Chlorella vulgaris as a Livestock Supplement and Animal Feed: A Comprehensive Review
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Searching Criteria
3. The Evolution and Global Trends of Chlorella vulgaris Production
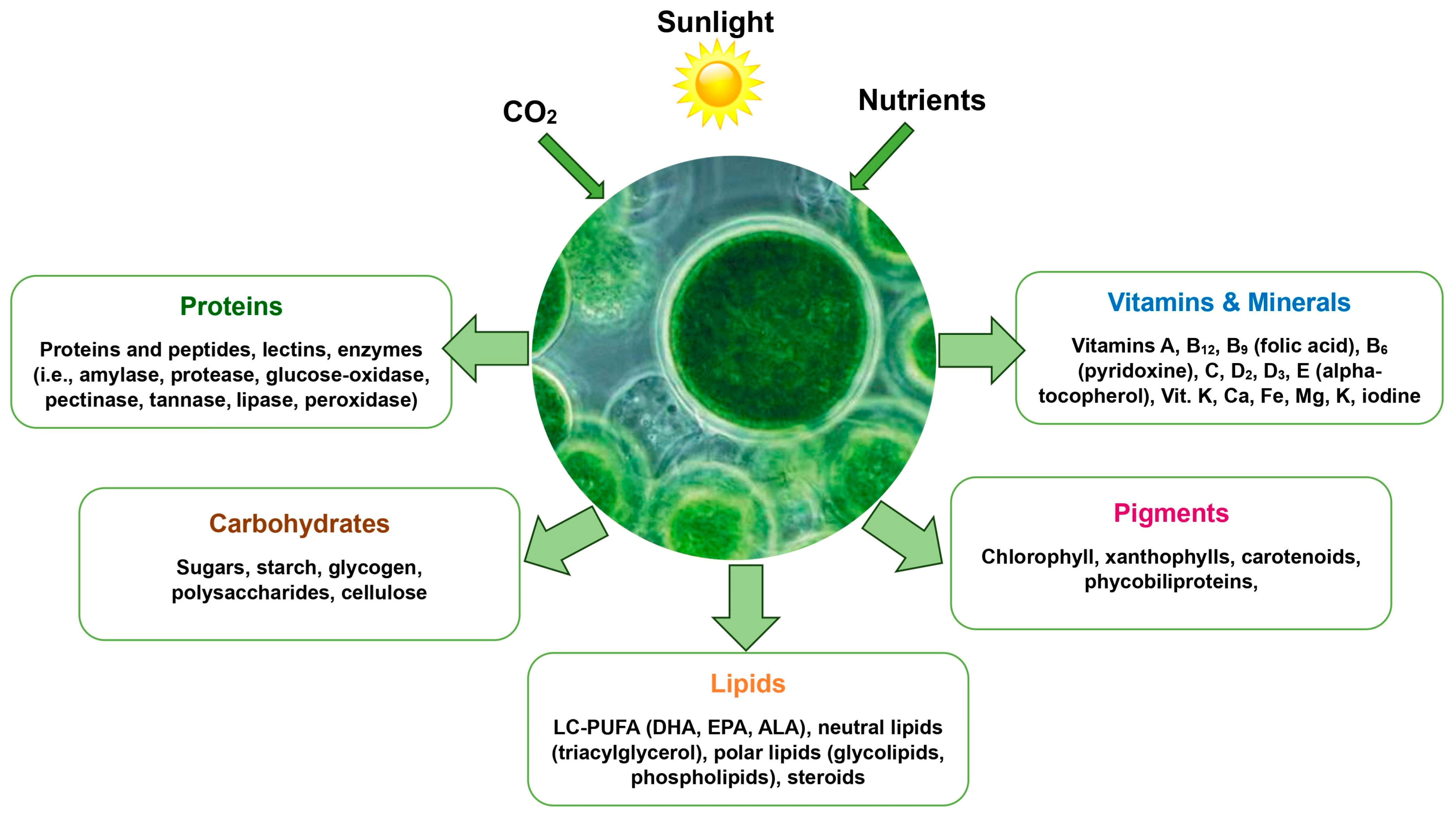
4. Chemical Composition of Chlorella vulgaris
| DM | OM | CP | EE | NDF | ADF | Ash | Energy | Reference |
|---|---|---|---|---|---|---|---|---|
| 93.1 | 81.3 | 42.8 | - | 32.2 | 19.4 | 11.8 | - | [3] |
| 93.1 | - | 46.0 | 9.4 | - | - | 12.7 | 4586 a | [51] |
| 95.5 | - | 22.7 | 14.2 | - | - | - | 434 b | [21] |
| 93.2 | 94.2 | 57.9 | 13.9 | 11.8 | 4.3 | - | - | [52] |
| 95.4 | - | 48.4 | 9.2 | 20.2 | - | 11.1 | 18.8 c | [44] |
| 94.6 | - | 60.8 | 9.5 | 0.0 | - | 5.7 | - | [53] |
| 94.2 | 90.5 | 51.5 | 12.2 | 9.2 | - | - | - | [22,23] |
| 92.7 | 84.8 | 67.7 | 10.5 | 12.8 | 4.2 | - | - | [50] |
| 94.6 | - | 60.6 | 12.8 | - | - | 4.5 | - | [54] |
| NR | - | 25.0 | 2.4 | - | - | - | - | [27] |
| 96.1 | - | 47.8 | 13.3 | - | - | 6.3 | 1427 d | [55] |
| Others | Amount | Reference | ||||||
| Starch | 4.4 | [44] | ||||||
| 4.3 | [53] | |||||||
| CF | 8.8 | [3] | ||||||
| 13.0 | [54] | |||||||
| 5.4 | [27] | |||||||
| CHO | 8.1 | [55] | ||||||
| NSC | 10.6 | [52] | ||||||
| 11.9 | [22,23] |
4.1. Fatty Acid Profile of Chlorella vulgaris
4.2. Mineral Composition of Chlorella vulgaris
5. In Vitro Ruminal Fermentation Parameters
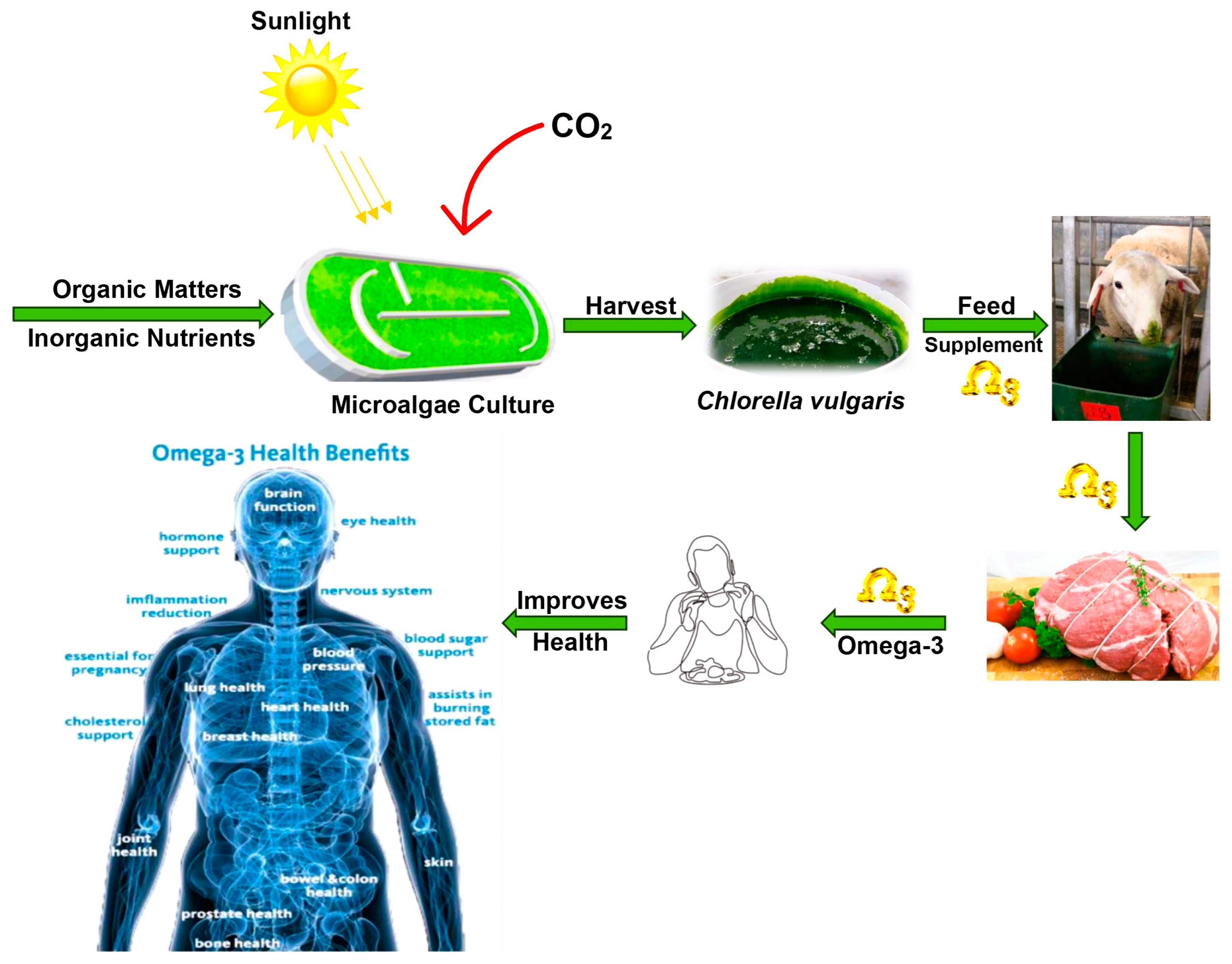
6. Chlorella vulgaris as Feed Supplement for Cattle
7. Impact of Chlorella vulgaris on Sheep
8. Chlorella vulgaris in Goat Diets
| Studies | CLV, g DM/d | Stage | Dose | Production | Ruminal Fermentation | Digestibility | Anti- Oxidant a | Milk FA | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DMI | ADG | MY | VFA | A:P | NH3-N | DM | NDF | ||||||
| [69] | 10 g (and/or 5 g N. sativa) | Pregnant | 10 g with 5 g N. sativa | - | - | +4% ↑ | - | +7% ↑ | - | +22% ↑ | |||
| [70] | 5 g (and/or 2 g Vit C) | Breeding buck | 5 g with 2 g Vit C | +27% ↑ | +10% ↑ | ||||||||
| [22] | 5 g and 10 g | Lactating | 10 g | +13% ↑ | +12% ↑ | ↑ | ↓ | ↓ | +3% ↑ | +5% ↑ | PUFA ↑ | ||
| [68] | 5 g, 10 g and 15 g (with CuSO4) | Lactating | 10 g with CuSO4 | - | +10% ↑ | ↑ | - | - | +10% ↑ | +9% ↑ | MUFA ↑ | ||
| [74] | 10 g | Lactating | - | ↓ | ω-6 ↑ | ||||||||
| [29] | 5 g and 10 g (dried) | Lactating | 10 g (Linear response) | PUFA ↑ ω-3 ↑ | |||||||||
9. The Effects of Chlorella vulgaris in Broiler Diets
| Summary of Main Findings | References |
|---|---|
| Broilers fed 1 g of CLV/kg diet for 32 d showed an increase in final body weight and weight gain as compared to control groups | [58] |
| Broilers fed 2.5% dried Chlorella vulgaris for 8 weeks had higher body weight in comparison to the control | [75] |
| Broilers fed with 0.8% CLV showed better final weight and feed conversion after 35 d than the control group | [21] |
| Birds fed Chlorella, in powder, Chlorella growth factor, or liquid form, gained more weight by 5 weeks of age than the control group, without affecting feed intake or efficiency | [76] |
| Chicks fed 0.05–0.5% Chlorella for 35 d had greater weight gains than the control group | [34] |
| Broilers fed CLV over 5 weeks had significantly higher body weight gain as compared to the control group | [77] |
| Broilers fed 1.0% E. coli fermented liquor with Chlorella showed a 2.6% higher weight gain and a 2.8% improved FCR than the control group | [78] |
| Broilers fed CLV over 5 weeks had similar feed intake and FCR | [77] |
| Broilers fed 10% CLV for 14 d showed similar growth and feed conversion rates, with or without added CAZymes | [79] |
| Broilers given 0, 10, or 20 g/kg of CLV had lower feed intake, yet their weight gain and FCR were similar | [26] |
| Birds on 15% and 20% CLV diets had reduced body weight, weight gain, and feed intake, unlike those on CLV10%, which were comparable to the control group | [51] |
| Birds fed with 15% and 20% CLV diets showed similar feed conversion ratios to the control group | [51] |
| Broilers receiving 1 g of CLV/kg of diet had similar feed intake and FCR as compared to the control groups | [58] |
| Adding 10% CLV and CAZymes to broiler diets did not significantly affect weight gain or feed efficiency | [83] |
| Chicks fed with 0.15% or 0.5% Chlorella or Chlorella growth factor showed increased serum IgG and IgM levels compared to the control group | [34] |
| Chicks fed 0.5% dried Chlorella powder had lower serum total lipid concentrations compared to the control | [34] |
| Broilers fed 0.8% CLV for 35 d showed reduced haptoglobin and interleukin-13 levels compared to the control | [21] |
| Supplementing broiler diets with 10 g/kg of Chlorella by-product enhances health, immunity, antioxidant capacity, and gut morphology | [26] |
10. Impact of Chlorella vulgaris on Meat Quality Parameters of Broiler Chickens
11. Impact on Laying Hen Performance and Egg Quality
12. Impact of Chlorella vulgaris on Pig
13. Chlorella vulgaris in Rabbit Diets
14. Impact of Chlorella vulgaris on Fish
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture: Trends and Challenges; FAO: Rome, Italy, 2017; Available online: https://openknowledge.fao.org/server/api/core/bitstreams/2e90c833-8e84-46f2-a675-ea2d7afa4e24/content (accessed on 10 December 2024).
- Gadzama, I.U.; Mugweru, I.M.; Makombe, W.S.; Madungwe, C.; Hina, Q.; Omofunmilola, E.O.; Panuel, P.; Olanrewaju, T.J.; Ray, S. Improving poultry production with black soldier fly larvae. Acta Sci. Agric. 2025, 9, 60–77. Available online: https://actascientific.com/ASAG/pdf/ASAG-08-1450.pdf (accessed on 16 March 2025).
- Martins, C.F.; Trevisi, P.; Coelho, D.F.; Correa, F.; Ribeiro, D.M.; Alfaia, C.M.; Pinho, M.; Pestana, J.M.; Mourato, M.P.; Almeida, A.M.; et al. Influence of Chlorella vulgaris on growth, digestibility and gut morphology and microbiota of weaned piglet. Sci. Rep. 2022, 12, 6012. [Google Scholar] [CrossRef] [PubMed]
- Spínola, M.P.; Costa, M.M.; Prates, J.A.M. Effect of Selected Mechanical/Physical Pre-Treatments on Chlorella vulgaris Protein Solubility. Agriculture 2023, 13, 1309. [Google Scholar] [CrossRef]
- Gadzama, I.U.; Hoffman, L.C.; Holman, B.; Chaves, A.V.; Meale, S.J. Effects of supplementing a feedlot diet with microalgae (Chlorella vulgaris) on the performance, carcass traits and meat quality of lambs. Livest. Sci. 2024, 288, 105552. [Google Scholar] [CrossRef]
- Gadzama, I.U.; Meale, S.J.; Chaves, A.V. Effect of microalgae on in vitro rumen fermentation, gas and methane production. In Proceedings of the Recent Advances in Animal Nutrition—Australia (RAAN-A) Conference, Gold Coast, QLD, Australia, 27–28 July 2023; Available online: https://www.researchgate.net/publication/383180460_Effect_of_microalgae_on_in_vitro_rumen_fermentation_gas_and_methane_production (accessed on 5 January 2025).
- Little, H.; Brown, R.M. The culture of Chlorella vulgaris in synthetic media of varying composition with special reference to heterotrophic growth on organic compounds. Ann. Bot. 1953, 17, 433–454. [Google Scholar]
