Modeling Spawning Habitats of Coreius guichenoti with Substrate Considerations: A Case Study of Pingdi Town in the Lower Jinsha River
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
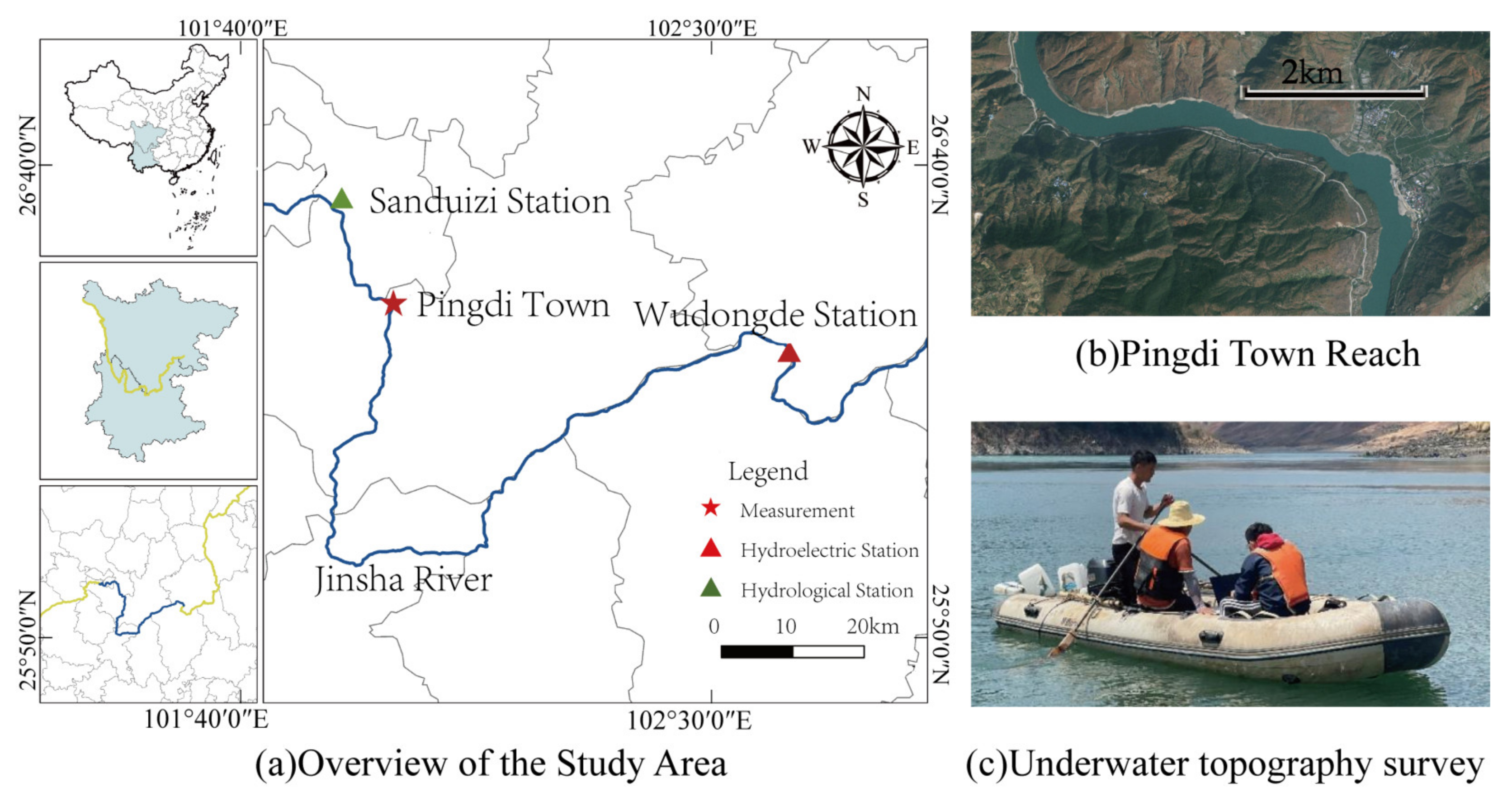
2.1. Overview of the Study Site
2.2. Habitat Model
- Threshold Requirements for Habitat Factors: The spawning of C. guichenoti requires specific environmental conditions. For example, spawning activity is minimal or does not occur when the water temperature is below 18 °C.
- Complementary and Trade-off Effects of Habitat Factors: Once all habitat factors meet the threshold requirements, their influence on spawning suitability may exhibit complementary or trade-off relationships. For instance, even if water temperature suitability is relatively low, the habitat may still be considered suitable for C. guichenoti spawning if flow velocity, water depth, and substrate conditions are highly favorable.
- Relative Importance of Habitat Factors: When assessing complementary or trade-off effects, different habitat factors have varying degrees of importance, ranked as follows: water temperature > flow velocity > water depth > substrate grain size.
