared with Multiparous Cows. Anim. Reprod. Sci. 2018, 197, 222–230. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayantoye, J.O.; Kolachi, H.A.; Zhang, X.; Shahzad, M.; Kandil, O.M.T.; Wan, P.; Zhao, X. Advances in Timed Artificial Insemination: Integrating Omics Technologies for Enhanced Reproductive Efficiency in Dairy Cattle. Animals 2025, 15, 816. https://doi.org/10.3390/ani15060816
Ayantoye JO, Kolachi HA, Zhang X, Shahzad M, Kandil OMT, Wan P, Zhao X. Advances in Timed Artificial Insemination: Integrating Omics Technologies for Enhanced Reproductive Efficiency in Dairy Cattle. Animals. 2025; 15(6):816. https://doi.org/10.3390/ani15060816
Chicago/Turabian StyleAyantoye, Jesse Oluwaseun, Hubdar Ali Kolachi, Xiaomeng Zhang, Muhammad Shahzad, Omaima Mohamed Tawfik Kandil, Pengcheng Wan, and Xueming Zhao. 2025. "Advances in Timed Artificial Insemination: Integrating Omics Technologies for Enhanced Reproductive Efficiency in Dairy Cattle" Animals 15, no. 6: 816. https://doi.org/10.3390/ani15060816
APA StyleAyantoye, J. O., Kolachi, H. A., Zhang, X., Shahzad, M., Kandil, O. M. T., Wan, P., & Zhao, X. (2025). Advances in Timed Artificial Insemination: Integrating Omics Technologies for Enhanced Reproductive Efficiency in Dairy Cattle. Animals, 15(6), 816. https://doi.org/10.3390/ani15060816