- Montoya, J.D.; Weathers, P.J.; McCabe, J.G. Engineering challenges in high-density algal production: A critical review of existing methods and future needs. J. Appl. Phycol. 2014, 26, 1519–1538. [Google Scholar]
- Liu, J.; Chen, F. Biology and Industrial Applications of Chlorella: Advances and Prospects. Adv. Biochem. Eng. Biotechnol. 2016, 153, 1–35. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.-Y.; Vaca-Garcia, C. Morphology, composition, production, processing and applications of Chlorella vulgaris: A review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; MacPherson, T.; McGinn, P.J.; Fredeen, A.H. In vitro digestion of microalgal biomass from freshwater species isolated in Alberta, Canada for monogastric and ruminant animal feed applications. Algal Res. 2016, 19, 324–332. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef]
- Saadaoui, I.; Rasheed, R.; Aguilar, A.; Cherif, M.; Al Jabri, H.; Sayadi, S.; Manning, S.R. Microalgal-based feed: Promising alternative feedstocks for livestock and poultry production. J. Anim. Sci. Biotechnol. 2021, 12, 76. [Google Scholar] [CrossRef] [PubMed]
- Brennan, L.; Owende, P. Biofuels from microalgae- a review of technologies for production, processing, and extractions of biofuels and co-products. Renew. Sustain. Energy Rev. 2010, 14, 557–577. [Google Scholar] [CrossRef]
- Panahi, Y.; Yari Khosroushahi, A.; Sahebkar, A.; Heidari, H.R. Impact of Cultivation Condition and Media Content on Chlorella vulgaris Composition. Adv. Pharm. Bull. 2019, 9, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, M.J.; Harrison, S.T.L. Lipid productivity as a key characteristic for choosing algal species for biodiesel production. J. Appl. Phycol. 2009, 21, 493–507. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-algae as a source of protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Guccione, A.; Biondi, N.; Sampietro, G.; Rodolfi, L.; Bassi, N.; Tredici, M.R. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol. Biofuels 2014, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, I.A.; Elbaily, Z.I. A review: Importance of chlorella and different applications. Alex. J. Vet. Sci. 2020, 65, 16. [Google Scholar] [CrossRef]
- Roques, S.; Koopmans, S.-J.; Mens, A.; van Harn, J.; van Krimpen, M.; Kar, S.K. Effect of Feeding 0.8% Dried Powdered Chlorella vulgaris Biomass on Growth Performance, Immune Response, and Intestinal Morphology during Grower Phase in Broiler Chickens. Animals 2022, 12, 1114. [Google Scholar] [CrossRef]
- Kholif, A.E.; Elghandour, M.M.Y.; Salem, A.Z.M.; Barbabosa, A.; Márquez, O.; Odongo, N.E. The effects of three total mixed rations with different concentrate to maize silage ratios and different levels of microalgae Chlorella vulgaris on in vitro total gas, methane and carbon dioxide production. J. Agric. Sci. 2017, 115, 494–507. [Google Scholar] [CrossRef]
- Kholif, A.E.; Morsy, T.A.; Matloup, O.H.; Anele, U.Y.; Mohamed, A.G.; El-Sayed, A.B. Dietary Chlorella vulgaris microalgae improves feed utilization, milk production and concentrations of conjugated linoleic acids in the milk of Damascus goats. J. Agric. Sci. 2017, 155, 508–518. [Google Scholar] [CrossRef]
- Ciliberti, M.G.; Albenzio, M.; Francavilla, M.; Neglia, G.; Esposito, L.; Caroprese, M. Extracts from Microalga Chlorella sorokiniana Exert an Anti-Proliferative Effect and Modulate Cytokines in Sheep Peripheral Blood Mononuclear Cells. Animals 2019, 9, 45. [Google Scholar] [CrossRef]
- Janczyk, P.; Halle, B.; Souffrant, W.B. Microbial community composition of the crop and ceca contents of laying hens fed diets supplemented with Chlorella vulgaris. Poult. Sci. 2009, 88, 2324–2332. [Google Scholar] [CrossRef]
- Mirzaie, S.; Sharifi, S.D.; Zirak-Khattab, F. The effect of a Chlorella by-product dietary supplement on immune response, antioxidant status, and intestinal mucosal morphology of broiler chickens. J. Appl. Phycol. 2020, 32, 1771–1777. [Google Scholar] [CrossRef]
- Zheng, L.; Oh, S.T.; Jeon, J.Y.; Moon, B.H.; Kwon, H.S.; Lim, S.U.; An, B.K.; Kang, C.W. The Dietary Effects of Fermented Chlorella vulgaris (CBT(®)) on Production Performance, Liver Lipids and Intestinal Microflora in Laying Hens. Asian-Australas. J. Anim. Sci. 2012, 25, 261–266. [Google Scholar] [CrossRef]
- Tsiplakou, E.; Abdullah, M.A.M.; Mavrommatis, A.; Chatzikonstantinou, M.; Skliros, D.; Sotirakoglou, K.; Flemetakis, E.; Labrou, N.E.; Zervas, G. The effect of dietary Chlorella vulgaris inclusion on goat’s milk chemical composition, fatty acids profile and enzymes activities related to oxidation. J. Anim. Physiol. Anim. Nutr. 2018, 102, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Kouřimská, L.; Vondráčková, E.; Fantová, M.; Nový, P.; Nohejlová, L.; Michnová, K. Effect of Feeding with Algae on Fatty Acid Profile of Goat’S Milk. Sci. Agric. Bohem. 2014, 45, 162–169. [Google Scholar] [CrossRef]
- Saleh, H.A.; Gaber, H.S.; El-Khayat, H.M.M.; Abdel-Motleb, A.; Mohammed, W.A.-A.; Okasha, H. Influences of Dietary Supplementation of Chlorella vulgaris and Spirulina platensis on Growth-Related Genes Expression and Antioxidant Enzymes in Oreochromis niloticus Fish Exposed to Heavy Metals. Aquac. Stud. 2021, 22, 445–456. [Google Scholar] [CrossRef]
- Xi, L.; Lu, Q.; Liu, Y.; Su, J.; Chen, W.; Gong, Y.; Han, D.; Yang, Y.; Zhang, Z.; Jin, J.; et al. Effects of fish meal replacement with Chlorella meal on growth performance, pigmentation, and liver health of largemouth bass (Micropterus salmoides). Anim. Nutr. 2022, 10, 26–40. [Google Scholar] [CrossRef]
- Pradhan, J.; Sahu, S.; Das, B.K. Protective Effects of Chlorella vulgaris Supplemented Diet on Antibacterial Activity and Immune Responses in Rohu Fingerlings, Labeo rohita (Hamilton), Subjected to Aeromonas hydrophila Infection. Life 2023, 13, 1028. [Google Scholar] [CrossRef]
- Mota, C.S.C.; Pinto, O.; Sá, T.; Ferreira, M.; Delerue-Matos, C.; Cabrita, A.R.J.; Almeida, A.; Abreu, H.; Silva, J.; Fonseca, A.J.M.; et al. A commercial blend of macroalgae and microalgae promotes digestibility, growth performance, and muscle nutritional value of European seabass (Dicentrarchus labrax L.) juveniles. Front. Nutr. 2023, 10, 1165343. [Google Scholar] [CrossRef] [PubMed]
- An, B.-K.; Kim, K.-E.; Jeon, J.-Y.; Lee, K.W. Effect of dried Chlorella vulgaris and Chlorella growth factor on growth performance, meat qualities and humoral immune responses in broiler chickens. Springerplus 2016, 5, 718. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Shariff, M.; Goh, Y.M.; Banerjee, S.; Yusoff, F.M. Interaction of low-level dietary supplementation of Chlorella vulgaris Beijerinck, 1890, and feeding duration on growth hormone, growth performance and serum biochemistry of red hybrid tilapia (Oreochromis mossambicus × Oreochromis niloticus). J. Fish Biol. 2023, 103, 715–726. [Google Scholar] [CrossRef]