2.3. Habitat Simulation
2.4. Evaluation of Spawning Habitat for C. guichenoti
2.5. Influence of Substrate on Model Performance
3. Results
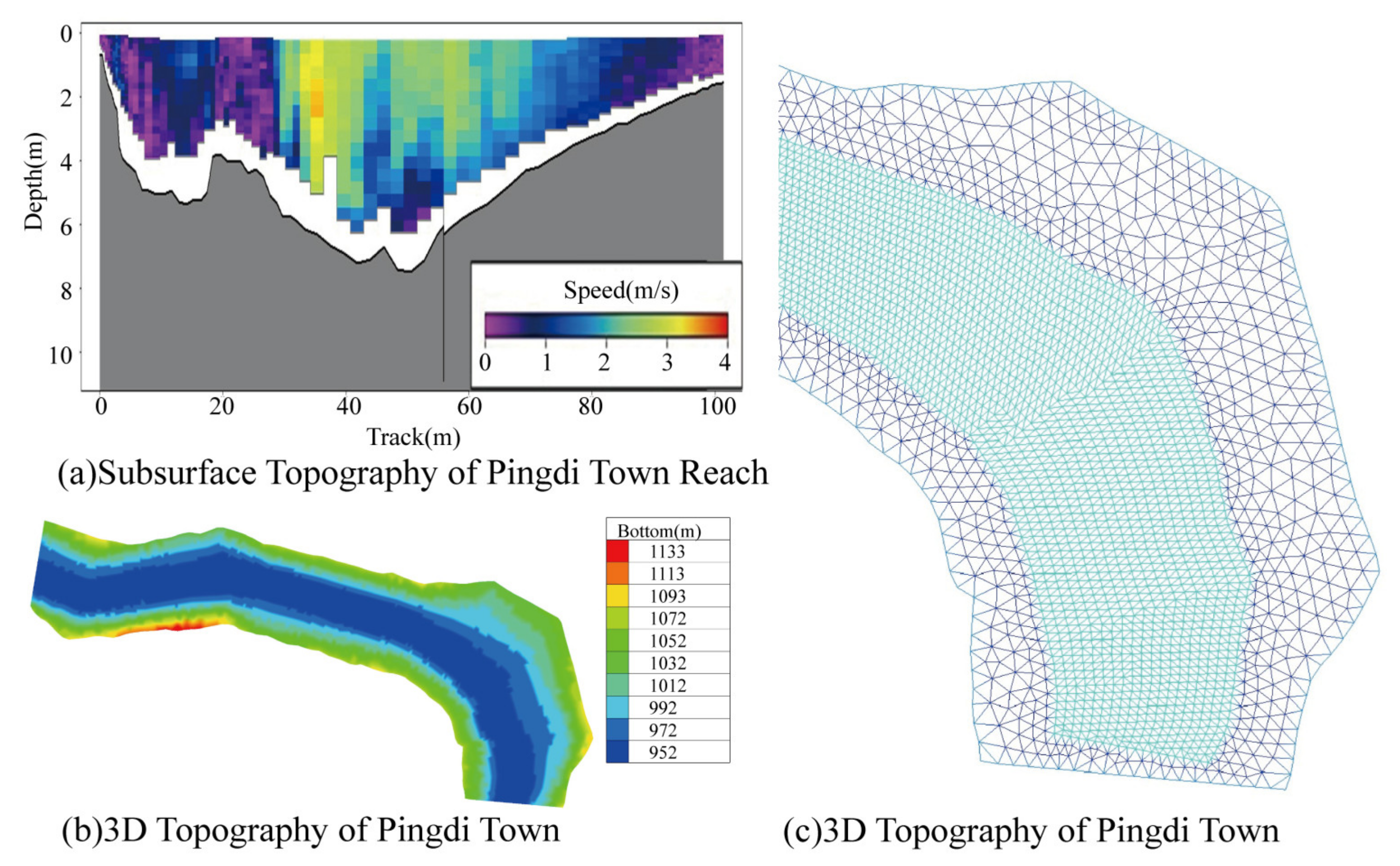
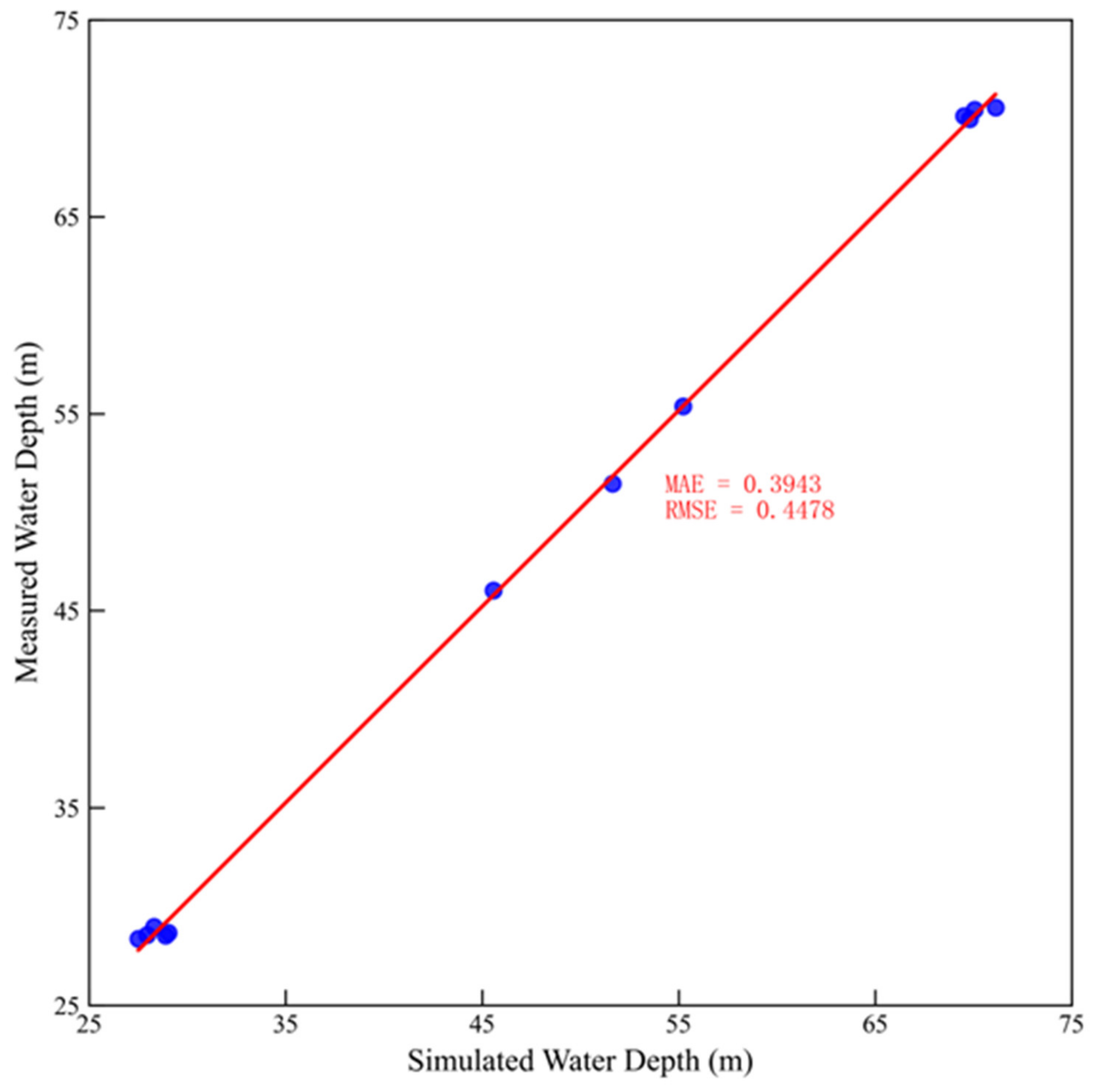
3.1. Validation and Results of the Hydrodynamic Model
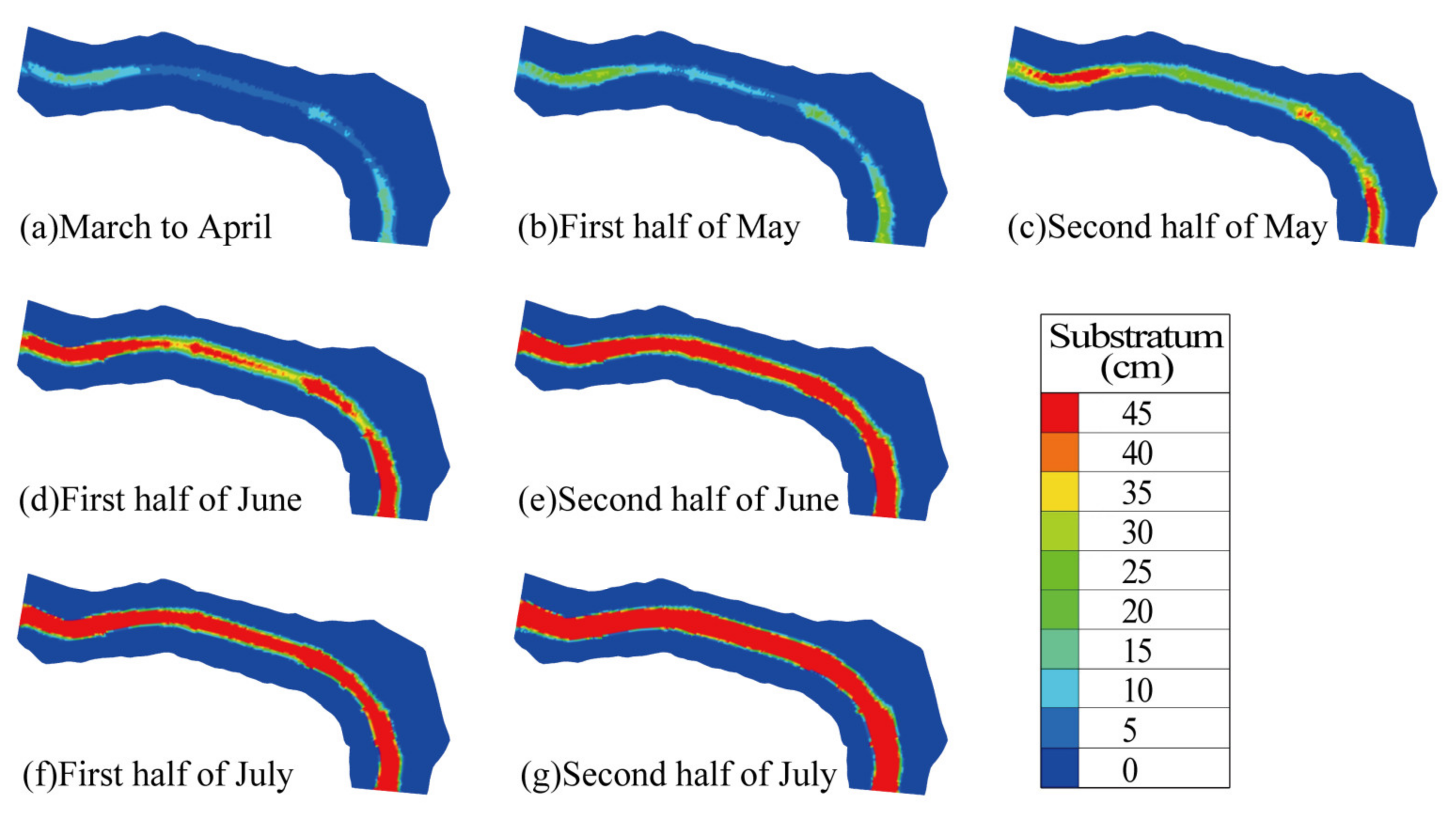
3.2. Evaluation of Spawning Habitat for C. guichenoti After Reservoir Impoundment at the Wudongde Hydropower Station
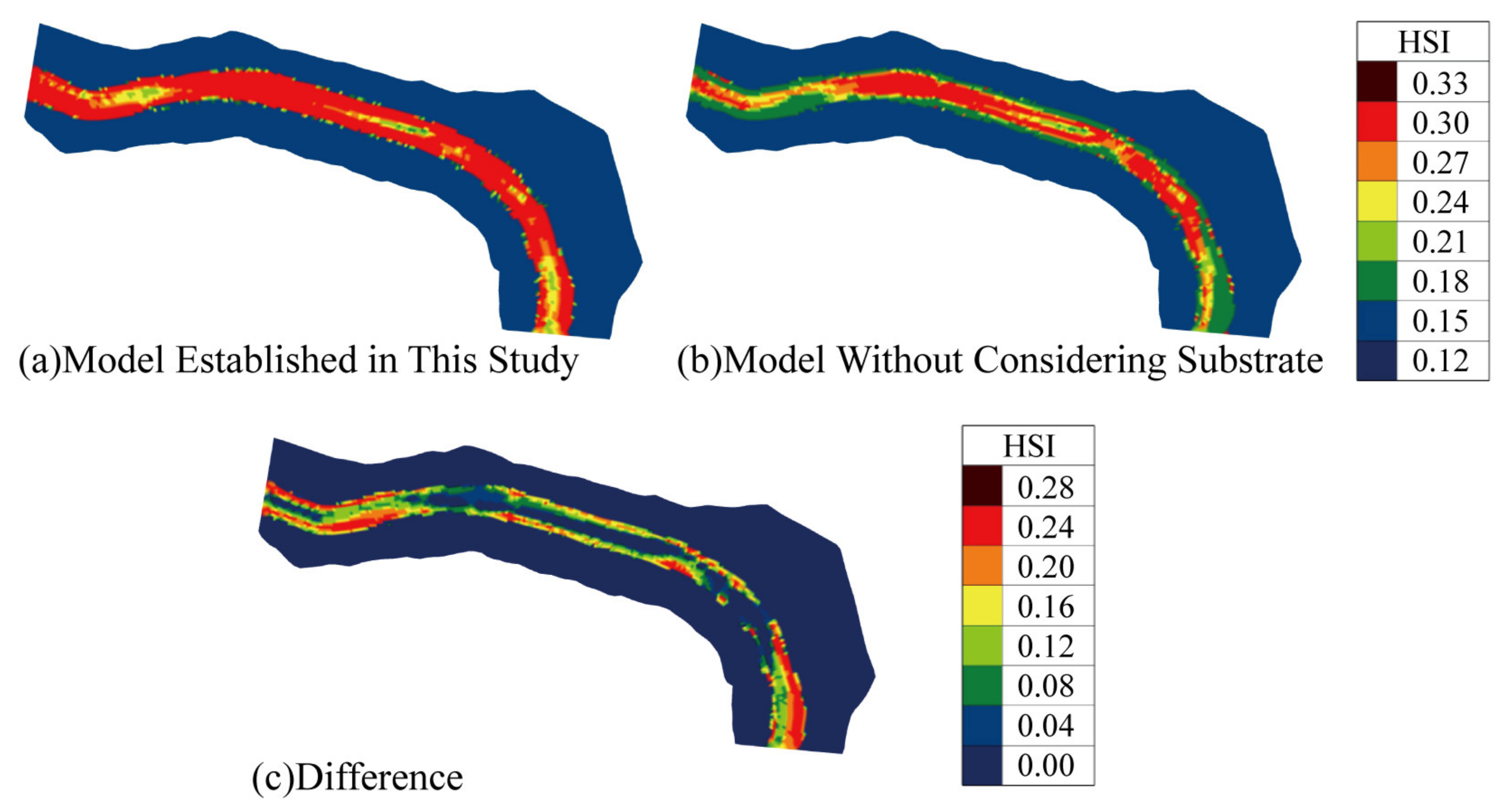
3.3. The Impact of InIncorporating Substrate into the Model
4. Discussion
4.1. The Necessity and Ecological Significance of Including Substrate in the Model
4.2. The Importance and Impact of Other Environmental Factors in the Model
4.3. Other Factors Affecting Spawning and Model Application
5. Conclusions
- Compensatory Effect of Substrate: Substrate plays a compensatory role in the spawning of C. guichenoti. Compared to traditional models, the model developed in this study provided a higher habitat quality evaluation for May. Specifically, the WUA and OSI increased by 42.31% and 38.73% in the first and second halves of May, respectively, while the MSP increased by 236.04% and 614.56%. These increases were mainly observed in the riverbank areas, with the HSI rising by approximately 0.25.
- Necessity of Substrate Inclusion: It is essential to incorporate substrate into the spawning habitat model for C. guichenoti. The model developed in this study effectively reflects the complex habitat requirements for the species’ spawning. It is a valuable tool for quantifying and assessing the spawning habitat of C. guichenoti in the study area.
