A Comprehensive Review of Canine and Feline Ventricular Septal Defects—From Pathogenesis to Long-Term Follow-Up
Simple Summary
Abstract
1. Introduction
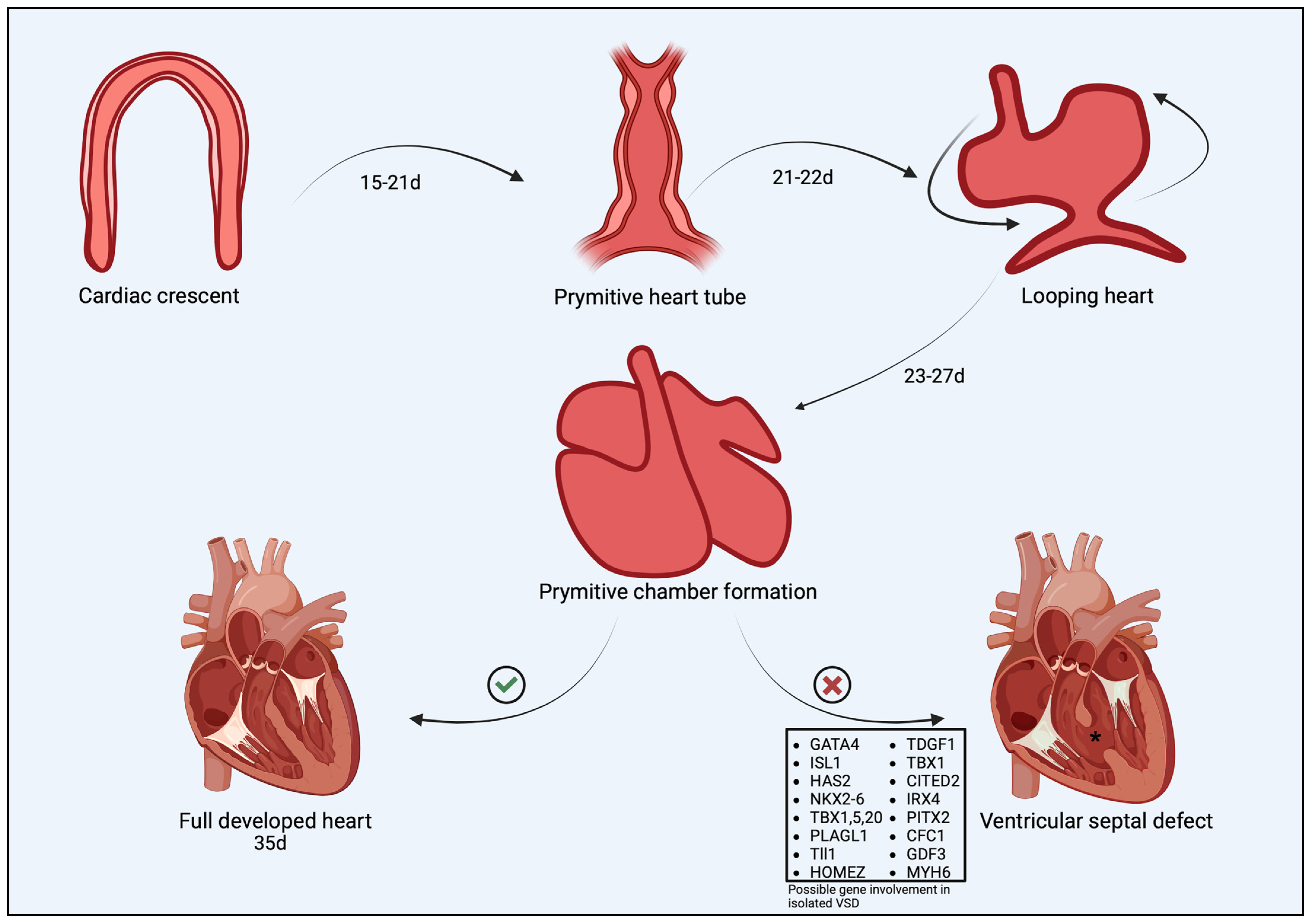
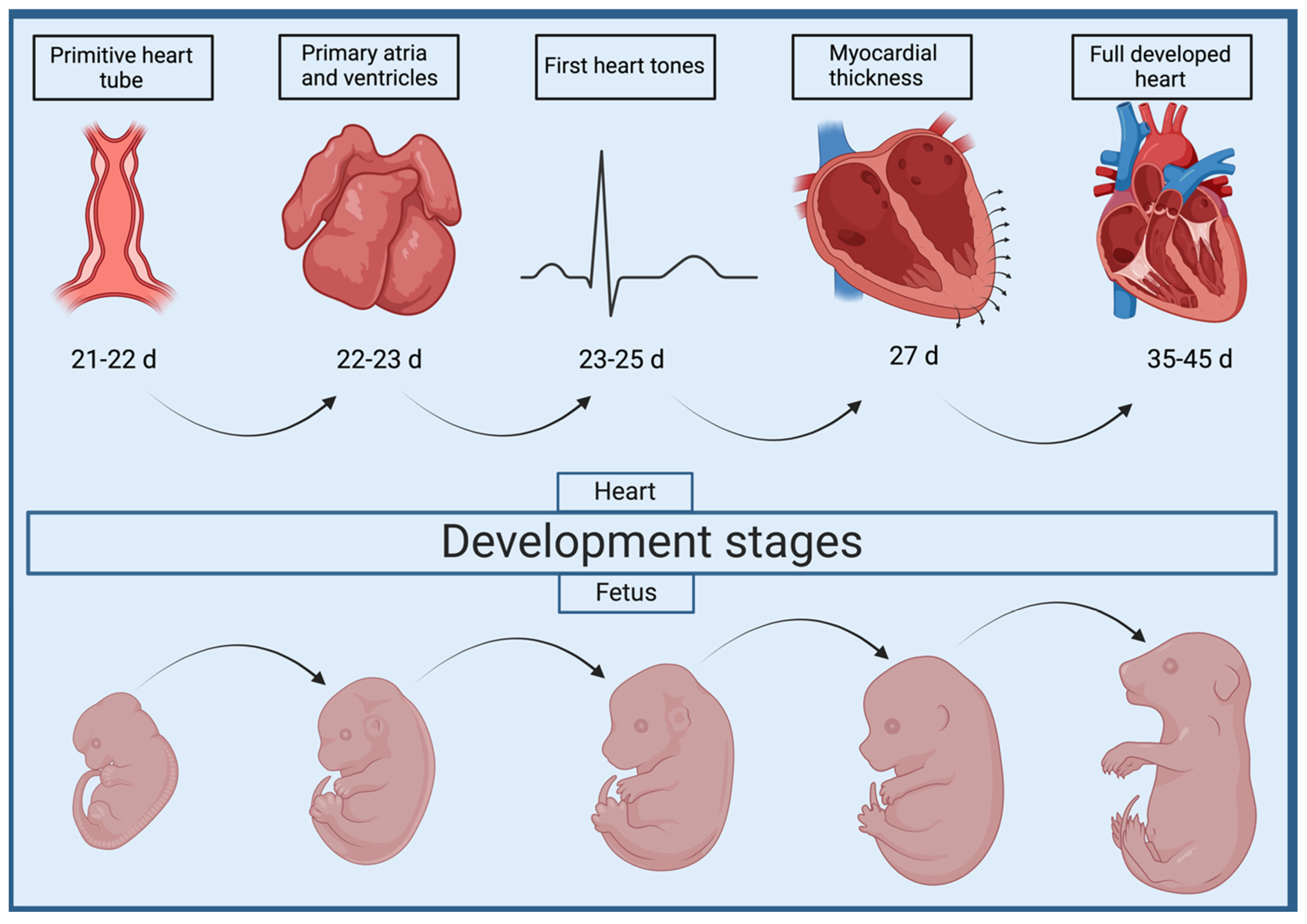
2. Cardiac Morphogenesis and Abnormal Development of the Interventricular Septum
Mechanisms Underlying VSD in Dogs and Cats
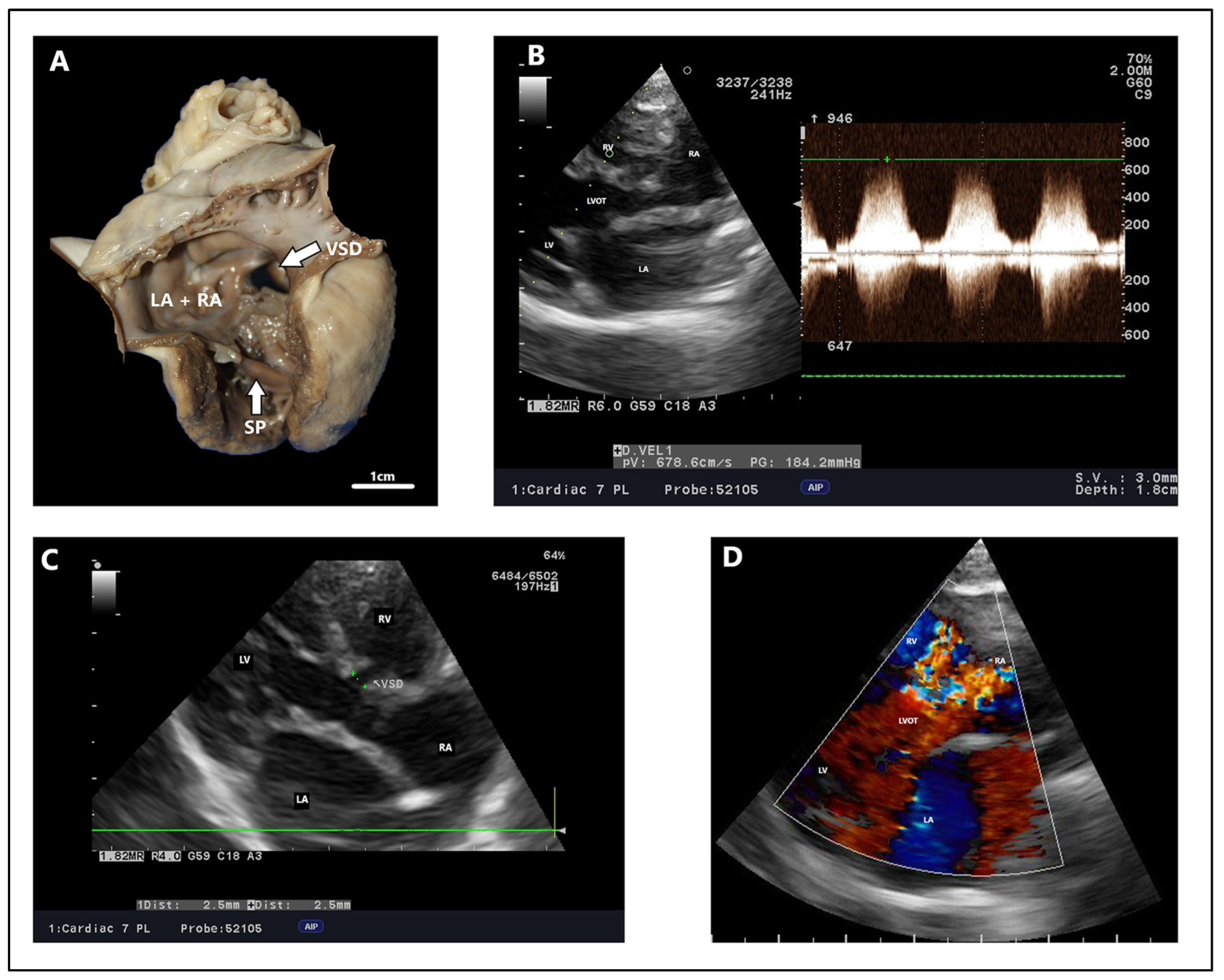
3. Nomenclature of Ventricular Septal Defects
4. VSD in Dogs and Cats
4.1. Clinical Findings
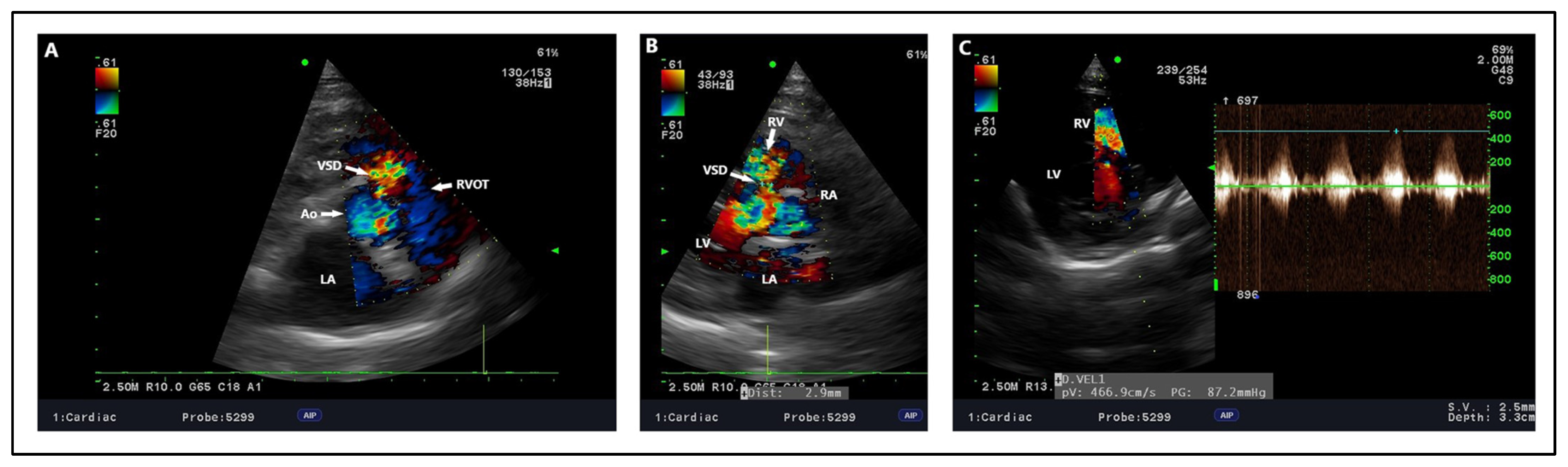
4.2. Echocardiography, Radiography, ECG Findings
4.3. Management of VSD
4.3.1. Pulmonary Artery Banding
4.3.2. Open Heart Surgery
4.3.3. Percutaneous Transcatheter VSD Closure
| Author | Breed | Age (Months) | Clinical Signs | Ventricular Septal Defect Type and Diameter (Millimetres) | Maximum Ventricular Septal Defect Velocity Peak | Treatment Method | Echocardiographic Findings | Radiographic Findings | Electrocardiographic Findings | Clinical Outcomes | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Before Procedure | After Procedure | ||||||||||
| [10] | Cavalier King Charles Spaniel | 3 | The loudest murmur on the right side | Perimembranous, 10 | NM | Open heart surgery with cross-circulation | NM | After surgery within normal limits, no residual shunt flow | Left-sided cardiomegaly, pulmonary over-perfusion, prominent main pulmonary artery, two months after the procedure—normal | After surgery persistent RBBB | Improved exercise tolerance, soft systolic murmur at the level of the TV after 2 months |
| [11] | Shiba Inu | 2 | III/VI right-sided holosytolic murmur | Partial AVSD with ventricular communication, NM | NM | Open heart surgery under cardiopulmonary bypass | LV and LA enlargement, MR with cleft of septal leaflet, L-to-R shunt | Mild MR, no residual shunt, reduced LVIDd | VHS before procedure—13.7, VHS after procedure—11.3 | Normal | Clinically normal after the year |
| [12] | Cavalier King Charles Spaniel | 7 | Shortness of breath when excited, V/VI right-sided systolic murmur | Muscular, 10 | NM | Percutaneous transcatheter closure (Amplatzer Muscular VSD occluder) | RV enlargement with wall thickness, bidirectional shunt flow | No residual flow through the device, Trivial TI, Trivial MI | Right ventricle enlargement, Loss of the cranial waist of the cardiac silhouette | NM | Clinically normal after 2 years |
| [13] | English Sheepdog | 12 | IV/VI right-sided holosystolic murmur, reduced exercise capability | Perimembranous, 6 | 4.8 m/s | Percutaneous transcatheter closure (Amplatzer occluder) | Qp:Qs: 1.76, EDVI—215 mL/m2, L-to-R shunt, Pressure gradient 92.2 mmHg | No residual flow through the device | Mild cardiomegaly with over-circulation of pulmonary vasculature, pulmonary venous congestion | VPCs—isolated ventricular premature beats, ECG after treatment was normal | Clinically normal after 1 month |
| [14] | Irish setter | 7 | Mild tachypnea, respiratory effort after strenuous exercise, VI/VI left basilar systolic murmur | Muscular, 12 | Bidirectional flow | Percutaneus transcatheter closure (amplatzer post-infarction muscular ventricular septal occluder) | RV hyperthrophy with systolic and diastolic septal flattening, moderate PI, severe post stenotic main and right pulmonary dilatation | Persistent mild L-to-R flow 5 months after procedure | Generalized cardiomegaly, VHS 11.25, dilation of the main pulmonary artery segment | NM | Died after 5 months after surgery due to acute neurological signs and respiratory failure |
| [15] | Beagles | 1 | III/VI right-sided systolic murmur | Perimembranous, NM | NM | Percutaneous transcatheter closure (detachable coil) | Qp:Qs—1.2, L-to-R shunt, aortic maximum velocity peak 0.93 m/s | Qp:Qs—1, aortic maximum velocity peak 1.20 m/s, small AR | No evidence of cardiomegaly, after the procedure, there was no evidence of abnormal cardiac silhouette | Normal rhythm | Diminished heart murmur |
| [16] | Mongrel | 12 | V/VI right-sided holosytolic murmur | Perimembranous, 7.8 from LV, 3.4–4.7 from RV | 4.7 m/s | Percutaneous transcatheter closure (canine duct occluder) | LA/Ao 1.8, Qp:Qs—1.7, Mild MR, LVIDdN—1.87 | LA/Ao—1.41, Qp:Qs—1.1, LVIDdN—1.69 | NM | NM | Clinically normal after 2 years |
| [16] | Staffordshire terrier | 6 | VI/VI right-sided systolic murmur | Perimembranous, 9.69 from LV, 5.78 from RV | NM | Percutaneous transcatheter closure (canine duct occluder) | LA/Ao 1.7 LVIDdN—1.79 Mild MR, PR, AR, | Qp:Qs 1.2, LVIDdN 1.66 | NM | NM | Clinically normal after 8 months |
| [18] | Bichon Fries | 4 | Tachypnoea during exercise, VI/VI right-sided systolic murmur | Outlet, 4.4 | 3.3 m/s | Percutaneous transcatheter closure (amplatzer duct occluder II) | LA/Ao—2.15, MR, Qp:Qs 2.8, Pressure gradient 43.6 mmHg LVIDd—26 mm LVIDs—15 mm | 17 days after the procedure: LVIDd—24 mm LVIDs—15 mm | Pulmonary vessel enlargement, cardiogenic edema in the caudal lung lobe, VHS before procedure—12.2 VHS 17 d after procedure—10.3 | NM | Clinically normal after 4 months |
| [19] | Domestic short hair cat | 3 | V/VI right parasternal systolic murmur | Perimembranous, 1.9 | 3.7 m/s | Pulmonary artery banding | LA/Ao 1.94, Qp:Qs 2.4, L-to-R, Pressure gradient 56 mmHg, LVIDd—15 mm, moderate concentrate thickening of RV | LA/Ao—2.1, VSD maximum velocity peak 2.6 m/s, Pressure gradient 26 mmHg, mild-to-moderate RV concentric hypertrophy | NM | NM | Clinically normal after 40 months |
| [19] | Domestic short hair cat | 10 | V/VI right parasternal systolic murmur | Perimembranous, 4 | 3.9 m/s | Pulmonary artery banding | LA/Ao 2.7, Qp;Qs 2.1, L-to-R flow, Pressure gradient 61 mmHg, LVIDd—26.2 mm | VSD maximum velocity peak 3.4 m/s, pressure gradient 37 mmHg, moderate dilation of LVIDd | Generalized cardiomegaly, severe LA enlargement, marked over-circulation of the pulmonary vessels | NM | Clinically normal after 9 months |
| [19] | Domestic short hair cat | 1 | Activity intolerance, resting tachypnea, V/VI right parasternal systolic murmur | Perimembranous, 2.5 | 2 m/s | Pulmonary artery banding | LA/Ao 1.9, LVIDd dilation, MR, PR, TR | LA/Ao 1.4, LVIDd—17.7 VSD maximum velocity peak 3.6 m/s, Pressure gradient 52 mmHg, | NM | NM | Clinically normal after 9 months |
| [20] | Maine Coon | 13 | Exercise intolerance, respiratory distress, abdominal and mouth breathing, tachypnea | Perimembranous, 7.6 | 4.9 m/s | Percutaneous transcatheter closure/pulmonary artery banding | Qp:Qs 3.82, LVIDd—34.4 | Qp:Qs 2.28, volume overload, CHF after 8 months | NM | NM | Clinically normal after 8 months |