- Kim, Y.-B.; Park, J.; Heo, Y.-J.; Lee, H.-G.; Kwon, B.-Y.; Joo, S.S.; Joo, S.Y.; Kim, M.; Kim, Z.-H.; Lee, K.-W. Effect of Dietary Chlorella vulgaris or Tetradesmus obliquus on Laying Performance and Intestinal Immune Cell Parameters. Animals 2023, 13, 1589. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gao, J.; Peng, C.; Song, J.; Xie, Z.; Jia, J.; Li, H.; Zhao, S.; Liang, Y.; Gong, B. The Effect of the Microalgae Chlorella vulgaris on the Gut Microbiota of Juvenile Nile Tilapia (Oreochromis niloticus) Is Feeding-Time Dependent. Microorganisms 2023, 11, 1002. [Google Scholar] [CrossRef] [PubMed]
- González-Arceo, M.; Trepiana, J.; Aguirre, L.; Ibarruri, J.; Martínez-Sanz, M.; Cebrián, M.; Recio, I.; Portillo, M.P.; Gómez-Zorita, S. Anti-Steatotic Effects of Chlorella vulgaris, Nannochloropsis gaditana and Gracilaria vermiculophylla Algae Extracts in AML-12 Hepatocytes. Nutrients 2023, 15, 1960. [Google Scholar] [CrossRef]
- Edrees, A.; Shaban, N.S.; Hassan, N.E.-H.Y.; Abdel-Daim, A.S.A.; Sobh, M.S.; Ibrahim, R.E. Acrylamide exposure induces growth retardation, neurotoxicity, stress, and immune/antioxidant disruption in Nile tilapia (Oreochromis niloticus): The alleviative effects of Chlorella vulgaris diets. Fish Shellfish Immunol. 2024, 146, 109411. [Google Scholar] [CrossRef]
- Ibrahim, D.; Rahman, M.M.I.A.; Abd El-Ghany, A.M.; Hassanen, E.A.; Al-Jabr, O.A.; Abd El-Wahab, R.A.; Zayed, S.; Abd El Khalek Salem, M.; Nabil El Tahawy, S.; Youssef, W.; et al. Chlorella vulgaris extract conjugated magnetic iron nanoparticles in nile tilapia (Oreochromis niloticus): Growth promoting, immunostimulant and antioxidant role and combating against the synergistic infection with Ichthyophthirius multifiliis and Aeromonashydrophila. Fish Shellfish Immunol. 2024, 145, 109352. [Google Scholar] [CrossRef]
- Han, K.J.; McCormick, M.E. Evaluation of nutritive value and in vitro rumen fermentation gas accumulation of de-oiled algal residues. J. Anim. Sci. Biotechnol. 2014, 5, 31. [Google Scholar] [CrossRef]
- Wild, K.J.; Trautmann, A.; Katzenmeyer, M.; Steingaß, H.; Posten, C.; Rodehutscord, M. Chemical composition and nutritional characteristics for ruminants of the microalgae Chlorella vulgaris obtained using different cultivation conditions. Algal Res. 2019, 38, 101385. [Google Scholar] [CrossRef]
- Vargas, J.d.J.; Tarnonsky, F.; Maderal, A.; Fernández-Marenchino, I.; Podversich, F.; Schulmeister, T.M.; DiLorenzo, N. Increasing levels of Chlorella spp. on in vitro fermentation and methane production in a corn silage-base diet. Rev. Colomb. Cienc. Pecu. 2023, 37, 42–51. [Google Scholar] [CrossRef]
- Meehan, D.J.; Cabrita, A.R.; Silva, J.L.; Fonseca, A.J.; Maia, M.R. Effects of Chlorella vulgaris, Nannochloropsis oceanica and Tetraselmis sp. supplementation levels on in vitro rumen fermentation. Algal Res. 2021, 56, 102284. [Google Scholar] [CrossRef]
- Furbeyre, H.; van Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of dietary supplementation with freshwater microalgae on growth performance, nutrient digestibility and gut health in weaned piglets. Animal 2017, 11, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Furbeyre, H.; van Milgen, J.; Mener, T.; Gloaguen, M.; Labussière, E. Effects of oral supplementation with Spirulina and Chlorella on growth and digestive health in piglets around weaning. Animal 2018, 12, 2264–2273. [Google Scholar] [CrossRef]
- Coelho, D.; Alfaia, C.M.; Lopes, P.A.; Pestana, J.M.; Costa, M.M.; Pinto, R.M.A.; Almeida, J.M.; Moreira, O.; Fontes, C.M.G.A.; Prates, J.A.M. Impact of Chlorella vulgaris as feed ingredient and carbohydrases on the health status and hepatic lipid metabolism of finishing pigs. Res. Vet. Sci. 2022, 144, 44–53. [Google Scholar] [CrossRef]
- Sucu, E. Effects of Microalgae Species on In Vitro Rumen Fermentation Pattern and Methane Production. Ann. Anim. Sci. 2020, 20, 207–218. [Google Scholar] [CrossRef]
- Carullo, D.; Abera, B.D.; Scognamiglio, M.; Donsì, F.; Ferrari, G.; Pataro, G. Application of Pulsed Electric Fields and High-Pressure Homogenization in Biorefinery Cascade of C. vulgaris Microalgae. Foods 2022, 11, 471. [Google Scholar] [CrossRef] [PubMed]
- Tsiplakou, E.; Abdullah, M.A.M.; Skliros, D.; Chatzikonstantinou, M.; Flemetakis, E.; Labrou, N.; Zervas, G. The effect of dietary Chlorella vulgaris supplementation on micro-organism community, enzyme activities and fatty acid profile in the rumen liquid of goats. J. Anim. Physiol. Anim. Nutr. 2017, 101, 275–283. [Google Scholar] [CrossRef]
- Cabrol, M.B.; Martins, J.C.; Malhão, L.P.; Alves, S.P.; Bessa, R.J.B.; Almeida, A.M.; Raymundo, A.; Lordelo, M.M.; Boskovic Cabrol, M.; Martins, J.C.; et al. Partial replacement of soybean meal with Chlorella vulgaris in broiler diets influences performance and improves breast meat quality and fatty acid composition. Poult. Sci. 2022, 101, 101955. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Abu Elella, A.A.; Patra, A.K. Replacing the concentrate feed mixture with Moringa oleifera leaves silage and Chlorella vulgaris microalgae mixture in diets of Damascus goats: Lactation performance, nutrient utilization, and ruminal fermentation. Animals 2022, 12, 1589. [Google Scholar] [CrossRef]
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12, 295–309. [Google Scholar] [CrossRef]
- Jeon, J.-Y.; Park, K.-K.; Lee, K.-W.; Jang, S.-W.; Moon, B.-H.; An, B.-K. Dietary effects of lutein-fortified chlorella on milk components of Holstein cows. Springerplus 2016, 5, 908. [Google Scholar] [CrossRef]
- Tokuşoglu, Ö.; Üunal, M.K. Biomass Nutrient Profiles of Three Microalgae: Spirulina platensis, Chlorella vulgaris, and Isochrisis galbana. J. Food Sci. 2003, 68, 1144–1148. [Google Scholar] [CrossRef]
- Gadzama, I.U. Evaluation of Fresh Microalgae in Ruminant Nutrition: Impact on Rumen Fermentation, Productive Performance and Meat Quality. Master’s Thesis, School of Agriculture and Food Sustainability, The University of Queensland, Gatton Campus, Gatton, QLD, Australia, 2024. [Google Scholar] [CrossRef]
- Gadzama, I.U. Microalgae Supplementation in Sheep Nutrition: Impact on Wool Production and Quality; Wikifarmer: Athens, Greece, 2024; Available online: https://www.researchgate.net/publication/384767611_Microalgae_Supplementation_in_Sheep_Nutrition_Impact_on_Wool_Production_and_Quality (accessed on 29 December 2024).
- El-Bahr, S.; Shousha, S.; Shehab, A.; Khattab, W.; Ahmed-Farid, O.; Sabike, I.; El-Garhy, O.; Albokhadaim, I.; Albosadah, K. Effect of Dietary Microalgae on Growth Performance, Profiles of Amino and Fatty Acids, Antioxidant Status, and Meat Quality of Broiler Chickens. Animals 2020, 10, 761. [Google Scholar] [CrossRef]
- Ötleş, S.; Pire, R. Fatty acid composition of Chlorella and Spirulina microalgae species. J. AOAC Int. 2001, 84, 1708–1714. [Google Scholar] [CrossRef]
- Kholif, A.E.; Gouda, G.A.; Morsy, T.A.; Matloup, O.H.; Sallam, S.M.; Patra, A.K. Associative effects between Chlorella vulgaris microalgae and Moringa oleifera leaf silage used at different levels decreased in vitro ruminal greenhouse gas production and altered ruminal fermentation. Environ. Sci. Pollut. Res. Int. 2023, 30, 6001–6020. [Google Scholar] [CrossRef]
- Luzzi, S.C.; Gardner, R.D.; Heins, B.J. Taste preference of Chlorella sp. algae from dairy wastewater by weaned dairy calves. JDS Commun. 2020, 1, 41–44. [Google Scholar] [CrossRef]