- Impact of Wudongde Reservoir Impoundment: Following the impoundment operations of the Wudongde Reservoir, C. guichenoti spawning in the downstream Pingdi Town reach of the Jinsha River occurred from May to July, with June being the peak spawning period. Suitable spawning areas were primarily concentrated along the riverbanks and progressively shrank towards the riverbanks as time went on.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McAdam, D.S.O. Retrospective weight-of-evidence analysis identifies substrate change as the apparent cause of recruitment failure in the upper Columbia River white sturgeon (Acipenser transmontanus). Can. J. Fish. Aquat. Sci. 2015, 72, 1208–1220. [Google Scholar] [CrossRef]
- Baril, A.-M.; Buszkiewicz, J.T.; Biron, P.M.; Phelps, Q.E.; Grant, J.W. Lake sturgeon (Acipenser fulvescens) spawning habitat: A quantitative review. Can. J. Fish. Aquat. Sci. 2018, 75, 925–933. [Google Scholar] [CrossRef]
- Wang, P.; Shen, Y.; Wang, C.; Hou, J.; Qian, J.; Yu, Y.; Kong, N. An improved habitat model to evaluate the impact of water conservancy projects on Chinese sturgeon (Acipenser sinensis) spawning sites in the Yangtze River, China. Ecol. Eng. 2017, 104, 165–176. [Google Scholar] [CrossRef]
- Du, H.; Wei, Q.; Zhang, H.; Liu, Z.; Wang, C.; Li, Y. Bottom substrate attributes relative to bedform morphology of spawning site of Chinese sturgeon Acipenser sinensis below the Gezhouba dam. J. Appl. Ichthyol. 2011, 27, 257–262. [Google Scholar] [CrossRef]
- Duerregger, A.; Pander, J.; Palt, M.; Mueller, M.; Nagel, C.; Geist, J. The importance of stream interstitial conditions for the early-life-stage development of the European nase (Chondrostoma nasus L.). Ecol. Freshw. Fish 2018, 27, 920–932. [Google Scholar] [CrossRef]
- Merz, J.E.; Setka, J.D.; Pasternack, G.B.; Wheaton, J.M. Predicting benefits of spawning-habitat rehabilitation to salmonid (Oncorhynchus spp.) fry production in a regulated California river. Can. J. Fish. Aquat. Sci. 2004, 61, 1433–1446. [Google Scholar] [CrossRef]
- Chapman, J.M.; Proulx, C.L.; Veilleux, M.A.; Levert, C.; Bliss, S.; Andre, M.-E.; Lapointe, N.W.; Cooke, S.J. Clear as mud: A meta-analysis on the effects of sedimentation on freshwater fish and the effectiveness of sediment-control measures. Water Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, Z.; An, R.; Cai, L.; Chen, X.; Zhao, X.; Zou, X. Water flow and substrate preferences of Schizothorax wangchiachii (Fang, 1936). Ecol. Eng. 2019, 138, 1–7. [Google Scholar] [CrossRef]
- Yao, W. Ecohydraulic tools for aquatic fauna habitat and population status assessment, analysis and monitoring aimed at promoting integrated river management. Ecol. Model. 2021, 456, 109682. [Google Scholar] [CrossRef]
- Wang, Q.; Han, Y.; Li, P.; Zhang, W.; Wang, Y.; Xi, Y.; Yao, W. Ecohydraulic modelling to evaluate cascade dam construction impact and support fish habitat restoration. Ecol. Eng. 2023, 192, 106974. [Google Scholar] [CrossRef]
- Armour, C.L.; Taylor, J.G. Evaluation of the instream flow incremental methodology by US Fish and Wildlife Service field users. Fisheries 1991, 16, 36–43. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, S. Review of aquatic species habitat simulation method and modelling. Sci. Sin. Technol. 2019, 49, 363–377. [Google Scholar] [CrossRef]
- Chen, Q.; Li, Q.; Lin, Y.; Zhang, J.; Xia, J.; Ni, J.; Cooke, S.J.; Best, J.; He, S.; Feng, T.; et al. River Damming Impacts on Fish Habitat and Associated Conservation Measures. Rev. Geophys. 2023, 61, e2023RG000819. [Google Scholar] [CrossRef]
- He, F.; Zarfl, C.; Tockner, K.; Olden, J.D.; Campos, Z.; Muniz, F.; Svenning, J.-C.; Jähnig, S.C. Hydropower impacts on riverine biodiversity. Nat. Rev. Earth Environ. 2024, 5, 755–772. [Google Scholar] [CrossRef]
- Csiki, S.J.; Rhoads, B.L. Influence of four run-of-river dams on channel morphology and sediment characteristics in Illinois, USA. Geomorphology 2014, 206, 215–229. [Google Scholar] [CrossRef]
- Kuriqi, A.; Pinheiro, A.N.; Sordo-Ward, A.; Bejarano, M.D.; Garrote, L. Ecological impacts of run-of-river hydropower plants—Current status and future prospects on the brink of energy transition. Renew. Sustain. Energy Rev. 2021, 142, 110833. [Google Scholar] [CrossRef]