| [21] | Domestic short hair cat | 8 | VI/VI systolic right-sided murmur, severe tachypnea, dry cough | Perimembranous, NM | 5.48 m/s | Pulmonary artery banding | Qp:Qs 3, MR, LVIDd 19 mm, LVIDs 12 mm | Qp:Qs 1.5 after 14 months | Left atrial enlargement, moderate RV enlargement, mild to moderate enlargement of the pulmonary vasculature, mild interstitial pattern in the cranial lung lobes, bronchointerstitial lung pattern in the perihilar region and caudodorsal lung fields | NM | Clinically normal after 14 months |
| [156] | Maltese and poodle mix | 5 | V/VI systolic right sided murmur, mild precordial thrill, jugular vein distension, nonproductive cough | Muscular, 5 | 1.1 m/s in systole, 0.9 m/s diastole | Transcatheter occlusion | Right ventricle enlargement with interventricular septal flattening, LVIDd/RVIDd < 1, MPA/AO ratio 1.41, RPA/AO ratio 0.72, PR 4.3 m/s (>74 mmHg), Qp:Qs—2.75 | LVIDd/RVIDd ratio 2.1, Ao/MPA ratio 0.81 Ao/RPA ratio 0.6 PR 1.84 m/s (13.5 mmHg), Qp:Qs ratio 1.3 | Enlargement of right cardiac silhouette (VHS 11.6), dilation of the main pulmonary artery and pulmonary vessels CVC/VL 1.8 | Sinus tachycardia (180–200 bpm), Deep S-wave | Clinically normal 9 months after procedure |
5. Long Term Follow-Up
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ao | aorta |
| AI | aortic insufficiency |
| ASD | atrial septal defect |
| AV | atrioventricular |
| AVSD | atrioventricular septal defect |
| ECG | electrocardiography |
| CHD | congenital heart defect |
| DCRV | double-chambered right ventricle |
| ISNPCHD | International Society for Nomenclature of Paediatric and Congenital Heart Disease |
| LV | left ventricle |
| LVOT | left ventricular outflow tract |
| PDA | patent ductus arteriosus |
| PS | pulmonic stenosis |
| Qp:Qs | pulmonary (Qp) to systemic flow (Qs) ratio |
| RBBB | right bundle branch block |
| RV | right ventricle |
| RVOT | right ventricular outflow tract |
| SAS | subaortic stenosis |
| VSD | ventricular septal defect |
| VSD:Ao | ventricular septal defect diameter to Aortic diameter ratio |
| Δp | pressure gradient |
References
- Hyun, C.; Lavulo, L. Congenital Heart Diseases in Small Animals: Part I. Genetic Pathways and Potential Candidate Genes. Vet. J. 2006, 171, 245–255. [Google Scholar] [CrossRef]
- Lee, S.A.; Lee, S.G.; Moon, H.S.; Lavulo, L.; Cho, K.O.; Hyun, C. Isolation, Characterization and Genetic Analysis of Canine GATA4 Gene in a Family of Doberman Pinschers with an Atrial Septal Defect. J. Genet. 2007, 86, 241–247. [Google Scholar] [CrossRef]
- Werner, P.; Raducha, M.G.; Prociuk, U.; Ostrander, E.A.; Spielman, R.S.; Kirkness, E.F.; Patterson, D.F.; Henthorn, P.S. The Keeshond Defect in Cardiac Conotruncal Development Is Oligogenic1. Hum. Genet. 2005, 116, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Lopez, L.; Houyel, L.; Colan, S.D.; Anderson, R.H.; Béland, M.J.; Aiello, V.D.; Bailliard, F.; Cohen, M.S.; Jacobs, J.P.; Kurosawa, H.; et al. Classification of Ventricular Septal Defects for the Eleventh Iteration of the International Classification of Diseases—Striving for Consensus: A Report From the International Society for Nomenclature of Paediatric and Congenital Heart Disease. Ann. Thorac. Surg. 2018, 106, 1578–1589. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Zühlke, L.; Black, G.C.; Choy, M.K.; Li, N.; Keavney, B.D. Global Birth Prevalence of Congenital Heart Defects 1970–2017: Updated Systematic Review and Meta-Analysis of 260 Studies. Int. J. Epidemiol. 2019, 48, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Riesen, S.C.; Kovacevic, A.; Lombard, C.W.; Amberger, C. Prevalence of Heart Disease in Symptomatic Cats: An Overview from 1998 to 2005. Schweiz. Arch. Für Tierheilkd. 2007, 149, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, P.; Domenech, O.; Silva, J.; Vannini, S.; Bussadori, R.; Bussadori, C. Retrospective Review of Congenital Heart Disease in 976 Dogs. J. Vet. Intern. Med. 2011, 25, 477–483. [Google Scholar] [CrossRef]
- Bomassi, E.; Misbach, C.; Tissier, R.; Gouni, V.; Trehiou-Sechi, E.; Petit, A.M.; Desmyter, A.; Damoiseaux, C.; Pouchelon, J.L.; Chetboul, V. Signalment, Clinical Features, Echocardiographic Findings, and Outcome of Dogs and Cats with Ventricular Septal Defects: 109 Cases (1992–2013). J. Am. Vet. Med. Assoc. 2015, 247, 166–175. [Google Scholar] [CrossRef]
- Shimizu, M.; Tanaka, R.; Hoshi, K.; Hirao, H.; Kobayashi, M.; Shimamura, S.; Yamane, Y. Surgical Correction of Ventricular Septal Defect with Aortic Regurgitation in a Dog. Aust. Vet. J. 2006, 84, 117–121. [Google Scholar] [CrossRef]
- HUNT, G.; PEARSON, M.; BELLENGER, C.; MALIK, R. Ventricular Septal Defect Repair in a Small Dog Using Cross-Circulation. Aust. Vet. J. 1995, 72, 379–382. [Google Scholar] [CrossRef]
- Akiyama, M.; Tanaka, R.; Maruo, K.; Yamane, Y. Surgical Correction of a Partial Atrioventricular Septal Defect With a Ventricular Septal Defect in a Dog. J. Am. Anim. Hosp. Assoc. 2005, 41, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Margiocco, M.L.; Bulmer, B.J.; Sisson, D.D. Percutaneous Occlusion of a Muscular Ventricular Septal Defect with an Amplatzer® Muscular VSD Occluder. J. Vet. Cardiol. 2008, 10, 61–66. [Google Scholar] [CrossRef]
- Bussadori, C.; Carminati, M.; Domenech, O. Transcatheter Closure of a Perimembranous Ventricular Septal Defect in a Dog. J. Vet. Intern. Med. 2007, 21, 1396–1400. [Google Scholar] [CrossRef] [PubMed]
- Durham, J.A.; Scansen, B.A.; Bonagura, J.D.; Schober, K.E.; Cheatham, S.L.; Cheatham, J.P. Iatrogenic Embolization and Transcatheter Retrieval of a Ventricular Septal Defect Occluder in a Dog. J. Vet. Cardiol. 2015, 17, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Fukuda, T.; Machida, N.; Yamane, T.; Wakao, Y. Transcatheter Closure of Congenital Ventricular Septal Defects in 3 Dogs with a Detachable Coil. J. Vet. Intern. Med. 2004, 18, 911–914. [Google Scholar] [CrossRef]
- Chi, I.J.B.; Scansen, B.A.; Potter, B.M.; Pierce, K.V.; Gagnon, A.L.; Sloan, C.Q. Transcatheter Closure of Aneurysmal Perimembranous Ventricular Septal Defect with the Canine Duct Occluder in Two Dogs. J. Vet. Cardiol. 2022, 43, 61–69. [Google Scholar] [CrossRef]
- Shimizu, M.; Tanaka, R.; Hirao, H.; Kobayashi, M.; Shimamura, S.; Maruo, K.; Yamane, Y. Percutaneous Transcatheter Coil Embolization of a Ventricular Septal Defect in a Dog. J. Am. Vet. Med. Assoc. 2005, 226, 69–72. [Google Scholar] [CrossRef]