- Shams, A.; Elsadany, A.; Abou-Aiana, R. Effects of Orally Chlorella vulgaris Algae Additive on Productive and Reproductive Performance of Lactating Friesian Cows. J. Anim. Poult. Prod. 2020, 11, 125–131. [Google Scholar] [CrossRef]
- Akhmedkhanova, R.; Dzhambulatov, Z.; Gadzhaeva, Z.; Shabanov, G.; Alieva, S. The influence of chlorella suspension on the quality of milk and its processing products. E3S Web Conf. 2020, 222, 2021. [Google Scholar] [CrossRef]
- Malyugina, S.; Černohous, M.; Látal, O. Characterization of Microalgae-Based Feed Supplement and Their Possible Influence on Cattle Rumen Microbial Ecosystem. Rural. Dev. 2020, 2019, 40–45. [Google Scholar] [CrossRef]
- Kuzmaitė, I.; Oberauskas, V.; Kantautaitė, J.; Žymantienė, J.; Želvytė, R.; Monkevičienė, I.; Sederevičius, A.; Bakutis, B. The effect of Chlorella vulgaris IFR-111 on microflora of the digestive system of neonate calves. Vet. Ir Zootech. 2009, 47, 44–49. [Google Scholar]
- Rabee, A.E.; Younan, B.R.; Kewan, K.Z.; Sabra, E.A.; Lamara, M. Modulation of rumen bacterial community and feed utilization in camel and sheep using combined supplementation of live yeast and microalgae. Sci. Rep. 2022, 12, 12990. [Google Scholar] [CrossRef]
- Slyusarenko, I.; Kitayeva, A.; Susol, R. Effect of Chlorella Microalgae Suspension on Dairy Productivity of Sheep Mothers and Growth Intensity of Lambs. Acta Biol. Univ. Daugavp. 2021, 21, 117–126. [Google Scholar]
- Kholif, A.E.; Kassab, A.Y.; Hamdon, H.A. Chlorella vulgaris Microalgae and Copper Mixture Supplementation Enhanced the Nutrient Digestibility and Milk Attributes in Lactating Boer Goats. Ann. Anim. Sci. 2021, 21, 939–957. [Google Scholar] [CrossRef]
- Ali, A.M.; Alshaheen, T.; Senosy, W.; Mohammed, A.E.N.; Kassab, A. Effects of feeding green microalgae and Nigella sativa on productive performance and metabolic profile of Boer goats during peripartum period in subtropics. Fresenius Environ. Bull. 2021, 30, 8203–8212. [Google Scholar]
- Abdel-Khalek, A.E.; El-Maghraby, M.M.; Elbialy, Z.I.; Al Wakeel, R.A.; Almadaly, E.A.; Shukry, M.; El-Badawy, A.A.; Zaghloul, H.K.; Assar, D.H. Mitigation of endogenous oxidative stress and improving growth, hemato-biochemical parameters, and reproductive performance of Zaraibi goat bucks by dietary supplementation with Chlorella vulgaris or/and vitamin C. Trop. Anim. Health Prod. 2023, 55, 267. [Google Scholar] [CrossRef]
- Silva, M.R.L.; Alves, J.P.M.; Fernandes, C.C.L.; Cavalcanti, C.M.; Conde, A.J.H.; Bezerra, A.F.; Soares, A.C.S.; Teixeira, D.Í.A.; do Rego, A.C.; Rondina, D. Effect of short-term nutritional supplementation of green microalgae on some reproductive indicators of Anglo-Nubian crossbred goats. Vet. World 2023, 16, 464–473. [Google Scholar] [CrossRef]
- Silva, M.R.L.; Alves, J.P.M.; Fernandes, C.C.L.; Cavalcanti, C.M.; Conde, A.J.H.; Bezerra, A.F.; Soares, A.C.S.; Tetaping, G.M.; de Sá, N.A.R.; Teixeira, D.Í.A.; et al. Use of green microalgae Chlorella as a nutritional supplement to support oocyte and embryo production in goats. Anim. Reprod. Sci. 2023, 256, 107296. [Google Scholar] [CrossRef]
- Kassab, A.; Hamdon, H.; Senosy, W.; Wardy, E.M. Impact of Chlorella vulgaris Microalgae as Anti Stress on Hematological Parameters and Thermoregulation of Boer Goats in Arid Subtropical Regions. New Val. J. Agric. Sci. 2023, 3, 21–27. [Google Scholar]
- Novotná, K.; Fantová, M.; Nohejlová, L.; Borková, M.; Stádník, L.; Ducháček, J. Effect of Chlorella vulgaris and Japonochytrium sp. Microalgae Supplementation on Composition and Fatty Acid Profile of Goat Milk. Acta Univ. Agric. Silvic. Mendelianae Brun. 2017, 65, 1585–1593. [Google Scholar] [CrossRef]
- Swati; Waidha, K.; Negam, S.; Parveen, N.; Chuskit, D.; Mayarngam, K.; Chaurasia, O.P. Effect of dietary supplementation of microalgae Spirulina and Chlorella on growth performance and blood profile of broiler chicken at high altitude. Indian J. Anim. Sci. 2022, 92, 995–998. [Google Scholar] [CrossRef]
- Kang, H.K.; Salim, H.M.; Akter, N.; Kim, D.W.; Kim, J.H.; Bang, H.T.; Kim, M.J.; Na, J.C.; Hwangbo, J.; Choi, H.C.; et al. Effect of various forms of dietary Chlorella supplementation on growth performance, immune characteristics, and intestinal microflora population of broiler chickens. J. Appl. Poult. Res. 2013, 22, 100–108. [Google Scholar] [CrossRef]
- Salim, H.M.; Kang, H.K.; Kim, D.W.; Choi, H.C.; Amin, M.R.; Khaleduzzaman, A.; Beg, M. Effect of Chlorella supplementation on growth performance, immune characteristics, and gut microbiota of broiler chickens. J. Appl. Poult. Res. 2019, 22, 100–108. [Google Scholar]
- Choi, H.; Jung, S.K.; Kim, J.S.; Kim, K.-W.; Oh, K.B.; Lee, P.-Y.; Byun, S.J. Effects of dietary recombinant chlorella supplementation on growth performance, meat quality, blood characteristics, excreta microflora, and nutrient digestibility in broilers. Poult. Sci. 2017, 96, 710–716. [Google Scholar] [CrossRef]
- Coelho, D.F.M.; Alfaia, C.M.R.P.M.; Assunção, J.M.P.; Costa, M.; Pinto, R.M.A.; de Andrade Fontes, C.M.G.; Lordelo, M.M.; Prates, J.A.M. Impact of dietary Chlorella vulgaris and carbohydrate-active enzymes incorporation on plasma metabolites and liver lipid composition of broilers. BMC Vet. Res. 2021, 17, 229. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Nomaguchi, T.; Mori, Y.; Ito, M.; Nakamura, Y.; Fujishima, M.; Fukuda, S. The nutritional efficacy of Chlorella supplementation depends on the individual gut environment: A randomised control study. Front. Nutr. 2021, 8, 648073. [Google Scholar] [CrossRef]
- Velankanni, P.; Go, S.H.; Jin, J.B.; Park, J.S.; Park, S.; Lee, S.B.; Lee, C.G. Chlorella vulgaris Modulates Gut Microbiota and Induces Regulatory T Cells to Alleviate Colitis in Mice. Nutrients 2023, 15, 3293. [Google Scholar] [CrossRef]
- Lee, J.Y.; Yoon, J.H.; An, S.H.; Cho, I.H.; Lee, C.W.; Jeon, Y.J.; Kong, C. Intestinal Immune Cell Populations, Barrier Function, and Microbiomes in Broilers Fed a Diet Supplemented with Chlorella vulgaris. Animals 2023, 13, 2380. [Google Scholar] [CrossRef]
- Alfaia, C.M.; Pestana, J.M.; Rodrigues, M.; Coelho, D.; Aires, M.J.; Ribeiro, D.M.; Major, V.T.; Martins, C.F.; Santos, H.; Lopes, P.A.; et al. Influence of dietary Chlorella vulgaris and carbohydrate-active enzymes on growth performance, meat quality and lipid composition of broiler chickens. Poult. Sci. 2021, 100, 926–937. [Google Scholar] [CrossRef]
- Kim, C.H.; Kang, H.K. Effect of dietary supplementation with a chlorella by-product on the performance, immune response and metabolic function in laying hens. Europ. Poult. Sci. 2015, 79. [Google Scholar] [CrossRef]
- Panaite, T.D.; Cornescu, G.M.; Predescu, N.C.; Cismileanu, A.; Turcu, R.P.; Saracila, M.; Soica, C. Microalgae (Chlorella vulgaris and Spirulina platensis) as a Protein Alternative and Their Effects on Productive Performances, Blood Parameters, Protein Digestibility, and Nutritional Value of Laying Hens’ Egg. Appl. Sci. 2023, 13, 10451. [Google Scholar] [CrossRef]