- Li, T.; Wang, S.; Liu, Y.; Fu, B.; Zhao, W. Driving forces and their contribution to the recent decrease in sediment flux to ocean of major rivers in China. Sci. Total Environ. 2018, 634, 534–541. [Google Scholar] [CrossRef]
- Wenxuan, C.; Jianbo, C.; Ye, Q.; Zhonghua, D. Fish Resources of Early Life History Stagrs in Yangze River; China WaterPower Press: Beijing, China, 2007. [Google Scholar]
- Li, D.; Lu, X.X.; Yang, X.; Chen, L.; Lin, L. Sediment load responses to climate variation and cascade reservoirs in the Yangtze River: A case study of the Jinsha River. Geomorphology 2018, 322, 41–52. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, C.; Zhang, J. Study on variations of runoff and sediment and effect to the lower Jinsha River. J. Sediment Res. 2016, 5, 20–27. [Google Scholar]
- Zhu, L.; Chen, D.; Yang, C.; Chen, K.; Li, S. Sediment depOSItion of cascade reservoirs in the lower Jinsha River and scouring of river channel under dam. J. Lake Sci. 2023, 35, 1097–1110. [Google Scholar]
- Du, Z.; Dong, X.; Zhang, F.; Qin, L. Study on runoff and sediment characteristics and reservoir depOSItion in Xiluodu Reservoir of the Jinsha River. J. Sediment Res. 2022, 47, 22–28. [Google Scholar]
- Yue, L. The Evolution of Flow and Sediment Regime in the Yangtze River and Its Fish Response Mechanism. Master’s Thesis, North China University of Water Resources and Hydropower, Zhengzhou, China, 2021. [Google Scholar]
- Liu, S.; Li, D.; Liu, D.; Zhang, X.; Wang, Z. Characteristics of sedimentation and sediment trapping efficiency in the Three Gorges Reservoir, China. Catena 2022, 208, 105715. [Google Scholar] [CrossRef]
- Rodriguez, A.; McKee, B.; Miller, C.; Bost, M.; Atencio, A. Coastal sedimentation across North America doubled in the 20th century despite river dams. Nat. Commun. 2020, 11, 3249. [Google Scholar] [CrossRef]
- Csiki, S.; Rhoads, B.L. Hydraulic and geomorphological effects of run-of-river dams. Prog. Phys. Geogr. 2010, 34, 755–780. [Google Scholar] [CrossRef]
- Lehe, L.; Guoxi, W.; Zhiling, W. Reproduction ecology of Coreius heterodon (Bleeker) and Coreius guichenoti (Sauvage et Dabry) in the mainstream of the Changjiang River after the construction of Gezhouba Dam. Acta Hydrobiol. Sin. 1990, 14, 205–215. [Google Scholar]
- Meihua, X.; Ke, S.; Weitao, L.; Bin, Z. Research progress on resources variation and protection of Coreius guichenoti. Yangtze River 2023, 54, 63–71. [Google Scholar] [CrossRef]
- Jiang, Z.; Jiang, J.; Wang, Y.; Zhang, E.; Zhang, Y.; Li, L.; Xie, F.; Cai, B.; Cao, L.; Zheng, G.; et al. Red List of China’s Vertebrates. Biodivers. Sci. 2016, 24, 501–551+615. [Google Scholar]
- Ren, Y.; Zhao, L.; Cao, H.; Ruan, Y. Influence of ecological regulation of cascade reservoirs in the lower Jinsha River. Ecol. Environ. Monit. Three Gorges 2020, 5, 8–13. [Google Scholar]
- Zhang, D.; Fan, H.; Wang, M.; Li, F.; Ruan, Y. Target Fish Screening for the Ecological Operation of Wudongde Hydropower Station on Jinsha River. J. Hydroecology 2022, 43, 73–82. [Google Scholar]
- Yichao, Z. Impactof Dam on Natural Reproduction of Coreius guichenoti and Rhinogobio ventralis in Upstream of Yangtze River. Ph.D. Thesis, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China, 2009. [Google Scholar]
- Yang, Z.; Zhang, P.; Tang, H.; Gong, Y.; Dong, C.; Chen, X.; Zhao, N. The formation of habitat suitability curves for Coreius guichenoti (Sauvage & Dabry de Thiersant,1874) of the lower Jinsha River. Ecol. Sci. 2017, 36, 129–137. [Google Scholar]
- Zhang, P.; Qiao, Y.; Schineider, M.; Chang, J.; Mutzner, R.; Fluixa-Sanmartin, J.; Yang, Z.; Fu, R.; Chen, X.; Cai, L.; et al. Using a hierarchical model framework to assess climate change and hydropower operation impacts on the habitat of an imperiled fish in the Jinsha River, China. Sci. Total Environ. 2019, 646, 1624–1638. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Hao, Z.; Wang, X.; Liu, M.; Wang, Y. Multi-objective assessment of the ecological flow requirement in the upper Yangtze national nature reserve in China using PHABSIM. Water 2018, 10, 326. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, P.; Li, H.; You, L.; Li, Y.; Li, J.; Liu, M.; Zhao, P.; Wang, K.; Zhu, Z. Assessment and conservation strategies for endemic fish with drifting eggs threatened by the cascade barrier effect: A case study in the Yalong River, China. Ecol. Eng. 2021, 170, 106364. [Google Scholar] [CrossRef]