- Saunders, A.B.; Carlson, J.A.; Nelson, D.A.; Gordon, S.G.; Miller, M.W. Hybrid Technique for Ventricular Septal Defect Closure in a Dog Using an Amplatzer® Duct Occluder II. J. Vet. Cardiol. 2013, 15, 217–224. [Google Scholar] [CrossRef]
- Sutherland, B.J.; Pierce, K.V.; Gagnon, A.L.; Scansen, B.A.; Orton, E.C. Dilatable Pulmonary Artery Banding for Ventricular Septal Defect: Surgical Technique and Case Report of Three Cats. J. Vet. Cardiol. 2019, 25, 32–40. [Google Scholar] [CrossRef]
- Tanaka, R.; Uemura, A. Hybrid Surgical Approach Using Amplatzer Occluder for Treatment Of Vsd In A Cat. Mac. Vet. Rev. 2017, 40, 15. [Google Scholar] [CrossRef]
- Cichocki, B.N.; Dugat, D.R.; Baumwart, R.D. Pulmonary Artery Banding in a Cat with a Perimembranous Ventricular Septal Defect and Left-Sided Congestive Heart Failure. J. Am. Vet. Med. Assoc. 2019, 254, 723–727. [Google Scholar] [CrossRef]
- Poelmann, R.E.; Gittenberger-De Groot, A.C.; Vicente-Steijn, R.; Wisse, L.J.; Bartelings, M.M.; Everts, S.; Hoppenbrouwers, T.; Kruithof, B.P.T.; Jensen, B.; De Bruin, P.W.; et al. Evolution and Development of Ventricular Septation in the Amniote Heart. PLoS ONE 2014, 9, e106569. [Google Scholar] [CrossRef]
- Christoffels, V.M.; Habets, P.E.M.H.; Franco, D.; Campione, M.; De Jong, F.; Lamers, W.H.; Bao, Z.Z.; Palmer, S.; Biben, C.; Harvey, R.P.; et al. Chamber Formation and Morphogenesis in the Developing Mammalian Heart. Dev. Biol. 2000, 223, 266–278. [Google Scholar] [CrossRef]
- Devine, W.P.; Wythe, J.D.; George, M.; Koshiba-Takeuchi, K.; Bruneau, B.G. Early Patterning and Specification of Cardiac Progenitors in Gastrulating Mesoderm. Elife 2014, 3, e03848. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, S.; Karra, R.; Passer, D.; Deutsch, M.A.; Krane, M.; Feistritzer, R.; Sturzu, A.; Domian, I.; Saga, Y.; Wu, S.M. Essential and Unexpected Role of Yin Yang 1 to Promote Mesodermal Cardiac Differentiation. Circ. Res. 2013, 112, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.; Jensen, B. Cardiac Morphogenesis: Specification of the Four-Chambered Heart. Cold Spring Harb. Perspect. Biol. 2020, 12, a037143. [Google Scholar] [CrossRef]
- Moorman, A.F.M.; Christoffels, V.M. Cardiac Chamber Formation: Development, Genes, and Evolution. Physiol. Rev. 2003, 83, 1223–1267. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, M.; Meilhac, S.; Zaffran, S. Building the Mammalian Heart from Two Sources of Myocardial Cells. Nat. Rev. Genet. 2005, 6, 826–835. [Google Scholar] [CrossRef]
- Moorman, A.F.M.; van den Berg, G.; Anderson, R.H.; Christoffels, V.M. Early Cardiac Growth and the Ballooning Model of Cardiac Chamber Formation. Heart Dev. Regen. Vol. I 2010, 1, 219–236. [Google Scholar] [CrossRef]
- Goddeeris, M.M.; Rho, S.; Petiet, A.; Davenport, C.L.; Johnson, G.A.; Meyers, E.N.; Klingensmith, J.; Goddeeris, M.M. Intracardiac Septation Requires Hedgehog-Dependent Cellular Contributions from Outside the Heart. Development 2008, 135, 1887–1895. [Google Scholar] [CrossRef]
- Boon, J. Veterinary Echocardiography; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Ayad, Y.; Almamoury, A. Ventricular Septal Defect. In Clinical and Surgical Aspects of Congenital Heart Diseases: Text and Study Guide; Springer: Berlin/Heidelberg, Germany, 2023; pp. 21–24. [Google Scholar] [CrossRef]
- Srivastava, D.; Olson, E.N. A Genetic Blueprint for Cardiac Development. Nature 2000, 407, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xin, Y.F.; Liu, X.Y.; Liu, Z.M.; Wang, X.Z.; Yang, Y.Q. A Novel NKX2-5 Mutation in Familial Ventricular Septal Defect. Int. J. Mol. Med. 2011, 27, 369–375. [Google Scholar] [CrossRef]
- Clark, T.G.; Conway, S.J.; Scott, I.C.; Labosky, P.A.; Winnier, G.; Bundy, J.; Hogan, B.L.M.; Greenspan, D.S. The Mammalian Tolloid-like 1 Gene, Tll1, Is Necessary for Normal Septation and Positioning of the Heart. Development 1999, 126, 2631–2642. [Google Scholar] [CrossRef]
- Zhu, X.; Deng, X.; Huang, G.; Wang, J.; Yang, J. A Novel Mutation of Hyaluronan Synthase 2 Gene in Chinese Children with Ventricular Septal Defect. PLoS ONE 2014, 9, 87437. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Q.; Chen, H.X.; Liu, X.C.; Yang, Q.; He, G.W. Identification of Variants of ISL1 Gene Promoter and Cellular Functions in Isolated Ventricular Septal Defects. Am. J. Physiol. Cell Physiol. 2021, 321, C443–C452. [Google Scholar] [CrossRef]
- Yang, Y.Q.; Li, L.; Wang, J.; Liu, X.Y.; Chen, X.Z.; Zhang, W.; Wang, X.Z.; Jiang, J.Q.; Liu, X.; Fang, W.Y. A Novel GATA4 Loss-of-Function Mutation Associated with Congenital Ventricular Septal Defect. Pediatr. Cardiol. 2012, 33, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Fang, M.; Liu, X.Y.; Xin, Y.F.; Liu, Z.M.; Chen, X.Z.; Wang, X.Z.; Fang, W.Y.; Liu, X.; Yang, Y.Q. A Novel GATA4 Mutation Responsible for Congenital Ventricular Septal Defects. Int. J. Mol. Med. 2011, 28, 557–564. [Google Scholar] [CrossRef]
- Wang, J.; Mao, J.H.; Ding, K.K.; Xu, W.J.; Liu, X.Y.; Qiu, X.B.; Li, R.G.; Qu, X.K.; Xu, Y.J.; Huang, R.T.; et al. A Novel NKX2.6 Mutation Associated with Congenital Ventricular Septal Defect. Pediatr. Cardiol. 2015, 36, 646–656. [Google Scholar] [CrossRef]
- Peng, T.; Wang, L.; Zhou, S.F.; Li, X. Mutations of the GATA4 and NKX2.5 Genes in Chinese Pediatric Patients with Non-Familial Congenital Heart Disease. Genetica 2010, 138, 1231–1240. [Google Scholar] [CrossRef]
- Salazar, M.; Consoli, F.; Villegas, V.; Caicedo, V.; Maddaloni, V.; Daniele, P.; Caianiello, G.; Pachón, S.; Nuñez, F.; Limongelli, G.; et al. Search of Somatic GATA4 and NKX2.5 Gene Mutations in Sporadic Septal Heart Defects. Eur. J. Med. Genet. 2011, 54, 306–309. [Google Scholar] [CrossRef]
- Liu, C.X.; Shen, A.D.; Li, X.F.; Jiao, W.W.; Bai, S.; Yuan, F.; Guan, X.L.; Zhang, X.G.; Zhang, G.R.; Li, Z.Z. Association of TBX5 Gene Polymorphism with Ventricular Septal Defect in the Chinese Han Population. Chin. Med. J. 2009, 122, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Sperling, S.; Grimm, C.H.; Dunkel, I.; Mebus, S.; Sperling, H.P.; Ebner, A.; Galli, R.; Lehrach, H.; Fusch, C.; Berger, F.; et al. Identification and Functional Analysis of CITED2 Mutations in Patients with Congenital Heart Defects. Hum. Mutat. 2005, 26, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Wang, J.; Su, D.; Pan, H.; Huang, G.; Li, X.; Li, Z.; Shen, A.; Xie, X.; Wang, B.; et al. Two Novel Mutations of the IRX4 Gene in Patients with Congenital Heart Disease. Hum. Genet. 2011, 130, 657–662. [Google Scholar] [CrossRef]