- Grigorova, S.; Surdjiiska, S.; Banskalieva, V.; Dimitrov, G. The effect of biomass from green algae of Chlorella genus on the biochemical characteristics of table eggs. J. Cent. Eur. Agric. 2006, 7, 111–116. [Google Scholar]
- Martins, C.F.; Pestana, J.M.; Alfaia, C.M.; Costa, M.; Ribeiro, D.M.; Coelho, D.; Lopes, P.A.; Almeida, A.M.; Freire, J.P.B.; Prates, J.A.M. Effects of Chlorella vulgaris as a Feed Ingredient on the Quality and Nutritional Value of Weaned Piglets’ Meat. Foods 2021, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Taranu, I.; Marin, D.E.; Untea, A.; Janczyk, P.; Motiu, M.; Criste, R.D.; Souffrant, W.B. Effect of dietary natural supplements on immune response and mineral bioavailability in piglets after weaning. Czech J. Anim. Sci. 2012, 57, 332–343. [Google Scholar] [CrossRef]
- Coelho, D.; Pestana, J.; Almeida, J.M.; Alfaia, C.M.; Fontes, C.M.G.A.; Moreira, O.; Prates, J.A.M. A High Dietary Incorporation Level of Chlorella vulgaris Improves the Nutritional Value of Pork Fat without Impairing the Performance of Finishing Pigs. Animals 2020, 10, 2384. [Google Scholar] [CrossRef]
- Yan, L.; Lim, S.U.; Kim, I.H. Effect of fermented chlorella supplementation on growth performance, nutrient digestibility, blood characteristics, fecal microbial and fecal noxious gas content in growing pigs. Asian-Australas. J. Anim. Sci. 2012, 25, 1742–1747. [Google Scholar] [CrossRef]
- Sikiru, A.B.; Arangasamy, A.; Alemede, I.C.; Egena, S.S.A.; Bhatta, R. Dietary supplementation effects of Chlorella vulgaris on performances, oxidative stress status and antioxidant enzymes activities of prepubertal New Zealand White rabbits. Bull. Natl. Res. Cent. 2019, 43, 162. [Google Scholar] [CrossRef]
- El Basuini, M.F.; Khattab, A.A.A.; Hafsa, S.H.A.; Teiba, I.I.; Elkassas, N.E.M.; El-Bilawy, E.H.; Dawood, M.A.O.; Atia, S.E.S. Impacts of algae supplements (Arthrospira & Chlorella) on growth, nutrient variables, intestinal efficacy, and antioxidants in New Zealand white rabbits. Sci. Rep. 2023, 13, 7891. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Sheiha, A.M.; Taha, A.E.; Swelum, A.A.; Alarifi, S.; Alkahtani, S.; Ali, D.; AlBasher, G.; Almeer, R.; Falodah, F.; et al. Impacts of Enriching Growing Rabbit Diets with Chlorella vulgaris Microalgae on Growth, Blood Variables, Carcass Traits, Immunological and Antioxidant Indices. Animals 2019, 9, 788. [Google Scholar] [CrossRef]
- Hassanein, H.; Arafa, M.M.; Abo Warda, M.A.; Abd–Elall, A. Effect of Using Spirulina Platensis and Chlorella Vulgaris As Feed Additives on Growing Rabbit Performance. Egypt. J. Rabbit. Sci. 2014, 24, 413–431. [Google Scholar] [CrossRef]
- Ahmad, M.T.; Shariff, M.; Yusoff, F.; Goh, Y.M.; Banerjee, S. Applications of microalga Chlorella vulgaris in aquaculture. Rev. Aquac. 2020, 12, 328–346. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Mousa, M.A.; Mamoon, A.; Abdelghany, M.F.; Abdel-Hamid, E.A.; Abdel-Razek, N.; Ali, F.S.; Shady, S.H.; Gewida, A.G. Dietary Chlorella vulgaris modulates the performance, antioxidant capacity, innate immunity, and disease resistance capability of Nile tilapia fingerlings fed on plant-based diets. Anim. Feed. Sci. Technol. 2022, 283, 115181. [Google Scholar] [CrossRef]
- Maliwat, G.C.; Velasquez, S.; Robil, J.L.; Chan, M.; Traifalgar, R.F.; Tayamen, M.; Ragaza, J.A. Growth and immune response of giant freshwater prawn Macrobrachium rosenbergii (De Man) postlarvae fed diets containing Chlorella vulgaris (Beijerinck). Aquac. Res. 2017, 48, 1666–1676. [Google Scholar] [CrossRef]
- Shi, X.; Luo, Z.; Chen, F.; Wei, C.C.; Wu, K.; Zhu, X.M.; Liu, X. Effect of fish meal replacement by Chlorella meal with dietary cellulase addition on growth performance, digestive enzymatic activities, histology and myogenic genes’ expression for crucian carp Carassius auratus. Aquac. Res. 2017, 48, 3244–3256. [Google Scholar] [CrossRef]
- Safari, O.; Paolucci, M.; Motlagh, H.A. Dietary supplementation of Chlorella vulgaris improved growth performance, immunity, intestinal microbiota and stress resistance of juvenile narrow clawed crayfish, Pontastacus leptodactylus Eschscholtz. Aquaculture 2022, 1823, 738138. [Google Scholar] [CrossRef]
- Abdelhamid, F.M.; Elshopakey, G.E.; Aziza, A.E. Ameliorative effects of dietary Chlorella vulgaris and β-glucan against diazinon-induced toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 96, 213–222. [Google Scholar] [CrossRef]
- Ramírez-Coronel, A.A.; Jasim, S.A.; Zadeh, A.H.A.; Jawad, M.A.; Al-Awsi, G.R.L.; Adhab, A.H.; Kodirov, G.; Soltanifar, Z.; Mustafa, Y.F.; Norbakhsh, M. Dietary Chlorella vulgaris mitigated the adverse effects of Imidacloprid on the growth performance, antioxidant, and immune responses of common carp (Cyprinus carpio). Ann. Anim. Sci. 2023, 23, 845–857. [Google Scholar] [CrossRef]
- Amar, E.C.; Kiron, V.; Satoh, S.; Watanabe, T. Enhancement of innate immunity in rainbow trout (Oncorhynchus mykiss Walbaum) associated with dietary intake of carotenoids from natural products. Fish Shellfish Immunol. 2004, 16, 527–537. [Google Scholar] [CrossRef]
- Quico, C.A.; Astocondor, M.M.; Ortega, R.A. ary supplementation with Chlorella peruviana improve the growth and innate immune response of rainbow trout Oncorhynchus mykiss fingerlings. Aquaculture 2021, 533, 736117. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Park, H.G.; Lee, S.M. Effects of dietary inclusion of Chlorella vulgaris on growth, blood biochemical parameters and antioxidant enzymes activity in olive flounder, Paralichthys olivaceus. Fish Shellfish Immunol. 2016, 53, 106. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; de Brabanter, J.; de Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Karapanagiotidis, I.T.; Metsoviti, M.N.; Gkalogianni, E.Z.; Psofakis, P.; Asimaki, A.; Katsoulas, N.; Papapolymerou, G.; Zarkadas, I. The effects of replacing fishmeal by Chlorella vulgaris and fish oil by Schizochytrium sp. and Microchloropsis gaditana blend on growth performance, feed efficiency, muscle fatty acid composition and liver histology of gilthead seabream (Sparus aurata). Aquaculture 2022, 561, 738709. [Google Scholar] [CrossRef]
- Raji, A.A.; Alaba, P.A.; Yusuf, H.; Bakar, N.H.A.; Taufek, N.M.; Muin, H.; Razak, S.A. Fishmeal replacement with Spirulina platensis and Chlorella vulgaris in African catfish (Clarias gariepinus) diet: Effect on antioxidant enzyme activities and haematological parameters. Res. Vet. Sci. 2018, 119, 67–75. [Google Scholar] [CrossRef]
- Rahimnejad, S.; Lee, S.-M.; Park, H.-G.; Choi, J. Effects of Dietary Inclusion of Chlorella vulgaris on Growth, Blood Biochemical Parameters, and Antioxidant Enzyme Activity in Olive Flounder, Paralichthys olivaceus. J. World Aquac. Soc. 2017, 48, 103–112. [Google Scholar] [CrossRef]
- Gouveia, L.; Gomes, E.; Empis, J. Use of Chlorella vulgaris in Rainbow Trout, Oncorhynchus mykiss, Diets to Enhance Muscle Pigmentation. J. Appl. Aquac. 1997, 7, 61–70. [Google Scholar] [CrossRef]
- Sleman, H.; Abdulrahman, N.M.; Hassan, N.; HamaSalih, H. Evaluation of blood, biochemical and biological effects of microalgae Chlorella and germinated barley powder as a source of prebiotic on common carp Cyprinus carpio L. Iraqi J. Vet. Sci. 2021, 35, 271–277. [Google Scholar] [CrossRef]