- Ruihua, S.; Anxiu, P. Variation of Water Level-discharge Relationship in Sanduizi Hydrological Station after Impoundment of Wudongde Power Station. Ecol. Environ. Monit. Three Gorges 2023, 8, 50–56. [Google Scholar] [CrossRef]
- Broekhoven, E.V.; Adriaenssens, V.; Baets, B.D.; Verdonschot, P.F.M. Fuzzy rule-based macroinvertebrate habitat suitability models for running waters. Ecol. Model. 2006, 198, 71–84. [Google Scholar] [CrossRef]
- Jorde, K.; Schneider, M.; Peter, A.; Zoellner, F. Fuzzy based models for the evaluation of fish habitat quality and instream flow assessment. In Proceedings of the 3rd International Symposium on Environmental Hydraulics, Tempe, AZ, USA, 5–8 December 2001; pp. 27–28. [Google Scholar]
- Theodoropoulos, C.; Vourka, A.; Skoulikidis, N.; Rutschmann, P.; Stamou, A. Evaluating the performance of habitat models for predicting the environmental flow requirements of benthic macroinvertebrates. J. Ecohydraulics 2018, 3, 30–44. [Google Scholar] [CrossRef]
- Li, T.; Mo, K.; Wang, J.; Chen, Q.; Zhang, J.; Zeng, C.; Zhang, H.; Yang, P. Mismatch between critical and accumulated temperature following river damming impacts fish spawning. Sci. Total Environ. 2021, 756, 144052. [Google Scholar] [CrossRef]
- Young, P.S.; Cech, J.J.; Thompson, L.C. Hydropower-related pulsed-flow impacts on stream fishes: A brief review, conceptual model, knowledge gaps, and research needs. Rev. Fish Biol. Fish. 2011, 21, 713–731. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, J.; Chen, Y.; Mo, K.; Wang, J.; Tang, L.; Lin, Y.; Chen, L.; Gao, Y.; Jiang, W. Inducing flow velocities to manage fish reproduction in regulated rivers. Engineering 2021, 7, 178–186. [Google Scholar] [CrossRef]
- Zhang, P.; Yang, Z.; Cai, L.; Qiao, Y.; Chen, X.; Chang, J. Effects of upstream and downstream dam operation on the spawning habitat suitability of Coreius guichenoti in the middle reach of the Jinsha River. Ecol. Eng. 2018, 120, 198–208. [Google Scholar] [CrossRef]
- Ruijing, Z. River Sediment Dynamics; ChinaWater&Power Press: Beijing, China, 1998. [Google Scholar]
- Ministry of Water Resources of the People’s Republic of China. China Hydrological Yearbook; China Water Power Press: Beijing, China, 2021. [Google Scholar]
- China Three Gorges Corporation. Reservoir Application and Power Station Operation Regulation for the Wudongde Hydropower Station on the Jinsha River; China Water & Power Press: Beijing, China, 2020. [Google Scholar]
- Ruan, Y.; Tuo, Y.; Deng, Y.; Xu, X. Prediction of water temperature in wudongde reservoir and mitigation measures of low-temperature water. Resour. Environ. Yangtze Basin 2017, 26, 1912–1918. [Google Scholar]
- Yao, W. Application of the Ecohydraulic Model on Hydraulic and Water Resources Engineering. Ph.D. Thesis, Technische Universität München, Munich, Germany, 2016. [Google Scholar]
- Yang, Z.; Tang, H.; Tao, J.; Zhao, N. The effect of cascaded huge dams on the downstream movement of Coreius guichenoti (Sauvage & Dabry de Thiersant, 1874) in the upper Yangtze River. Environ. Biol. Fishes 2017, 100, 1507–1516. [Google Scholar]
- Zhang, P.; Cai, L.; Yang, Z.; Chen, X.; Qiao, Y.; Chang, J. Evaluation of fish habitat suitability using a coupled ecohydraulic model: Habitat model selection and prediction. River Res. Appl. 2018, 34, 937–947. [Google Scholar] [CrossRef]
- Yu, L.; Lin, J.; Chen, D.; Duan, X.; Peng, Q.; Liu, S. Ecological flow assessment to improve the spawning habitat for the four major species of carp of the Yangtze River: A study on habitat suitability based on ultrasonic telemetry. Water 2018, 10, 600. [Google Scholar] [CrossRef]
- Zhitang, Y.; Zhixin, L.; Bolu, Y. The early development of Coreius heterodon and Coreius guichenoti. Acta Hydrobiol. Sin. 1984, 8, 371–388. [Google Scholar]
- Kolar, C.S.; Chapman, D.; Courtenay, W.R., Jr.; Housel, C.M.; Williams, J.D.; Jennings, D.P. Bigheaded Carps: A Biological Synopsis and Environmental Risk Assessment; American Fisheries Society: Baltimore, MD, USA, 2007. [Google Scholar]
- Kocovsky, P.M.; Chapman, D.C.; McKenna, J.E. Thermal and hydrologic suitability of Lake Erie and its major tributaries for spawning of Asian carps. J. Great Lakes Res. 2012, 38, 159–166. [Google Scholar] [CrossRef]