- Wei, D.; Gong, X.H.; Qiu, G.; Wang, J.; Yang, Y.Q. Novel PITX2c Loss-of-Function Mutations Associated with Complex Congenital Heart Disease. Int. J. Mol. Med. 2014, 33, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wang, Z.G.; Liu, X.Y.; Zhao, H.; Zhou, N.; Zheng, G.F.; Qiu, X.B.; Li, R.G.; Yuan, F.; Shi, H.Y.; et al. A Novel TBX1 Loss-of-Function Mutation Associated with Congenital Heart Disease. Pediatr. Cardiol. 2015, 36, 1400–1410. [Google Scholar] [CrossRef]
- Smemo, S.; Campos, L.C.; Moskowitz, I.P.; Krieger, J.E.; Pereira, A.C.; Nobrega, M.A. Regulatory Variation in a TBX5 Enhancer Leads to Isolated Congenital Heart Disease. Hum. Mol. Genet. 2012, 21, 3255–3263. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wang, J.; Liu, S.; Han, X.; Xie, X.; Tao, Y.; Yan, J.; Ma, X. CFC1 Mutations in Chinese Children with Congenital Heart Disease. Int. J. Cardiol. 2011, 146, 86–88. [Google Scholar] [CrossRef]
- Kirk, E.P.; Sunde, M.; Costa, M.W.; Rankin, S.A.; Wolstein, O.; Castro, M.L.; Butler, T.L.; Hyun, C.; Guo, G.; Otway, R.; et al. Mutations in Crdiac T-Box Factor Gene TBX20 Are Associated with Diverse Cardiac Pathologies, Including Defects of Septation and Valvulogenesis and Cardiomyopathy. Am. J. Hum. Genet. 2007, 81, 280–291. [Google Scholar] [CrossRef]
- Xiao, J.; Kang, G.; Wang, J.; Li, T.; Chen, J.; Wang, J.; Li, W.; Wang, B. A Novel Variation of GDF3 in Chinese Han Children with a Broad Phenotypic Spectrum of Non-Syndromic CHDs. Cardiol. Young 2015, 25, 1263–1267. [Google Scholar] [CrossRef]
- Wang, B.; Yan, J.; Peng, Z.; Wang, J.; Liu, S.; Xie, X.; Ma, X. Teratocarcinoma-Derived Growth Factor 1 (TDGF1) Sequence Variants in Patients with Congenital Heart Defect. Int. J. Cardiol. 2011, 146, 225–227. [Google Scholar] [CrossRef]
- Xuan, C.; Jia, K.G.; Wang, B.; Bai, X.Y.; Gao, G.; Yang, Q.; Wang, X.L.; Liu, X.C.; Ma, X.; He, G.W. Identification of Two Novel Mutations of the HOMEZ Gene in Chinese Patients with Isolated Ventricular Septal Defect. Gen. Test. Mol. Biomark. 2013, 17, 390–394. [Google Scholar] [CrossRef]
- Xuan, C.; Wang, B.; Gao, G.; Bai, X.Y.; Yang, Q.; Liu, X.C.; Jing, W.B.; Ma, X.; He, G.W. A Novel Variation of PLAGL1 in Chinese Patients with Isolated Ventricular Septal Defect. Gen. Test. Mol. Biomark. 2012, 16, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Chaithra, S.; Agarwala, S.; Ramachandra, N.B. High-Risk Genes Involved in Common Septal Defects of Congenital Heart Disease. Gene 2022, 840, 146745. [Google Scholar] [CrossRef]
- Zuo, J.Y.; Chen, H.X.; Liu, Z.G.; Yang, Q.; He, G.W. Identification and Functional Analysis of Variants of MYH6 Gene Promoter in Isolated Ventricular Septal Defects. BMC Med. Genom. 2022, 15, 213. [Google Scholar] [CrossRef] [PubMed]
- Perrot, A.; Rickert-Sperling, S. Human Genetics of Ventricular Septal Defect. Adv. Exp. Med. Biol. 2024, 1441, 505–534. [Google Scholar] [CrossRef] [PubMed]
- Pieri, N.C.G.; Souza, A.F.; Casals, J.B.; Roballo, K.C.S.; Ambrósio, C.E.; Martins, D.S. Comparative Development of Embryonic Age by Organogenesis in Domestic Dogs and Cats. Reprod. Domest. Anim. 2015, 50, 625–631. [Google Scholar] [CrossRef]
- Martins, A.A.; Favaron, P.O.; de Jesus Oliveira, L.; Schäfer, B.T.; Oliveira, F.D.; Miglino, M.A. Development of the Cardiorespiratory System in Dogs from Days 16 to 46 of Pregnancy. Reprod. Domest. Anim. 2016, 51, 804–812. [Google Scholar] [CrossRef]
- Yeager, A.E.; Concannon, P.W. Association between the Preovulatory Luteinizing Hormone Surge and the Early Ultrasonographic Detection of Pregnancy and Fetal Heartbeats in Beagle Dogs. Theriogenology 1990, 34, 655–665. [Google Scholar] [CrossRef]
- Kim, B.S.; Son, C.H. Time of Initial Detection of Fetal and Extra-Fetal Structures by Ultrasonographic Examination in Miniature Schnauzer Bitches. J. Vet. Sci. 2007, 8, 289. [Google Scholar] [CrossRef]
- Pretzer, S.D. Canine Embryonic and Fetal Development: A Review. Theriogenology 2008, 70, 300–303. [Google Scholar] [CrossRef]
- Patterson, D.F.; Pyle, R.L.; Van Mierop, L.; Melbin, J.; Olson, M. Hereditary Defects of the Conotruncal Septum in Keeshond Dogs: Pathologic and Genetic Studies. Am. J. Cardiol. 1974, 34, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Van Mierop, L.H.S.; Patterson, D.F.; Schnarr, W.R. Hereditary Conotruncal Septal Defects in Keeshond Dogs: Embryologic Studies. Am. J. Cardiol. 1977, 40, 936–950. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.P. Echocardiographic Diagnosis of Congenital Membranous Ventricular Septal Aneurysm in the Dog and Cat. J. Am. Anim. Hosp. Assoc. 2005, 41, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Peddle, G.D.; Boger, L.; Van Winkle, T.J.; Oyama, M.A. Gerbode Type Defect and Third Degree Atrioventricular Block in Association with Bacterial Endocarditis in a Dog. J. Vet. Cardiol. 2008, 10, 133–139. [Google Scholar] [CrossRef]
- Sykes, J.E.; Kittleson, M.D.; Chomel, B.B.; MacDonald, K.A.; Pesavento, P.A. Clinicopathologic Findings and Outcome in Dogs with Infective Endocarditis: 71 Cases (1992–2005). J. Am. Vet. Med. Assoc. 2006, 228, 1735–1747. [Google Scholar] [CrossRef]
- Patterson, D.F.; Pexieder, T.; Schnarr, W.R.; Navratil, T.; Alaili, R. A Single Major-Gene Defect Underlying Cardiac Conotruncal Malformations Interferes with Myocardial Growth during Embryonic Development: Studies in the CTD Line of Keeshond Dogs. Am. J. Hum. Genet. 1993, 52, 388. [Google Scholar]
- Diez-Prieto, I.; García-Rodríguez, B.; Ríos-Granja, A.; Cano-Rábano, M.; Peña-Penabad, M.; García, C.P. Cardiac Conotruncal Malformations in a Family of Beagle Dogs. J. Small Anim. Pract. 2009, 50, 597–603. [Google Scholar] [CrossRef]
- Brown, W. Ventricular septal defects in the English springer spaniel. In Kirk’s Current Veterinary Therapy XII; Bonagura, J.D., Ed.; W B Saunders: Philadelphia, PA, USA, 1995; pp. 827–830. [Google Scholar]
- Wang, J.M.H.; Rai, R.; Carrasco, M.; Sam-Odusina, T.; Salandy, S.; Gielecki, J.; Zurada, A.; Loukas, M. An Anatomical Review of the Right Ventricle. Transl. Res. Anat. 2019, 17, 100049. [Google Scholar] [CrossRef]
- Kurosawa, H.; Becker, A.E. Atrioventricular Conduction in Congenital Heart Disease: Surgical Anatomy, 1st ed.; Springer Nature: Cham, Switzerland, 2012. [Google Scholar]
- Jacobs, J.P.; Burke, R.P.; Quintessenza, J.A.; Mavroudis, C. Congenital Heart Surgery Nomenclature and Database Project: Atrioventricular Canal Defect. Ann. Thorac. Surg. 2000, 69, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Becu, L.M.; Burchell, H.B.; Dushane, J.W.; Edwards, J.E.; Fontana, R.S.; Kirklin, J.W. Anatomic and Pathologic Studies in Ventricular Septal Defect. Circulation 1956, 14, 349–364. [Google Scholar] [CrossRef]