- Mahmoud, E.A.; El-Sayed, B.M.; Mahsoub, Y.H.; El-Murr, A.E.I.; Neamat-Allah, A.N.F. Effect of Chlorella vulgaris enriched diet on growth performance, hemato-immunological responses, antioxidant and transcriptomics profile disorders caused by deltamethrin toxicity in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2020, 102, 422–429. [Google Scholar] [CrossRef]
| Source | Location | References |
|---|---|---|
| Cultured CLV biomass produced by Phycom, Utrecht | The Netherlands | [21] |
| CLV produced by the Algal Biotechnology Unit, National Research Centre, Giza | Egypt | [22,23] |
| Chlorella (UTEX 2805) from Aqualgae’s vertical photobioreactor in Almerìa | Spain | [24] |
| CLV grown in a photobioreactor with sunlight at IGV, Nuthetal | Germany | [25] |
| Dried CLV biomass from SurNature Biological Technology Co., Ltd., Xi’an | China | [26] |
| Fresh CLV cultivated by Genesis Co. Pty Ltd., Bowen | Australia | [6] |
| A commercial product of CLV produced by fermentation (CBT®), Celltech Co., Ltd., Eumseong-gun | Republic of Korea | [27] |
| CLV cultivated in a photobioreactor at Athens’ Agricultural University’s Molecular Biology Lab., Athens | Greece | [28] |
| CLV (Beij., 1996/H 14) produced in the laboratory of the Institute of Botany, Třeboň | Czech Republic | [29] |
| Dried CLV produced by commercial algae culture in Menoufia governorate | Egypt | [30] |
| CLV meal produced by Demeter Bio-Tech Co., Ltd., Wuhan | China | [31] |
| CLV cultured from the algal culture unit of CIFA, Bhubaneswar | India | [32] |
| Commercial CLV (Algaessence® feed) by ALGAplus, Ílhavo and Allmicroalgae, Pataias | Portugal | [33] |
| Dried CLV powder and Chlorella Growth Factor from Daesang Corp., Icheon | Republic of Korea | [34] |
| Dry CLV powder produced by Daesang Corporation, Seoul | Republic of Korea | [35] |
| CLV strain (PKVL7422) from Korean Collection for Type Cultures (13361BP), Daejeon | Republic of Korea | [36] |
| CLV from FACHB’s Freshwater Algae Collection, Wuhan | China | [37] |
| CLV produced by NEOALGAE, Gijón, Asturias | Spain | [38] |
| Dried CLV powder produced by the Institute of National Research Center, Cairo | Egypt | [39] |
| CLV powder Organic Traditions Company, Advantage Health Matters Inc., Toronto, ON | Canada | [40] |
| CLV cultivated from Baton Rouge, Louisiana | USA | [41] |
| CLV strain (SAG 211–12) grown in 500 mL flasks on an orbital shaker (KS 501 digital, Ika-Werke) in Staufen | Germany | [42] |
| Dried and pelleted CLV produced by Origo, LLC, Venus, FL | USA | [43] |
| CLV from UTEX Algae Collection, Algoteca, University of Texas, Austin, TX | USA | [44] |
| CLV powder from Setalg©, Pleubian | France | [45] |
| CLV processed by Algosource Technologies, Saint-Nazaire | France | [46] |
| CLV produced by Allmicroalgae (Natural Products, Portugal), Leiria | Portugal | [47] |
| CLV cultivated in a flat panel photobioreactor under controlled conditions without any contamination | Turkey | [48] |
| CLV strain (CCAP 211) from the Culture Collection of Algae and Protozoa, Argyll | UK | [49] |
| Fatty Acids | % of Total Fatty Acids | Reference |
|---|---|---|
| Butyric (C4:0) | 0.20 | [59] |
| Caproic (C6:0) | 2.77 | [59] |
| Caprylic (C8:0) | 0.26 | [59] |
| Undecanoic (C11:0) | 1.39 | [59] |
| Undecenoic (C11:1) | 2.17 | [59] |
| Lauric acid (C12:0) | 0.87 6.78 | [59] [44] |
| Lauroleic (C12:1) | 0.41 | [59] |
| Tridecanoic (C13:0) | 1.03 | [59] |
| Myristic acid (C14:0) | 0.38 0.69 1.13 6.91 15.90 | [55] [59] [3] [58] [44] |
| Pentadecanoic (C15:0) | 1.70 | [59] |
| Pentadecenoic (C15:1) | 3.53 | [59] |
| Palmitic acid (C16:0) | 14.42 15.41 17.20 20.90 37.10 59.85 | [59] [55] [3] [44] [50] [58] |
| Palmitoleic (C16:1) | 1.17 3.52 3.90 4.04 14.30 | [55] [58] [3] [59] [44] |
| Hexadecadienoic (C16:2) | 5.34 | [59] |
| Hexadecatrienoic (C16:3) | 4.90 | [59] |
| Margaric acid (C17:0) | 0.12 0.23 | [59] [3] |
| Heptadecenoic (C17:1) | 0.27 0.61 | [59] [3] |
| Stearic acid (C18:0) | 1.35 1.57 3.00 6.24 13.40 15.27 | [50] [59] [3] [55] [44] [58] |
| Oleic acid (C18:1) | 2.43 6.36 11.70 17.62 50.90 | [50] [58] [3] [59] [44] |
| Vaccenic acid (C18:1n-7) | 1.13 | [55] |
| Elaidic acid (C18:1n-9) | 33.14 | [55] |
| Linoleic acid (C18:2n-6) | 9.73 11.20 11.97 12.30 22.04 26.37 | [55] [3] [59] [44] [50] [58] |
| Alpha-Linolenic acid (C18:3n-3) | 1.93 10.10 11.82 15.79 19.10 22.10 | [55] [3] [58] [59] [44] [50] |
| Arachidic acid Eicosanoic (C20:0) | 0.14 0.17 0.19 26.22 | [3] [59] [55] [58] |
| Gondoic acid (C20:1) | 0.13 | [3] |
| Eicosapentaenoic acid-EPA (C20:5n-3) | 1.26 1.61 3.23 ND a | [58] [44] [55] [59] |
| Lignoceric acid (C22:0) | 0.06 | [3] |
| Docosapentaenoic-DPA (C22:5n-3) | 3.11 | [55] |
| Docosahexaenoic acidDHA(C22:6n-3) | 0.30 1.99 20.94 | [59] [58] [55] |
| Tetracosanoic (C24:0) | 0.22 | [59] |
| ∑ SFA | 22.22 | [55] |
| ∑ MUFA | 35.44 | [55] |
| ∑ PUFA | 38.94 | [55] |
| ∑ n-3 | 29.21 | [55] |
| ∑ n-6 | 9.73 | [55] |
| ∑ n-3/n-6 | 3.00 | [55] |
| DHA/EPA | 6.73 | [55] |
| Tokuşoglu and Üunal [55] | Zheng et al. [27] | Sucu [48] | Martins et al. [3] | |
|---|---|---|---|---|
| Indices | mg/kg | mg/kg | mg/kg | mg/kg |
| Na | 13,464 | NR | 16,450 | 3820 |
| P | 17,615 | 6500 | 27,080 | 20,400 |
| Ca | 5937 | 2000 | 940 | 7030 |
| K | 499.2 | NR | 132,950 | 29,200 |
| Mg | 3443 | NR | 12,360 | NR |
| Fe | 2591 | NR | 5400 | NR |
| Cr | 0.2 | NR | NR | NR |
| Cu | 0.6 | NR | 0 | NR |
| Zn | 11.9 | NR | 530 | NR |
| Mn | 20.9 | NR | 1270 | NR |
| Se | 0.7 | NR | NR | NR |
| B | NR | NR | 1640 | NR |
| Parameters | Gadzama et al. [6] a | Vargas et al. [43] b | Kholif et al. [60] c | Sucu [48] d |
|---|---|---|---|---|
| CLV (% DM) | 0, 0.5, and 1% | 0, 1, 5, and 10% | 0, 1, 2 and 3% | 0 and 25% |
| Incubation time | 24 h | 24 h | 48 h | 48 h |
| Key findings | ||||
| Total gas production | No effect | No effect | 1% CLV ↑ | 25% CLV ↓ |
| Methane production | No effect | No effect | 1% CLV ↓ | 25% CLV ↓ |
| Total VFA | No effect | No effect | No effect | 25% CLV ↓ |
| % of VFA | ||||
| Acetate | No effect | No effect | No effect | 25% CLV ↓ |
| Propionate | No effect | No effect | No effect | No effect |
| Butyrate | No effect | No effect | No effect | No effect |
| Ammonia-N | N/A | No effect | 1% CLV ↑ | 25% CLV ↑ |
| NDF digestibility | N/A | N/A | 1% CLV ↑ | N/A |
| Summary of Main Findings | References |
|---|---|
| Holstein cows in mid-lactation fed 30 g each of either conventional or lutein-fortified Chlorella for 3 weeks showed no significant differences in feed consumption, milk production, or milk fat percentage when compared to a control group | [54] |
| Cows fed Chlorella showed increased concentration of milk protein and non-fat solids than those in the control group | [54] |
| Milk from cows fed lutein-enriched Chlorella showed higher lutein levels than milk from cows fed standard Chlorella or control | [54] |
| Feeding Holstein heifer calves Chlorella spp. (60 g/d) led to a decrease in their daily feed intake compared to calves that did not receive Chlorella | [61] |