- Stanley, J.G.; Miley, W.W.; Sutton, D.L. Reproductive requirements and likelihood for naturalization of escaped grass carp in the United States. Trans. Am. Fish. Soc. 1978, 107, 119–128. [Google Scholar] [CrossRef]
- Leslie, A.J., Jr.; Van Dyke, J.M.; Nall, L.E.; Miley, W.W. Current velocity for transport of grass carp eggs. Trans. Am. Fish. Soc. 1982, 111, 99–101. [Google Scholar] [CrossRef]
- George, A.E.; Chapman, D.C.; Deters, J.E.; Erwin, S.O.; Hayer, C.A. Effects of sediment burial on grass carp, Ctenopharyngodon idella (Valenciennes, 1844), eggs. J. Appl. Ichthyol. 2015, 31, 1120–1126. [Google Scholar] [CrossRef]
- Garcia, T.; Zamalloa, C.Z.; Jackson, P.R.; Murphy, E.A.; Garcia, M.H. A Laboratory Investigation of the Suspension, Transport, and Settling of Silver Carp Eggs Using Synthetic Surrogates. PLoS ONE 2015, 10, e0145775. [Google Scholar] [CrossRef]




| Substrate | Temperature | Velocity | Depth | HSI |
|---|---|---|---|---|
| L/M/H | M | L/H | L/H | M |
| L/M/H | L/H | M | L/H | M |
| M | L/H | L/H | M | M |
| L/H | L/H | L/H | M | L |
| L/M/H | M | M | L/H | H |
| L/M/H | M | L/H | M | H |
| M | L/H | M | M | H |
| L/H | L/H | M | M | M |
| L/M/H | M | M | M | VH |
| VL | AC | AC | AC | L |
| L/M/H | VL/VH | AC | AC | L |
| L/M/H | L/M/H | VL/VH | AC | L |
| L/M/H | L/M/H | L/M/H | VL/VH | L |
| Time | Discharge (m3/s) | Water Level (m) | Manning Coefficient | Temperature (°C) |
|---|---|---|---|---|
| First half of March | 1884 | 970.46 | 0.1780 | 13.40 |
| Second half of March | 2023.63 | 969.94 | 0.1780 | 13.40 |
| First half of April | 1824 | 970.26 | 0.1810 | 16.10 |
| Second half of April | 2066.67 | 970.19 | 0.1810 | 16.10 |
| First half of May | 1840.67 | 968.17 | 0.1704 | 18.70 |
| Second half of May | 2805 | 957.74 | 0.1704 | 18.70 |
| First half of June | 3610.67 | 950.03 | 0.1071 | 20.60 |
| Second half of June | 5778.67 | 952.14 | 0.1071 | 20.60 |
| First half of July | 4654.67 | 951.51 | 0.0883 | 21.10 |
| Second half of July | 9596.25 | 973.66 | 0.0883 | 21.10 |
| Time | WUA (105 m2) | OSI | ISP | MSP | LSP |
|---|---|---|---|---|---|
| March to April | 2.41 | 0.06 | 0.00% | 0.00% | 100.00% |
| First half of May | 4.80 | 0.11 | 0.00% | 22.58% | 77.42% |
| Second half of May | 4.70 | 0.11 | 0.00% | 18.47% | 81.53% |
| First half of June | 6.79 | 0.15 | 3.81% | 20.68% | 75.51% |
| Second half of June | 6.11 | 0.14 | 2.72% | 16.37% | 80.92% |
| First half of July | 5.59 | 0.13 | 2.63% | 14.89% | 82.48% |
| Second half of July | 5.40 | 0.12 | 1.29% | 13.07% | 85.63% |
| Time | WUA (105 m2) | OSI | ISP | MSP | LSP |
|---|---|---|---|---|---|
| March to April | 0.00% | 0.00% | 0.00% | 0.00% | 0.00% |
| First half of May | 42.31% | 42.31% | 0.00% | 236.04% | −17.00% |
| Second half of May | 38.73% | 38.73% | 0.00% | 614.56% | −16.30% |
| First half of June | −0.13% | −0.13% | −1.68% | 0.21% | 0.03% |
| Second half of June | −0.17% | −0.17% | −1.74% | −0.18% | 0.10% |
| First half of July | −0.16% | −0.16% | −1.33% | −0.26% | 0.09% |
| Second half of July | −0.19% | −0.19% | −3.01% | −0.46% | 0.12% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Chen, D.; Zhu, L.; Liu, T.; Wang, H.; Zhang, L.; Han, R.; Yang, Z.; Yan, J.; Yang, H.; et al. Modeling Spawning Habitats of Coreius guichenoti with Substrate Considerations: A Case Study of Pingdi Town in the Lower Jinsha River. Animals 2025, 15, 881. https://doi.org/10.3390/ani15060881
Li W, Chen D, Zhu L, Liu T, Wang H, Zhang L, Han R, Yang Z, Yan J, Yang H, et al. Modeling Spawning Habitats of Coreius guichenoti with Substrate Considerations: A Case Study of Pingdi Town in the Lower Jinsha River. Animals. 2025; 15(6):881. https://doi.org/10.3390/ani15060881
Chicago/Turabian StyleLi, Wenchao, Dong Chen, Lekui Zhu, Tong Liu, Hanyue Wang, Litao Zhang, Rui Han, Zhi Yang, Jun Yan, Hongyi Yang, and et al. 2025. "Modeling Spawning Habitats of Coreius guichenoti with Substrate Considerations: A Case Study of Pingdi Town in the Lower Jinsha River" Animals 15, no. 6: 881. https://doi.org/10.3390/ani15060881
APA StyleLi, W., Chen, D., Zhu, L., Liu, T., Wang, H., Zhang, L., Han, R., Yang, Z., Yan, J., Yang, H., Guo, A., & Liu, L. (2025). Modeling Spawning Habitats of Coreius guichenoti with Substrate Considerations: A Case Study of Pingdi Town in the Lower Jinsha River. Animals, 15(6), 881. https://doi.org/10.3390/ani15060881