- WEIRICH, W.E.; BLEVINS, W.E. Ventricular Septal Defect Repair. Vet. Surg. 1978, 7, 2–7. [Google Scholar] [CrossRef]
- HAGLER, D.J.; EDWARDS, W.D.; SEWARD, J.B.; TAJIK, A.J. Standardized Nomenclature of the Ventricular Septum and Ventricular Septal Defects, With Applications for Two-Dimensional Echocardiography. Mayo Clin. Proc. 1985, 60, 741–752. [Google Scholar] [CrossRef]
- Van Praagh, R.; Geva, T.; Kreutzer, J. Ventricular Septal Defects: How Shall We Describe, Name and Classify Them? * Components of the Interventricular Septum. J. Am. Coll. Cardiol. 1989, 14, 1298–1307. [Google Scholar] [CrossRef] [PubMed]
- Soto, B.; Becker, A.E.; Moulaert, A.J.; Lie, J.T.; Anderson, R.H. Classification of Ventricular Septal Defects. Heart 1980, 43, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Soto, B.; Ceballos, R.; Kirklin, J.W. Ventricular Septal Defects: A Surgical Viewpoint. J. Am. Coll. Cardiol. 1989, 14, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Tidholm, A. Retrospective Study of Congenital Heart Defects in 151 Dogs. J. Small Anim. Pract. 1997, 38, 94–98. [Google Scholar] [CrossRef]
- Garncarz, M.; Parzeniecka-Jaworska, M.; Szaluś-Jordanow, O. Congenital Heart Defects in Dogs: A Retrospective Study of 301 Dogs. Med. Weter. 2017, 73, 651–656. [Google Scholar] [CrossRef]
- Baumgartner, C.; Glaus, T.M. Congenital Cardiac Diseases in Dogs: A Retrospective Analysis. Schweiz. Arch. Tierheilkd. 2003, 145, 527–533, 535. [Google Scholar] [CrossRef]
- Lucina, S.B.; Sarraff, A.P.; Wolf, M.; Silva, V.B.C.; Sousa, M.G.; Froes, T.R. Congenital Heart Disease in Dogs: A Retrospective Study of 95 Cases. Top. Companion Anim. Med. 2021, 43, 100505. [Google Scholar] [CrossRef]
- Brambilla, P.G.; Polli, M.; Pradelli, D.; Papa, M.; Rizzi, R.; Bagardi, M.; Bussadori, C. Epidemiological Study of Congenital Heart Diseases in Dogs: Prevalence, Popularity, and Volatility throughout Twenty Years of Clinical Practice. PLoS ONE 2020, 15, e0230160. [Google Scholar] [CrossRef]
- Schrope, D.P. Prevalence of Congenital Heart Disease in 76,301 Mixed-Breed Dogs and 57,025 Mixed-Breed Cats. J. Vet. Cardiol. 2015, 17, 192–202. [Google Scholar] [CrossRef]
- Scurtu, I.; Tabaran, F.; Mircean, M.; Giurgiu, G.; Nagy, A.; Catoi, C.; Ohad, D.G. Combined Double Chambered Right Ventricle, Tricuspid Valve Dysplasia, Ventricular Septal Defect, and Subaortic Stenosis in a Dog. BMC Vet. Res. 2017, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Tou, S.P.; Keene, B.W.; Barker, P.C.A. Pulmonary Atresia and Ventricular Septal Defect with Aortopulmonary Collaterals in an Adult Dog. J. Vet. Cardiol. 2011, 13, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Tidholm, A.; Ljungvall, I.; Michal, J.; Häggström, J.; Höglund, K. Congenital Heart Defects in Cats: A Retrospective Study of 162 Cats (1996–2013). J. Vet. Cardiol. 2015, 17, S215–S219. [Google Scholar] [CrossRef] [PubMed]
- Peterson, O.; Torres, M.P.; White, R.; Manson, E.; Tropf, M.; Ward, J. Multimodal Imaging of Congenital Double-Inlet Left Ventricle with Hypoplastic Right Ventricle and Ventricular Septal Defect in a Young Cat. J. Vet. Cardiol. 2025, 59, 8–14. [Google Scholar] [CrossRef]
- Navalón, I.; Coromoto Verdugo, B.; Tursi, M. Tricuspid Atresia with Atrial and Ventricular Septal Defects in a Kitten. J. Vet. Cardiol. 2024, 51, 138–144. [Google Scholar] [CrossRef]
- Fox, P.R.; Sisson, D.D.; Moise, N.S. Textbook of Canine and Feline Cardiology: Principles and Clinical Practice, 2nd ed.; Saunders: Philadelphia, PA, USA, 1999. [Google Scholar]
- Mazurek, Ł.; Kalinowksi, M.; Szadkowski, M.; Pisarek, M.; Adaszek, Ł. Ventricular septal defect in cats: A retrospective study. Med. Wet. 2025, 81, 115–118. [Google Scholar]
- Schrope, D.P. Atrioventricular Septal Defects: Natural History, Echocardiographic, Electrocardiographic, and Radiographic Findings in 26 Cats. J. Vet. Cardiol. 2013, 15, 233–242. [Google Scholar] [CrossRef]
- Calkoen, E.E.; Hazekamp, M.G.; Blom, N.A.; Elders, B.B.L.J.; Gittenberger-De Groot, A.C.; Haak, M.C.; Bartelings, M.M.; Roest, A.A.W.; Jongbloed, M.R.M. Atrioventricular Septal Defect: From Embryonic Development to Long-Term Follow-Up. Int. J. Cardiol. 2016, 202, 784–795. [Google Scholar] [CrossRef]
- Amin, Z.; Gu, X.; Berry, J.M.; Bass, J.L.; Titus, J.L.; Urness, M.; Han, Y.M.; Amplatz, K. New Device for Closure of Muscular Ventricular Septal Defects in a Canine Model. Circulation 1999, 100, 320–328. [Google Scholar] [CrossRef]
- Ramírez, G.A.; De Los Espinosa Monteros, A.; Rodríguez, F.; Weisbrode, S.E.; Jaber, J.R.; Herráez, P. Left Ventricular Outflow Tract-Right Atrial Communication (Gerbode Type Defect) Associated with Bacterial Endocarditis in a Dog. Vet. Pathol. 2003, 40, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Gardner, L.; Silva, J.; Novo Matos, J. Spontaneous Closure of a Traumatic Acquired Gerbode Defect in a Dog. J. Vet. Cardiol. 2022, 41, 194–198. [Google Scholar] [CrossRef]
- Maneval, K.L.; Winter, R.L.; Hlusko, K.C.; Rajeev, M. Thoracic Trauma Causing an Acquired Gerbode Defect, Aortic Sinus Rupture, and Third-Degree Atrioventricular Block With Secondary Endocarditis in a Dog. CASE 2024, 8, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Wasserman, S.M.; Fann, J.I.; Atwood, J.E.; Burdon, T.A.; Fadel, B.M. Acquired Left Ventricular-Right Atrial Communication Gerbode-Type Defect. Echocardiography 2002, 19, 67–72. [Google Scholar] [CrossRef]
- Vörös, K.; Seehusen, F.; Hungerbühler, S.; Meyer-Lindenberg, A.; von der Hoeh, N. Ventricular Septal Defect with Aortic Valve Insufficiency in a New Zealand White Rabbit. J. Am. Anim. Hosp. Assoc. 2011, 47, e42–e49. [Google Scholar] [CrossRef]
- Di Girolamo, N.; Critelli, M.; Zeyen, U.; Selleri, P. Ventricular Septal Defect in a Ferret (Mustela Putorius Furo). J. Small Anim. Pract. 2012, 53, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Westcott, C.E.; Markovic, L.E.; Folmar, C.N.; Ryan, C.A.; Hammond, H.K. Multimodal Imaging Diagnosis of Doubly Committed Juxta-Arterial Ventricular Septal Defect and Persistent Left Cranial Vena Cava in a Goat. CASE 2024, 8, 395–400. [Google Scholar] [CrossRef]
- Buczinski, S.; Fecteau, G.; DiFruscia, R. Ventricular Septal Defects in Cattle: A Retrospective Study of 25 Cases. Can. Vet. J. 2006, 47, 246. [Google Scholar]
- De Lange, L.; Vera, L.; Decloedt, A.; Van Steenkiste, G.; Vernemmen, I.; van Loon, G. Prevalence and Characteristics of Ventricular Septal Defects in a Non-Racehorse Equine Population (2008–2019). J. Vet. Intern. Med. 2021, 35, 1573–1581. [Google Scholar] [CrossRef]
