Copeptin Hormone Concentrations in Dogs with Heart Disease and Relationship with Antidiuretic Hormone
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Electrolyte Measurements and Calculated Osmolality
2.3. Assay Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADH | antidiuretic hormone |
| ACVIM | American College of Veterinary Internal Medicine |
| calOsm | calculated serum osmolality |
| Cl− | chloride |
| CHF | congestive heart failure |
| cCl− | corrected serum chloride concentration |
| DCM | dilated cardiomyopathy |
| LOA | limit of agreement |
| RAAS | renin-angiotensin aldosterone system |
| SD | standard deviation |
| Na+ | sodium |
References
- Ames, M.K.; Atkins, C.E.; Pitt, B. The renin-angiotensin-aldosterone system and its suppression. J. Vet. Intern. Med. 2019, 33, 363–382. [Google Scholar] [CrossRef] [PubMed]
- Keene, B.W.; Atkins, C.E.; Bonagura, J.D.; Fox, P.R.; Häggström, J.; Fuentes, V.L.; Oyama, M.A.; Rush, J.E.; Stepien, R.; Uechi, M. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 2019, 33, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Riegger, A.J.; Liebau, G. The renin-angiotensin-aldosterone system, antidiuretic hormone and sympathetic nerve activity in an experimental model of congestive heart failure in the dog. Clin. Sci. 1982, 62, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Beaumier, A.; Rush, J.E.; Yang, V.K.; Freeman, L.M. Clinical findings and survival time in dogs with advanced heart failure. J. Vet. Intern. Med. 2018, 32, 944–950. [Google Scholar] [CrossRef]
- Iwanaga, K.; Araki, R.; Isaka, M. A retrospective study of 14 dogs with advanced heart failure treated with loop diuretics and hydrochlorothiazide. Open Vet. J. 2021, 11, 342–345. [Google Scholar] [CrossRef]
- Adin, D.; Kurtz, K.; Atkins, C.; Papich, M.G.; Vaden, S. Role of electrolyte concentrations and renin-angiotensin-aldosterone activation in the staging of canine heart disease. J. Vet. Intern. Med. 2020, 34, 53–64. [Google Scholar] [CrossRef]
- Oyama, M.A.; Adin, D. Toward quantification of loop diuretic responsiveness for congestive heart failure. J. Vet. Intern. Med. 2023, 37, 12–21. [Google Scholar] [CrossRef]
- Loughran, K.A.; Larouche-Lebel, É.; Huh, T.; Testani, J.M.; Rao, V.S.; Oyama, M.A. Prediction and measurement of diuretic responsiveness after oral administration of furosemide to healthy dogs and dogs with congestive heart failure. J. Vet. Intern. Med. 2020, 34, 2253–2264. [Google Scholar] [CrossRef]
- Roche-Catholy, M.; Van Cappellen, I.; Locquet, L.; Broeckx, B.J.G.; Paepe, D.; Smets, P. Clinical relevance of serum electrolytes in dogs and cats with acute heart failure: A retrospective study. J. Vet. Intern. Med. 2021, 35, 1652–1662. [Google Scholar] [CrossRef]
- Vantyghem, M.C.; Balavoine, A.S.; Wémeau, J.L.; Douillard, C. Hyponatremia and antidiuresis syndrome. Ann. Endocrinol. 2011, 72, 500–512. [Google Scholar] [CrossRef]
- Riegger, G.A.; Liebau, G.; Kochsiek, K. Antidiuretic hormone in congestive heart failure. Am. J. Med. 1982, 72, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Szczepanska-Sadowska, E.; Czarzasta, K.; Cudnoch-Jedrzejewska, A. Dysregulation of the Renin-Angiotensin System and the Vasopressinergic System Interactions in Cardiovascular Disorders. Curr. Hypertens. Rep. 2018, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Tidholm, A.; Häggström, J.; Hansson, K. Vasopressin, cortisol, and catecholamine concentrations in dogs with dilated cardiomyopathy. Am. J. Vet. Res. 2005, 66, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Scollan, K.F.; Bulmer, B.J.; Sisson, D.D. Validation of a commercially available enzyme immunoassay for measurement of plasma antidiuretic hormone concentration in healthy dogs and assessment of plasma antidiuretic hormone concentration in dogs with congestive heart failure. Am. J. Vet. Res. 2013, 74, 1206–1211. [Google Scholar] [CrossRef]