| Holstein cows receiving 1 to 1.5 L of CLV suspension daily showed higher concentrations of protein, fat, and iodine in their milk than the control groups | [63] |
| Neonate calves fed 400 mL daily of CLV IFR-111 for 30 d showed no significant difference in microflora compared to the control group | [65] |
| Cows receiving 90 and 170 g of lyophilized CLV in their TMR had a higher ciliate protozoa population after 21 d, surpassing that of the control diet | [64] |
| Dietary inclusion of CLV at 30, 90, and 170 g per diet enhanced the ruminal protozoa population, boosting genera like Isotricha, Dasytricha, Charonina, Buetschlia, Ostracodinium, and Ophryoscolex | [64] |
| Multiparous Friesian cows that received 2 mL or 4 mL of CLV per kg BW showed increased feed intake up to day 120 of lactation, compared to cows that did not receive CLV | [62] |
| For cows fed 2 mL and 4 mL of CLV, feed efficiency per kg for 4% FCM decreased | [62] |
| Cows fed 2 mL and 4 mL of CLV showed a decrease in DMI by 10.66% and 18.85%, TDN by 8% and 13.33%, CP by 10.17% and 18.55%, and DCP by 7.58% and 13.32%, respectively, compared to the control group | [62] |
| Parameters | Gadzama et al. [5] | Rabee et al. [66] | Slyusarenko et al. [67] |
|---|---|---|---|
| CLV form | Fresh (g/100 g DM) | Combination with yeast a (g/100 g DM) | Suspension (mL/kg of ewe BW) |
| CLV dose | 0, 0.5, and 1% | 0 and 1% | 0, 3, 5, 7, and 9 mL/kg BW |
| Stage | Lamb (4 months) | Ram (5 years) | Lactating ewe b |
| Key findings c | Response in lamb | ||
| DMI | - | +31% ↑ | +66% ↑ |
| ADG | - | +2X ↑ | |
| Total VFA | - | - | |
| % of VFA | |||
| Acetate | - | −24% ↓ | |
| Propionate | - | +88% ↑ | |
| Butyrate | 15.91% ↓ | −16% ↓ | |
| Isobutyrate | - | +109% ↑ | |
| Ammonia-N | - | ||
| NDF digestibility | +14% ↑ |
| Summary of Main Findings | References |
|---|---|
| Broilers fed diets containing 10, 15, or 20% CLV showed higher ileal digesta viscosity and greater gastrointestinal size, which also led to increased breast muscle yield | [51] |
| Feeding CLV to broilers raised levels of DHA, EPA, and n-3 PUFA in their breast meat and lowered the n-6/n-3 PUFA ratio | [51] |
| Adding 10% CLV and CAZymes to broiler diets increased plasma total lipids and improved the n-6/n-3 ratio and total carotenoids | [79] |
| Adding CLV led to a yellower breast muscle, significantly boosting chlorophyll a, b, and total carotenoids | [51] |
| Dietary CLV enhanced the color and carotenoid content in poultry meat | [83] |
| Broilers given a 20% CLV diet showed improved meat water-holding capacity and reduced cooking loss | [51] |
| Broilers fed dried Chlorella powder and Chlorella growth factor had similar meat qualities such as pH, color, and cooking loss | [34] |
| Broilers fed CLV at 1 g/kg diet showed lower levels of malondialdehyde and protein carbonyl, reduced cooking loss and bacterial counts, and higher SOD activity than the control group | [58] |
| Dietary CLV reduced bacteria levels in meat in comparison to control groups | [51] |
| Broilers given 10% CLV in their feed for 2 weeks showed comparable breast muscle quality, tenderness, juiciness, and taste to those on a standard diet | [83] |
| Broiler breast meat, enhanced with 10% CLV in their feed for 40 d, showed higher acceptance ratings | [51] |
| CLV supplementation led to lower HDL-cholesterol in broilers’ meat | [79] |
| Summary of Main Findings | References |
|---|---|
| Hen-day egg production and feed intake improved with higher levels of Chlorella by-product at 75 g/kg of basal diet | [84] |
| Lohmann Brown hens fed 2.0% CLV for 8 weeks had similar feed intake, final body weight and cholesterol levels as compared to control | [85] |
| Hy-Line Brown laying hens fed a 5 g CLV/kg diet had a similar laying performance as the control group | [36] |
| CLV-supplemented hens had greater egg weight (62.4 g) as compared to (59.8 g) in the control | [85] |
| Feeding spray-dried or bullet-milled spray-dried CLV at 5.0 g/kg increased the diversity of lactobacilli in the crop of Lohmann Brown laying hens as compared to the control group | [25] |
| Dietary CLV in the diet of hens resulted in a more diverse bacterial community in the ceca of Lohmann Brown hens | [25] |
| CLV levels of 0, 1000, or 2000 mg/kg positively affected the contents of hepatic triacylglycerol and the profiles of cecal microflora in Hy-line Brown hens | [27] |
| Hens fed CLV at 5 g of diet did not differ in laying performance, jejunal histology, cecal short-chain fatty acids, and antioxidant/immune markers in ileal mucosa | [36] |
| Indicators of egg freshness (Haugh unit) and eggshell quality (i.e., strength and thickness) were not altered by 5 g dietary CLV in the diets of Hy-Line Brown laying hens | [36] |
| A total of 80 weeks Hy-line Brown layers fed fermented CLV levels of 0, 1000, or 2000 mg/kg diet for 42 d showed a linear increase in egg production, egg yolk color, and Haugh unit | [27] |
| CLV supplementation improved the color parameters (L*, a*, and b*) of fresh and 10 min boiled eggs compared to the control group | [85] |
| Dietary CLV significantly influenced egg yolk color in laying hens compared to the control group | [36] |
| Dietary CLV increased fatty acid content, ß-carotene concentration, antioxidant capacity, yolk color intensity, and boiling eggs enhanced the b* colour | [85] |
| A total of 74 weeks Bovans Braun laying hens fed Chlorella at 2% and 10% of the diet for 37 days had similar total cholesterol content in 100 g of yolk as compared to the control | [86] |
| Chlorella supplementation increased palmitic and linoleic acid concentration but decreased docosatetraenic acid in egg yolk | [86] |
| Summary of Main Findings | References |
|---|---|
| Weaned male piglets fed 5% CLV in their diet for 15 d had higher ADFI compared to the control but no changes in final weight, ADG, and FCR | [3] |
| Pigs fed 0.1% levels of fermented Chlorella for 6 weeks had higher ADG and DM digestibility but similar ADFI and G:F ratio as compared to the control group, or the 0.2% CLV-supplemented group | [90] |
| Growth performance of finishing pigs fed 5% CLV was not different from the control group, receiving a soybean meal-based diet | [89] |
| Weaned piglets fed 1.0% of CLV for 11 days had similar body weight as compared to the control | [88] |
| Pigs supplemented with 1% CLV from 28 to 42 d had similar ADFI, ADG, and gain: feed (G:F) ratio compared to control | [45] |
| Oral administration of CLV to suckling piglets at 385 mg/kg BW per day resulted in a similar post-weaning ADG, feed intake, and G:F ratio | [46] |
| Post-weaned male piglets fed CLV at 5% levels of diet for 2 weeks had similar growth as the control | [87] |
| CLV had no significant effect on pigs’ carcass characteristics | [89] |
| Pigs fed CLV-based diets had increased IgG and decreased IgM levels | [3] |
| A high dietary level of CLV at 5% of the diet impacts the blood parameters of finishing pigs, with a notable immunosuppressive effect increasing susceptibility to infectious diseases | [89] |