- Shimamura, S.; Kutsuna, H.; Shimizu, M.; Kobayashi, M.; Hirao, H.; Tanaka, R.; Takashima, K.; Machida, N.; Yamane, Y. Comparison of Right Atrium Incision and Right Ventricular Outflow Incision for Surgical Repair of Membranous Ventricular Septal Defect Using Cardiopulmonary Bypass in Dogs. Vet. Surg. 2006, 35, 382–387. [Google Scholar] [CrossRef]
- Rausch, W.P.; Keene, B.W. Spontaneous Resolution of an Isolated Ventricular Septal Defect in a Dog. J. Am. Vet. Med. Assoc. 2003, 223, 197, 219–220. [Google Scholar] [CrossRef] [PubMed]
- van de Watering, A.; Szatmári, V. Spontaneous Closure of an Isolated Congenital Perimembranous Ventricular Septal Defect in Two Dogs. BMC Vet. Res. 2022, 18, 162. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeong, H.; Lee, S. Spontaneous closure of perimembranous ventricular septal defect in a cat. J. Vet. Res. 2023, 63, 36. [Google Scholar] [CrossRef]
- Breznock, E.M. Spontaneous Closure of Ventricular Septal Defects in the Dog. J. Am. Vet. Med. Assoc. 1973, 162, 399–403. [Google Scholar]
- Yun, S.; Kim, B.; Youn, H.; Choi, M.; Yoon, J. Right-to-left shunting ventricular septal defect in a dog. J. Vet. Clin. 2015, 32, 343–346. [Google Scholar] [CrossRef]
- Greet, V.; Bode, E.F.; Dukes-McEwan, J.; Oliveira, P.; Connolly, D.J.; Sargent, J. Clinical Features and Outcome of Dogs and Cats with Bidirectional and Continuous Right-to-Left Shunting Patent Ductus Arteriosus. J. Vet. Intern. Med. 2021, 35, 780–788. [Google Scholar] [CrossRef]
- van Loon, A.C.J.; Locquet, L.J.N.; Bosseler, L.; Mortier, F.; Paepe, D.; Smets, P.M.Y. Infective Vegetative Endocarditis of the Mitral, Aortic, and Pulmonary Valves Due to Enterococcus Hirae in a Cat with a Ventricular Septal Defect. J. Vet. Cardiol. 2020, 30, 69–76. [Google Scholar] [CrossRef]
- Winter, R.L.; Gordon, S.G.; Zhang, S.; Hariu, C.D.; Miller, M.W. Mural Endocarditis Caused by Corynebacterium Mustelae in a Dog With a VSD. J. Am. Anim. Hosp. Assoc. 2014, 50, 366–372. [Google Scholar] [CrossRef]
- Quintavalla, C.; Mavropoulou, A.; Buratti, E. Aortic Endocarditis Associated with a Perforated Septal Membranous Aneurysm in a Boxer Dog. J. Small Anim. Pract. 2007, 48, 330–334. [Google Scholar] [CrossRef]
- Ploegaert, W.W.M. Review and Retrospective Study of Ventricular Septal Defect in Dogs. Master’s Thesis, Utrecht University, Utrecht, The Nederland, 2014. [Google Scholar]
- Yilmaz, Z.; Levent, P.; Saril, A.; Uemura, A.; Kocatürk, M.; Tanaka, R. Ventricular Septal Defect and Pulmonic Stenosis in a Dog (Bir Köpekte Ventriküler Septal Defekt ve Pulmonik Stenozis). Kafkas Univ Vet Fak Derg 2019, 25, 729–730. [Google Scholar] [CrossRef]
- MacDonald, K.A. Congenital Heart Diseases of Puppies and Kittens. Vet. Clin. North. Am. Small Anim. Pract. 2006, 36, 503–531. [Google Scholar] [CrossRef] [PubMed]
- Piantedosi, D.; Cortese, L.; Meomartino, L.; Di Loria, A.; Ciaramella, P. Situs Inversus Totalis Associated with Subaortic Stenosis, Restrictive Ventricular Septal Defect, and Tricuspid Dysplasia in an Adult Dog. Can. Vet. J. 2011, 52, 1237. [Google Scholar]
- Schrope, D. Acquired Infundibular Pulmonary Stenosis Associated with a Congenital Membranous Ventricular Septal Defect (Gasul Phenomenon) in a Dog and Discussion Regarding Causes of Infundibular Stenosis. J. Vet. Cardiol. 2023, 47, 64–69. [Google Scholar] [CrossRef]
- Galie, N.; Manes, A.; Palazzini, M.; Negro, L.; Marinelli, A.; Gambetti, S.; Mariucci, E.; Donti, A.; Branzi, A.; Picchio, F.M. Management of Pulmonary Arterial Hypertension Associated with Congenital Systemic-to-Pulmonary Shunts and Eisenmenger’s Syndrome. Drugs 2008, 68, 1049–1066. [Google Scholar] [CrossRef] [PubMed]
- Daliento, L.; Somerville, J.; Presbitero, P.; Menti, L.; Brach-Prever, S.; Rizzoli, G.; Stone, S. Eisenmenger Syndrome. Factors Relating to Deterioration and Death. Eur. Heart J. 1998, 19, 1845–1855. [Google Scholar] [CrossRef]
- Kozlik-Feldmann, R.; Hansmann, G.; Bonnet, D.; Schranz, D.; Apitz, C.; Michel-Behnke, I. Pulmonary Hypertension in Children with Congenital Heart Disease (PAH-CHD, PPHVD-CHD). Expert Consensus Statement on the Diagnosis and Treatment of Paediatric Pulmonary Hypertension. The European Paediatric Pulmonary Vascular Disease Network, Endorsed by ISHLT and DGPK. Heart 2016, 102, ii42–ii48. [Google Scholar] [CrossRef] [PubMed]
- Beijerink, N.J. Congenital Heart Disease. In Textbook of Veterinary Internal Medicine; Ettinger, S.J., Feldman, E.C., Cote, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 2952–3032. [Google Scholar]
- Pacó, T.R.; Rocha, C.C.; de Souza, Z.J.; de Paula Antunes, J.M.A. Echocardiographic Diagnosis of Interventricular Septum Defect with Eisenmenger Syndrome in an Adult Dog—Case Report. Acta Vet. Bras. 2022, 16, 5. [Google Scholar] [CrossRef]
- Bruno, B.; Savarino, P.; Bussadori, C.; Degiovanni, A.; Lardone, E.; Bertero, A.; Tarducci, A. Case Report: Eisenmenger Syndrome in a Dog with Ventricular Septal Defect: Long Term Management and Complications. Front. Vet. Sci. 2024, 11, 1393919. [Google Scholar] [CrossRef]
- Chowdhury, D. Pathophysiology of Congenital Heart Diseases. Ann. Card. Anaesth. 2007, 10, 19–26. [Google Scholar] [CrossRef]
- Sommer, R.J.; Hijazi, Z.M.; Rhodes, J.F. Pathophysiology of Congenital Heart Disease in the Adult Part I: Shunt Lesions. Circulation 2008, 117, 1090–1099. [Google Scholar] [CrossRef]
- Houston, A.B.; Lim, M.K.; Doig, W.B.; Reid, J.M.; Coleman, E.N. Doppler Assessment of the Interventricular Pressure Drop in Patients with Ventricular Septal Defects. Br. Heart J. 1988, 60, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Stout, K.K.; Daniels, C.J.; Aboulhosn, J.A.; Bozkurt, B.; Broberg, C.S.; Colman, J.M.; Crumb, S.R.; Dearani, J.A.; Fuller, S.; Gurvitz, M.; et al. 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 73, 1494–1563. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Relan, J.; Agarwal, R.; Awasthy, N.; Azad, S.; Chakrabarty, M.; Dagar, K.; Devagourou, V.; Dharan, B.; Gupta, S.; et al. Indian Guidelines for Indications and Timing of Intervention for Common Congenital Heart Diseases: Revised and Updated Consensus Statement of the Working Group on Management of Congenital Heart Diseases. Ann. Pediatr. Cardiol. 2019, 12, 254–286. [Google Scholar] [CrossRef] [PubMed]
- Serres, F.; Chetboul, V.; Tissier, R.; Gouni, V.; Desmyter, A.; Sampedrano, C.C.; Pouchelon, J.L. Quantification of Pulmonary to Systemic Flow Ratio by a Doppler Echocardiographic Method in the Normal Dog: Repeatability, Reproducibility, and Reference Ranges. J. Vet. Cardiol. 2009, 11, 23–29. [Google Scholar] [CrossRef]
- Sisson, D.; Luethy, M.; Thomas, W.P. Ventricular Septal Defect Accompanied by Aortic Regurgitation in Five Dogs. J. Am. Anim. Hosp. Assoc. 1991, 27, 441–448. [Google Scholar]
- Reinero, C.; Visser, L.C.; Kellihan, H.B.; Masseau, I.; Rozanski, E.; Clercx, C.; Williams, K.; Abbott, J.; Borgarelli, M.; Scansen, B.A. ACVIM Consensus Statement Guidelines for the Diagnosis, Classification, Treatment, and Monitoring of Pulmonary Hypertension in Dogs. J. Vet. Intern. Med. 2020, 34, 549–573. [Google Scholar] [CrossRef]
- Bodh, D.; Hoque, M.; Saxena, A.C.; Gugjoo, M.B.; Bist, D.; Chaudhary, J.K. Vertebral Scale System to Measure Heart Size in Thoracic Radiographs of Indian Spitz, Labrador Retriever and Mongrel Dogs. Vet. World 2016, 9, 371. [Google Scholar] [CrossRef]
- Kim, Y.; Kwon, D.; Kim, S.S.; Lee, K.; Yoon, H. Echocardiographic Changes in the Progress of Reverse Shunt and Improvement to Left-to-Right Shunt after Medical Treatment in Dogs with Bidirectional Patent Ductus Arteriosus or Ventricular Septal Defect: A Report of Two Cases. Vet. Med. Sci. 2023, 9, 1044–1052. [Google Scholar] [CrossRef]
- Orton, C.E. Congenital Septal Defect. In Small Animal Thoracic Surgery, 1st ed.; Orton, C.E., Monet, E., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2017; pp. 211–244. [Google Scholar]
- Sreeram, N.; Jagtap, R.; Silove, E.D.; Brawn, W.J.; Sethia, B. Intraoperative Epicardial Echocardiography in Assessing Pulmonary Artery Banding Procedures. Ann. Thorac. Surg. 1995, 60, 1778–1782. [Google Scholar] [CrossRef]
- Nakayama, T.; Wakao, Y.; Uechi, M.; Muto, M.; Kageyama, T.; Tanaka, K.; Kawabata, M.; Takahashi, M. A Case Report of Surgical Treatment of a Dog with Atrioventricular Septal Defect (Incomplete Form of Endocardial Cushion Defect). J. Vet. Med. Sci. 1994, 56, 981–984. [Google Scholar] [CrossRef]
- Mihara, K.; Kanemoto, I.; Ando, T.; Kawase, K.; Iguchi, K.; Yokoyama, S.; Asai, A.; Hoshi, K. Simultaneous Surgical Repair of a Cardiac Myxoma Causing Left Ventricular Outflow Tract Obstruction and a Ventricular Septal Defect in a Small Dog. Open Vet. J. 2024, 14, 743. [Google Scholar] [CrossRef]
- Mampaey, G.; Bové, T.; De Somer, F.; Devriendt, N.; Bouchez, S.; Bosmans, T.; Stammeleer, L.; Panzer, J.; Hellemans, A.; Smets, P. Surgical Correction of an Infundibular Pulmonic Stenosis and Ventricular Septal Defect in a Shetland Sheepdog. J. Vet. Cardiol. 2023, 49, 29–37. [Google Scholar] [CrossRef]
- Butera, G.; Carminati, M.; Chessa, M.; Piazza, L.; Micheletti, A.; Negura, D.G.; Abella, R.; Giamberti, A.; Frigiola, A. Transcatheter Closure of Perimembranous Ventricular Septal Defects: Early and Long-Term Results. J. Am. Coll. Cardiol. 2007, 50, 1189–1195. [Google Scholar] [CrossRef]
- Bacha, E.A.M.; Hijazi, Z.M. Hybrid Procedures in Pediatric Cardiac Surgery. Semin. Thorac. Cardiovasc. Surg. Pediatr. Card. Surg. Annu. 2005, 8, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Trehan, V.; Kumar, A.; Kalra, G.S.; Nigam, M. Transcatheter Closure of Congenital Ventricular Septal Defects: Experience with Various Devices. J. Interv. Cardiol. 2003, 16, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Saxena, A.; Choudhary, S.K. Residual VSD Closure with an ADO II Device in an Infant. Congenit. Heart Dis. 2011, 6, 60–63. [Google Scholar] [CrossRef] [PubMed]
- Predescu, D.; Chaturvedi, R.R.; Friedberg, M.K.; Benson, L.N.; Ozawa, A.; Lee, K.J. Complete Heart Block Associated with Device Closure of Perimembranous Ventricular Septal Defects. J. Thorac. Cardiovasc. Surg. 2008, 136, 1223–1228. [Google Scholar] [CrossRef]
- Yang, L.; Tai, B.C.; Khin, L.W.; Quek, S.C. A Systematic Review on the Efficacy and Safety of Transcatheter Device Closure of Ventricular Septal Defects (VSD). J. Interv. Cardiol. 2014, 27, 260–272. [Google Scholar] [CrossRef]
- Haddad, R.N.; Daou, L.; Saliba, Z. Device Closure of Perimembranous Ventricular Septal Defect: Choosing between Amplatzer Occluders. Front. Pediatr. 2019, 7, 461777. [Google Scholar] [CrossRef]
- Sadiq, M.; Qureshi, A.U.; Younas, M.; Arshad, S.; Hyder, S.N. Percutaneous Closure of Ventricular Septal Defect Using LifeTech TM Konar-MF VSD Occluder: Initial and Short-Term Multi-Institutional Results. Cardiol. Young 2022, 32, 755–761. [Google Scholar] [CrossRef]
- Ece, İ.; Bağrul, D.; Kavurt, A.V.; Terin, H.; Torun, G.; Koca, S.; Gül, A.E.K. Transcatheter Ventricular Septal Defect Closure with LifetechTM Konar-MF Occluder in Infants Under 10 Kg with Only Using Venous Access. Pediatr. Cardiol. 2023, 45, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kong, P.; Liu, X.; Feng, S.; Ouyang, W.; Wang, S.; Hu, X.; Xie, Y.; Zhang, F.; Zhang, Y.; et al. A Fully Biodegradable Polydioxanone Occluder for Ventricle Septal Defect Closure. Bioact. Mater. 2023, 24, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Bu, H.; Yang, Y.; Hu, S.; Wu, Q.; Gong, X.; Zhao, T. A Novel Biodegradable Occluder for the Closure of Ventricular Septal Defects: Immediate and Medium-Term Results in a Canine Model. Interact. Cardiovasc. Thorac. Surg. 2019, 29, 783–792. [Google Scholar] [CrossRef]
- Guo, G.; Hu, J.; Wang, F.; Fu, D.; Luo, R.; Zhang, F.; Hu, C.; Chen, J.; Pan, X.; Yang, L.; et al. A Fully Degradable Transcatheter Ventricular Septal Defect Occluder: Towards Rapid Occlusion and Post-Regeneration Absorption. Biomaterials 2022, 291, 121909. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, L.; Liu, Y.; Leng, J. Recent Developments in Next-Generation Occlusion Devices. Acta Biomater. 2021, 128, 100–119. [Google Scholar] [CrossRef]
- Gordon, S.G.; Miller, M.W.; Roland, R.M.; Saunders, A.B.; Achen, S.E.; Drourr, L.T.; Nelson, D.A. Transcatheter Atrial Septal Defect Closure with the AMPLATZER® Atrial Septal Occluder in 13 Dogs: Short- and Mid-Term Outcome. J. Vet. Intern. Med. 2009, 23, 995–1002. [Google Scholar] [CrossRef]
- Gordon, S.G.; Nelson, D.A.; Achen, S.E.; Miller, M.M.; Roland, R.M.; Saunders, A.B.; Drourr, L.T. Open Heart Closure of an Atrial Septal Defect by Use of an Atrial Septal Occluder in a Dog. J. Am. Vet. Med. Assoc. 2010, 236, 434–439. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Sohn, J.-H.; Kim, J.; Hyun, C. Successful Interventional Occlusion of Muscular Ventricular Septal Defect in a Dog. Can. Vet. 2024, 65, 221–226. [Google Scholar]

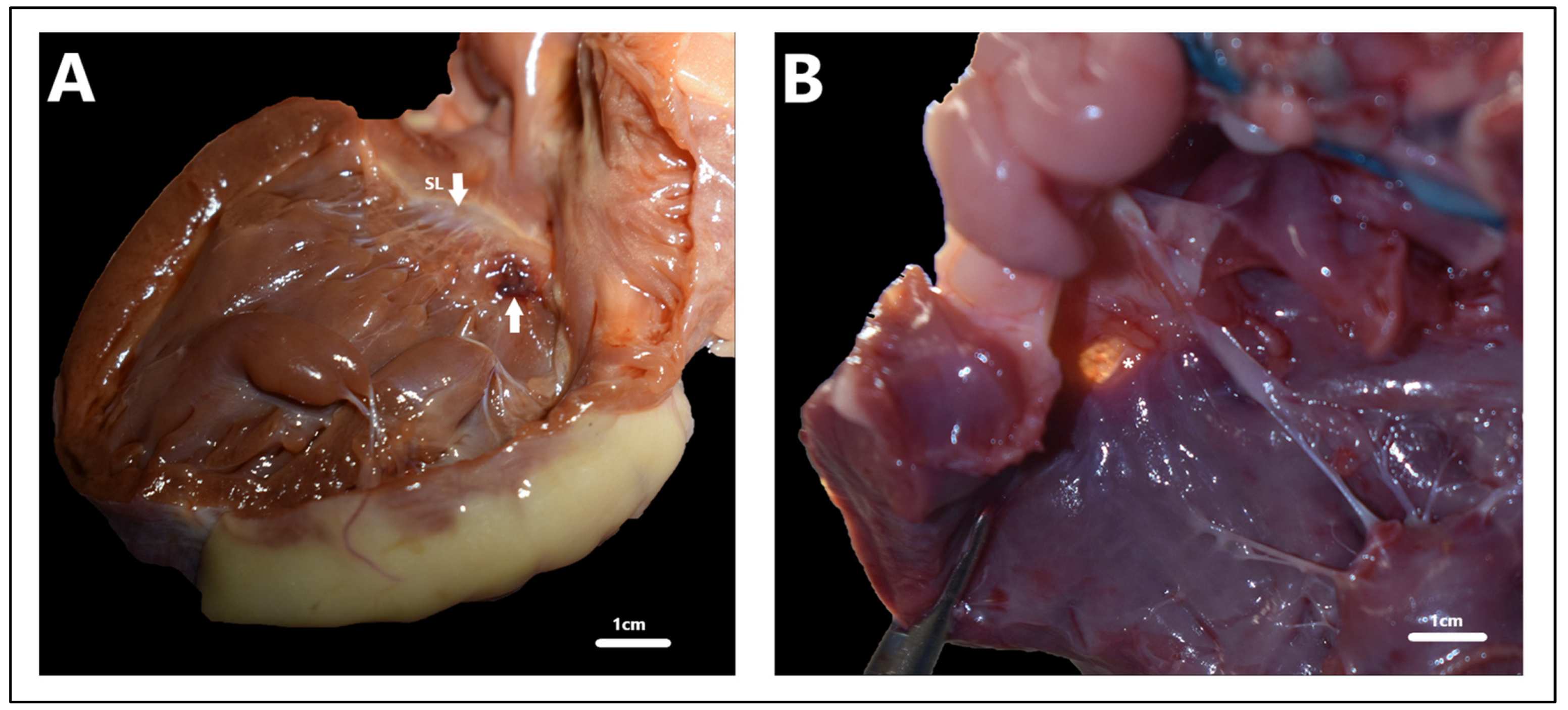
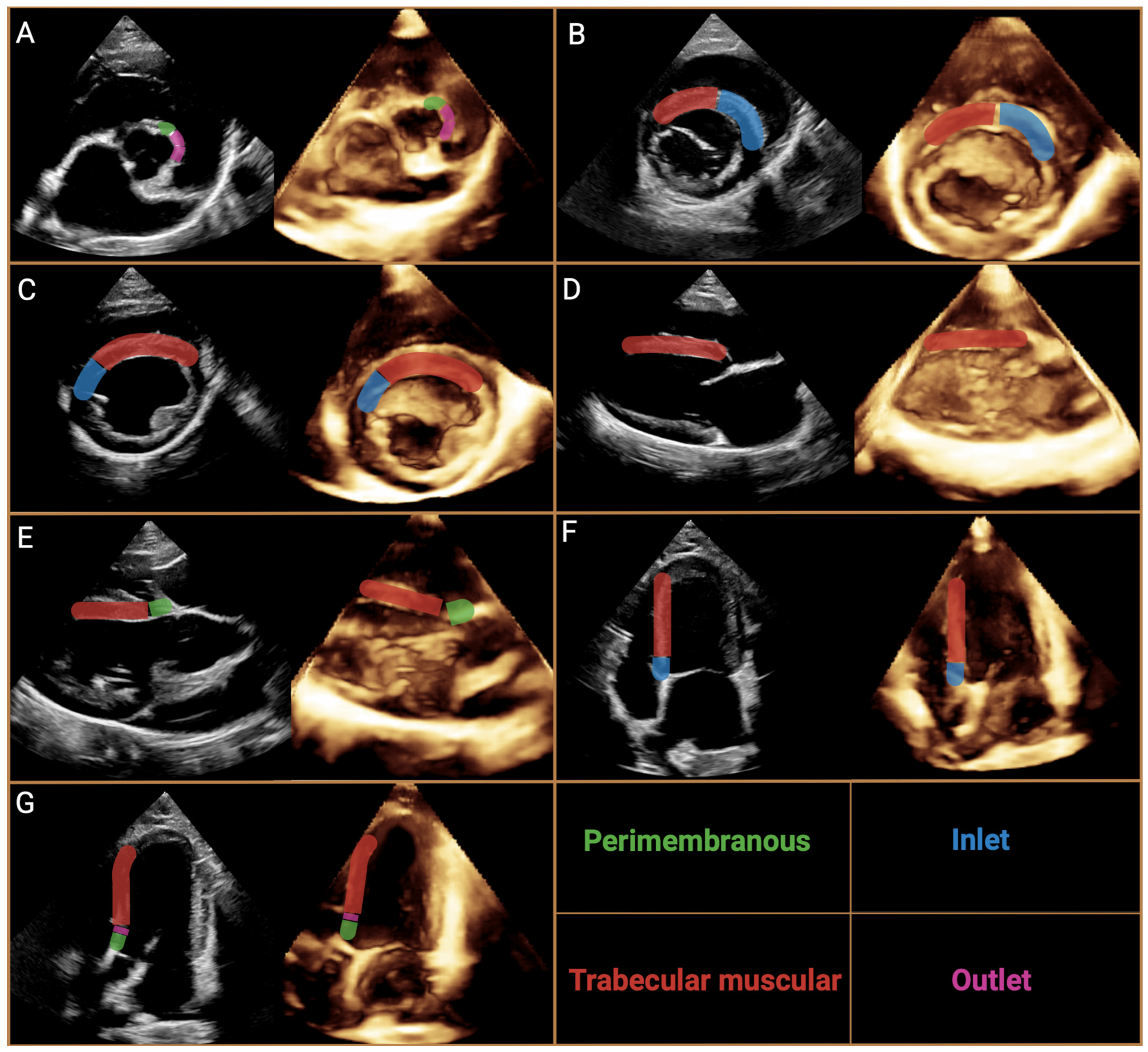
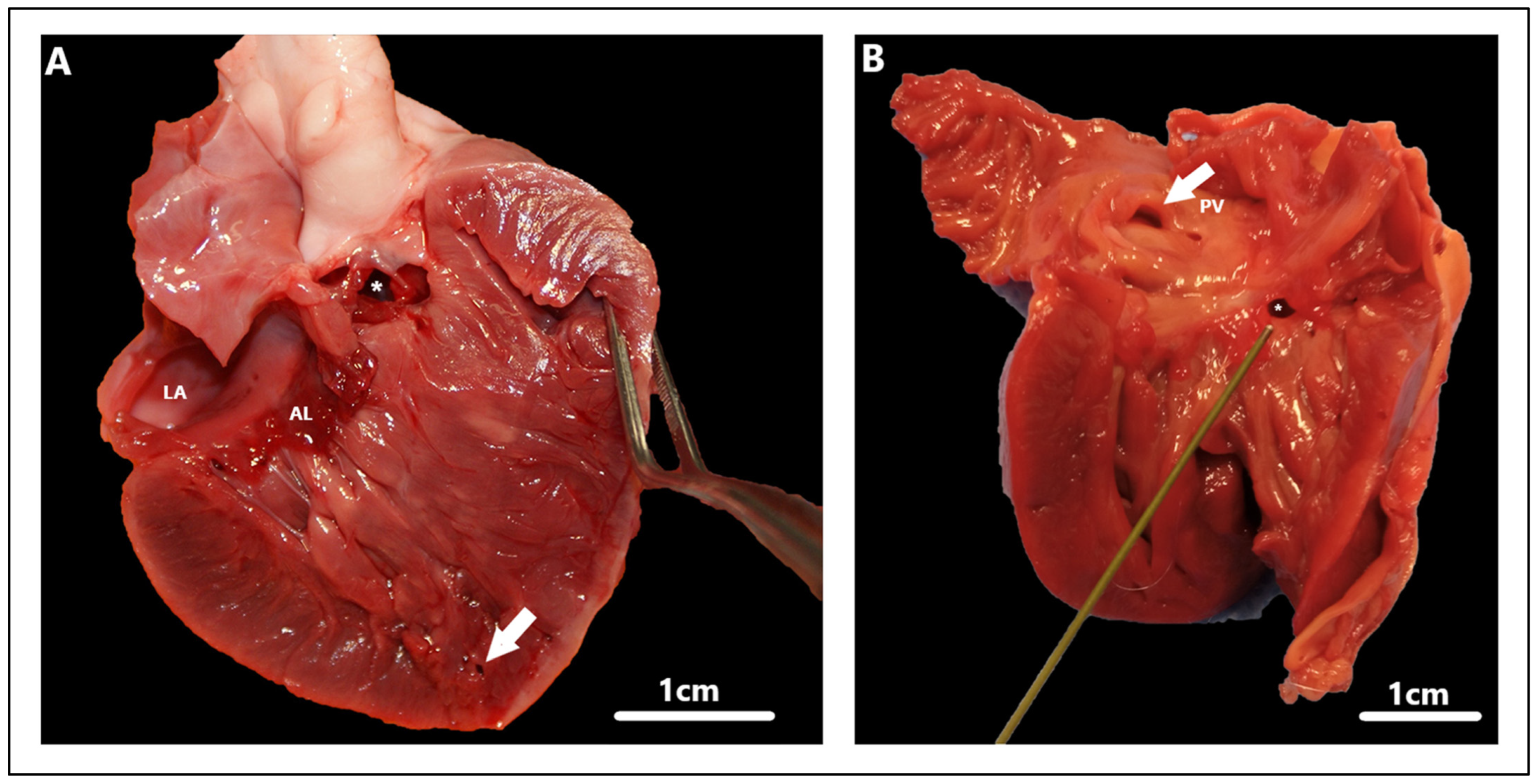
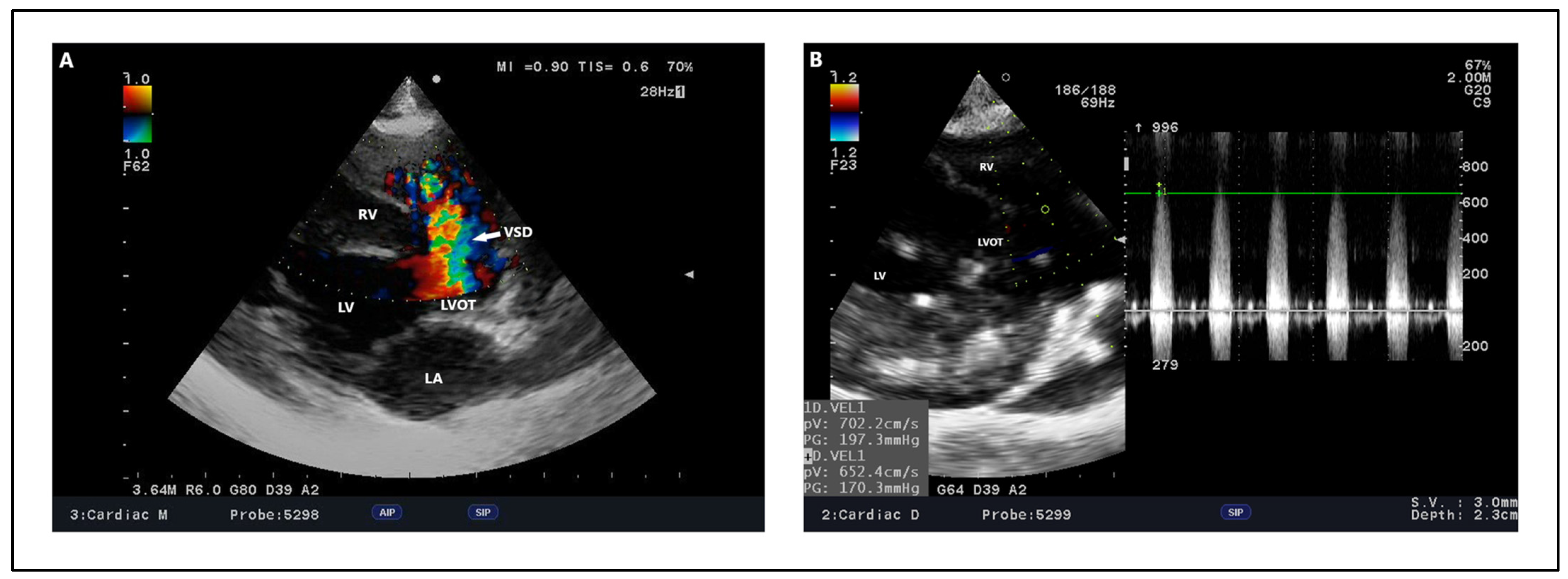
| Approach | Author | VSD Classification | Description | |
|---|---|---|---|---|