| Piglets fed CLV levels of 0%, 5%, 5% + Rovabio, and 5% + CAZyme mixture had increased total LDL-, and VLDL-cholesterol while HDL-cholesterol decreased after 15 days | [3] |
| CLV had no significant effect on pigs’ meat quality traits | [89] |
| Finishing pigs fed CLV showed increased lipid-soluble antioxidant pigments and n-3 PUFA in pork fat | [89] |
| Dietary CLV decreased n-6 PUFA, and increased n-3 PUFA, improving the n-6/n-3 ratio in the liver of pigs | [3] |
| CLV decreased n-6/n-3 fatty acid ratio, improving pork fat nutritional value | [89] |
| Pigs fed 5% CLV for 2 weeks had greater total carotenoids and n-3 PUFA, and better n-6/n-3 fatty acid ratio | [87] |
| CLV reduced systemic antioxidant capacity in pigs, and increased hepatic n-3 PUFA content, reducing the n-6/n-3 ratio | [47] |
| Summary of Main Findings | References |
|---|---|
| Rabbits fed 200–500 mg CLV/kg BW had a higher final body weight and feed-to-gain ratio than the control | [91] |
| Rabbits showed similar live weight, weight gain, feed consumption, and FCR when given 0.5 g, 1.0 g, or 1.5 g of CLV powder/kg of diet over 8 weeks | [93] |
| Rabbits fed 300 and 500 mg/kg of CLV for 8 weeks had higher final weight and weight gain than the control | [92] |
| Rabbits on a diet with 0.75 g/kg of CLV had a better FCR than the control group | [94] |
| Dietary CLV levels of 300 and 500 mg/kg enhanced FCR as compared to the control group | [92] |
| New Zealand White rabbits showed no significant differences in feed intake, final body weight, or weight gain when fed diets with 0.75 or 1.5 g/kg of CLV compared to the control group over 12 weeks | [94] |
| New Zealand White rabbits fed 500 mg/kg BW of CLV biomass showed a decrease in feed consumption relative to the control group | [91] |
| New Zealand male rabbits fed a high dose of CLV 500 mg/kg diet for 8 weeks had reduced feed intake | [92] |
| Rabbits fed CLV showed no significant differences in nutrient digestibility (DM, OM, CP, CF, EE, and NFE) | [94] |
| Rabbits fed CLV levels of 0.75 or 1.5 g/kg diet had similar dressing percentages, liver, kidney, heart, and total giblet weights and percentages compared to the control | [94] |
| Malondialdehyde levels, an indicator of oxidative stress, were lower in rabbits fed CLV | [93] |
| CLV levels of 500 mg/kg BW reduced malondialdehyde, and protein carbonyl concentrations compared to the control | [91] |
| Rabbits fed CLV had improved immunoglobulins (IgG and IgM), and glutathione activities compared to the control | [93] |
| Rabbits fed a 1.0 g CLV/kg diet had enhanced immune responses and antioxidant status compared to the control | [93] |
| Rabbits fed CLV levels of 0, 0.5, 1.0, or 1.5 g/kg diet showed reduced serum triglycerides and low-density lipoprotein compared to control after 8 weeks | [93] |
| Summary of Main Findings | References |
|---|---|
| Increasing levels of CLV 0, 2.5, 5, 10, 15, and 20 g/kg diet enhanced feed consumption, growth rate, and SGR in Tilapia for 70 d | [96] |
| Fish fed diets with 5%, 10%, and particularly 15% CLV showed a greater final body weight after 8 weeks, compared to the control group | [30] |
| Juvenile seabass fed from 0 to 6% CLV blend showed a 70% increase in final body weight compared to the control group over 12 weeks | [33] |
| Juvenile fish fed 75% CLV at 2% body weight had improved FCR and PER compared to the control group | [107] |
| Olive flounder had higher growth rates over 8 weeks when fed with increasing levels of CLV 0, 5, 10, and 15% | [108] |
| Catfish fed 75% CLV diet at 2% BW had higher body weight and SGR compared to the control group | [107] |
| Largemouth bass showed a quadratic increase in body weight, growth rate, and feed consumption over 8 weeks when fed a diet with 0, 25, 50, 75 and 100% CLV meal as a replacement for fishmeal | [31] |
| Fingerlings fed increasing levels (0, 0.1, 0.5, and 1%) of CLV per kg diet for 90 d had improved growth rates and FCR as compared to the control | [32] |
| Nile Tilapia on a 5% CLV diet for 60 days showed improved survival and growth over the control group | [100] |
| Fish survival rates remained consistent across varying CLV concentrations (0, 5, 10, and 15%) | [108] |
| Tilapia fingerlings fed diets with CLV levels ranging from 0 to 20 g/kg had comparable FCR and survival rates | [96] |
| Seabreams given 10–30% CLV in their diet as a fishmeal substitute showed similar growth, feed conversion, and protein efficiency after 12 weeks | [106] |
| As dietary CL levels rose from 0% to 100%, fish FCR increased linearly | [31] |
| Common carp given a diet with 5% CLV for 56 d, showed increased final weight and SGR and a reduced FCR, in contrast to the control group | [101] |
| Tilapia fingerlings receiving a diet with 4.76% CLV powder for 90 d had higher SGR over the control group | [35] |
| Fish fed with a 5% CLV diet for 60 d, showed SGR, FCR, and PER values like those of the control group | [100] |
| Rainbow trout fed 0.2% CLV or synthetic carotenoids for 9 weeks had similar feed intake and weight gain | [109] |
| African catfish fed 50% and 75% CLV for 12 weeks, as a fishmeal substitute at 2% BW showed a decrease in feed consumption | [107] |
| A diet with 2% and 4% CLV blend decreased fish FCR after 12 weeks | [33] |
| Common carp fed dietary CLV levels of 5% and 10% showed blood parameters Hb, RBC, and WBC comparable to the control group | [110] |
| A 5% concentration of CLV enhanced growth, blood health, antioxidant levels, immunity, survival, and gene activity in Nile tilapia, countering the effects of 15 μg/L deltamethrin over 8 weeks | [111] |
| Dietary CLV resulted in a minor reduction of muscle carotenoid levels in rainbow trout, recording 11.9 mg/kg compared to 13.3 mg/kg observed with synthetic carotenoids | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadzama, I.U.; Ray, S.; Méité, R.; Mugweru, I.M.; Gondo, T.; Rahman, M.A.; Redoy, M.R.A.; Rohani, M.F.; Kholif, A.E.; Salahuddin, M.; et al. Chlorella vulgaris as a Livestock Supplement and Animal Feed: A Comprehensive Review. Animals 2025, 15, 879. https://doi.org/10.3390/ani15060879
Gadzama IU, Ray S, Méité R, Mugweru IM, Gondo T, Rahman MA, Redoy MRA, Rohani MF, Kholif AE, Salahuddin M, et al. Chlorella vulgaris as a Livestock Supplement and Animal Feed: A Comprehensive Review. Animals. 2025; 15(6):879. https://doi.org/10.3390/ani15060879
Chicago/Turabian StyleGadzama, Ishaya Usman, Saraswati Ray, René Méité, Isaac Maina Mugweru, Takudzwa Gondo, Md Atikur Rahman, Md Rahat Ahmad Redoy, Md Fazle Rohani, Ahmed Eid Kholif, Md Salahuddin, and et al. 2025. "Chlorella vulgaris as a Livestock Supplement and Animal Feed: A Comprehensive Review" Animals 15, no. 6: 879. https://doi.org/10.3390/ani15060879
APA StyleGadzama, I. U., Ray, S., Méité, R., Mugweru, I. M., Gondo, T., Rahman, M. A., Redoy, M. R. A., Rohani, M. F., Kholif, A. E., Salahuddin, M., & Brito, A. F. (2025). Chlorella vulgaris as a Livestock Supplement and Animal Feed: A Comprehensive Review. Animals, 15(6), 879. https://doi.org/10.3390/ani15060879