| Geographic | [74] | Related to ventricular outflow tracts | Ventricular septal defect is located anteriorly from the supraventricular crest involving the fibrous annulus region of the pulmonary valve and the aortic valve. The second is located posterior to the supraventricular crest involving the membranous portion of the interventricular septum and adjacent septal muscular portion | |
| Not related to ventricular outflow tracts | Ventricular septal defect associated with the muscular portion of the inflow ventricular septum and defects not associated with any of the atrioventricular valves | |||
| [75] | Located above supraventricular crest—“supracristal” | Ventricular septal defect located above the supraventricular crest, i.e., in the ventricular outflow tract | ||
| Located beneath supraventricular crest—“infracristal” | Ventricular septal defect located below the supraventricular crest, i.e., within the inflow tract of the ventricle | |||
| Multiple muscular | Ventricular septal defect involving different localizations of the muscular portion of the interventricular septum | |||
| [76] | Membranous septum | Membranous | Ventricular septal defect around the membranous part of the interventricular septum, often extended into the adjacent myocardium. Cradled by the limbs of a septal band and roofed by an infundibular septum. Malalignment between infundibular and ventricular septa with overriding of at least one semilunar valve may occur | |
| Perimembranous | ||||
| Paramembranous | ||||
| Inlet and trabecular | Muscular | Ventricular septal defect involves the basal part of the interventricular septum at the inflow region. Its posterior surface is the fibrous junction between the mitral and tricuspid valves. It may be associated with lateral or rotational (or both) malalignment between atrial and ventricular septa. Malalignment may be associated with overriding or straddling (or both) of either atrioventricular valve | ||
| Muscular inlet | ||||
| Muscular trabecular | ||||
| Infundibular septum | Infundibular | Ventricular septal defect in the region of the infundibular septum with borders completely muscular or partially limited by the leaflets of the semilunar valves. | ||
| Outlet | ||||
| Supraristal | ||||
| [77] | Atrioventricular canal type | The septum of atrioventricular canal is completely absent with straddling of the septal leaflet of the tricuspid valve. | ||
| Muscular | Openings in the muscular ventricular septum, above and below the septal band | |||
| Conoventricular | Ventricular septal defect lying between the conal septum and the ventricular septum. Partially encompassing the membranous portion of the ventricular septum and extending to the infundibular septum | |||
| Conal | Ventricular septal defect just at the orifice of the great vessels | |||
| Borders | [78] | Perimembranous | Inlet | Ventricular septal defect in the portion of the membranous part with extension to the inflow region |
| Trabecular | Ventricular septal defect in the portion of the membranous part with extension towards the apex of the heart | |||
| Infundibular | Ventricular septal defect in the portion of the membranous part with extension towards the infundibulum and prolapse (overriding) of the non-coronary leaflet of the aortic valve | |||
| Muscular | Posterior (inlet) | Ventricular septal defect below the septal leaflet of the tricuspid valve but with entirely muscular rims | ||
| Trabecular | Ventricular septal defect around septal–marginal trabeculae, usually multiple | |||
| Infundibular | Ventricular septal defect opening into outflow tract with completely muscular rims without prolapsing non-coronary valve leaflet of the aorta | |||
| Subarterial infundibular | Ventricular septal defect in the infundibulum septal area where the aortic and pulmonary artery valves formed its upper border | |||
| [79] | Conoventricular | Ventricular septal defect in the area of the mid portion of right ventricle and outlet portion of left ventricle. They can be juxtatricuspid, juxtamitral, or juxtaaortic. They may also be associated with the membranous part of the septum. Sometimes there are posteriori with leftward malalignment of the conal septum | ||
| In RV outlet | Ventricular septal defect in the outflow portion area of left ventricle and right ventricle. They can be juxtapulmonary, juxtaaortic (with possible aortic valve leaflet prolapse), juxtaarterial or with completely muscular borders | |||
| Inlet septal | Ventricular septal defect in the area of the inflow portion to the ventricle. They could be juxtatricuspid or juxtamitral, associated with the membranous part of the interventricular septum or with completely muscular rims. Sometimes associated with malalignment of the atrial to ventricular septum | |||
| Trabecular | Ventricular septal defect in the anterior, middle or apical region | |||
| Hybrid | [73] | Type 1—subarterial, supracristal, conal, infundibular | Ventricular septal defect in the ventricular outflow tract area or conal septum below the semilunar valves | |
| Type 2—perimembranous, paramembranous, conoventricular | Ventricular septal defect associated with the membranous part of the septum bounded by atrioventricular valves, excluding cases classified as type 3 | |||
| Type 3—inlet, AV canal type | Ventricular septal defect of the inflow portion to the right ventricle, located just below the atrioventricular valve apparatus | |||
| Type 4—Muscular | Ventricular septal defect with completely muscular borders unrelated to previous types. They may be multiple. | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graczyk, S.; Grzeczka, A.; Pasławska, U. A Comprehensive Review of Canine and Feline Ventricular Septal Defects—From Pathogenesis to Long-Term Follow-Up. Animals 2025, 15, 850. https://doi.org/10.3390/ani15060850
Graczyk S, Grzeczka A, Pasławska U. A Comprehensive Review of Canine and Feline Ventricular Septal Defects—From Pathogenesis to Long-Term Follow-Up. Animals. 2025; 15(6):850. https://doi.org/10.3390/ani15060850
Chicago/Turabian StyleGraczyk, Szymon, Arkadiusz Grzeczka, and Urszula Pasławska. 2025. "A Comprehensive Review of Canine and Feline Ventricular Septal Defects—From Pathogenesis to Long-Term Follow-Up" Animals 15, no. 6: 850. https://doi.org/10.3390/ani15060850
APA StyleGraczyk, S., Grzeczka, A., & Pasławska, U. (2025). A Comprehensive Review of Canine and Feline Ventricular Septal Defects—From Pathogenesis to Long-Term Follow-Up. Animals, 15(6), 850. https://doi.org/10.3390/ani15060850