- Ter Maaten, J.M.; Damman, K.; Hanberg, J.S.; Givertz, M.M.; Metra, M.; O’Connor, C.M.; Teerlink, J.R.; Ponikowski, P.; Cotter, G.; Davison, B.; et al. Hypochloremia, Diuretic Resistance, and Outcome in Patients With Acute Heart Failure. Circ. Heart Fail. 2016, 9, e003109. [Google Scholar] [CrossRef]
- Bellino, M.C.; Massari, F.; Albanese, M.; Ursi, R.; Angelini, G.; Lisi, F.; Amato, L.; Scicchitano, P.; Guida, P.; Brunetti, N.D.; et al. Baseline and incident hypochloremia in chronic heart failure outpatients: Clinical correlates and prognostic role. Eur. J. Intern. Med. 2021, 84, 32–37. [Google Scholar] [CrossRef]
- Li, Z.; Xing, C.; Li, T.; Du, L.; Wang, N. Hypochloremia is associated with increased risk of all-cause mortality in patients in the coronary care unit: A cohort study. J. Int. Med. Res. 2020, 48, 300060520911500. [Google Scholar] [CrossRef]
- Adin, D.; Atkins, C.; Londoño, L.; Del Nero, B. Correction of serum chloride concentration in dogs with congestive heart failure. J. Vet. Intern. Med. 2021, 35, 51–57. [Google Scholar] [CrossRef]
- Harris, A.N.; Hanner, C.; Cooper, A.; Castro, R.A.; Adin, D.B. Antidiuretic hormone concentrations in dogs with heart disease and relationship to serum chloride. J. Vet. Cardiol. 2025, 59, 15–23. [Google Scholar] [CrossRef]
- Alehagen, U.; Dahlström, U.; Rehfeld, J.F.; Goetze, J.P. Association of copeptin and N-terminal proBNP concentrations with risk of cardiovascular death in older patients with symptoms of heart failure. JAMA 2011, 305, 2088–2095. [Google Scholar] [CrossRef]
- Jalleh, R.; Torpy, D.J. The Emerging Role of Copeptin. Clin. Biochemist. Rev. 2021, 42, 17–25. [Google Scholar] [CrossRef]
- Yuen, T.; Gouda, P.; Margaryan, R.; Ezekowitz, J. Do Heart Failure Biomarkers Influence Heart Failure Treatment Response? Curr. Heart Fail. Rep. 2023, 20, 358–373. [Google Scholar] [CrossRef]
- Burckhardt, M.A.; Gotta, V.; Beglinger, S.; Renggli, L.; Bachmann, S.; Hess, M.; Rentsch, K.; Pfister, M.; Koch, G.; Davis, E.A.; et al. Copeptin Kinetics and Its Relationship to Osmolality During Rehydration for Diabetic Ketoacidosis in Children. J. Clin. Endocrinol. Metab. 2020, 105, e4169–e4178. [Google Scholar] [CrossRef]
- Refardt, J.; Winzeler, B.; Christ-Crain, M. Copeptin and its role in the diagnosis of diabetes insipidus and the syndrome of inappropriate antidiuresis. Clin. Endocrinol. 2019, 91, 22–32. [Google Scholar] [CrossRef]
- Neuhold, S.; Huelsmann, M.; Strunk, G.; Stoiser, B.; Struck, J.; Morgenthaler, N.G.; Bergmann, A.; Moertl, D.; Berger, R.; Pacher, R. Comparison of copeptin, B-type natriuretic peptide, and amino-terminal pro-B-type natriuretic peptide in patients with chronic heart failure: Prediction of death at different stages of the disease. J. Am. Coll. Cardiol. 2008, 52, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.; Squire, I.B.; Khan, S.Q.; Quinn, P.; Struck, J.; Morgenthaler, N.G.; Davies, J.E.; Ng, L.L. C-terminal provasopressin (copeptin) is associated with left ventricular dysfunction, remodeling, and clinical heart failure in survivors of myocardial infarction. J. Card. Fail. 2008, 14, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.Q.; Dhillon, O.S.; O’Brien, R.J.; Struck, J.; Quinn, P.A.; Morgenthaler, N.G.; Squire, I.B.; Davies, J.E.; Bergmann, A.; Ng, L.L. C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) study. Circulation 2007, 115, 2103–2110. [Google Scholar] [CrossRef]
- Reichlin, T.; Hochholzer, W.; Stelzig, C.; Laule, K.; Freidank, H.; Morgenthaler, N.G.; Bergmann, A.; Potocki, M.; Noveanu, M.; Breidthardt, T.; et al. Incremental value of copeptin for rapid rule out of acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 60–68. [Google Scholar] [CrossRef]
- Pelligand, L.; Guillot, E.; Geneteau, A.; Guyonnet, J.; Magnier, R.; Elliott, J.; Peyrou, M.; Jacobs, M. Population Pharmacokinetics and Pharmacodynamics Modeling of Torasemide and Furosemide After Oral Repeated Administration in Healthy Dogs. Front. Vet. Sci. 2020, 7, 151. [Google Scholar] [CrossRef]
- Balanescu, S.; Kopp, P.; Gaskill, M.B.; Morgenthaler, N.G.; Schindler, C.; Rutishauser, J. Correlation of plasma copeptin and vasopressin concentrations in hypo-, iso-, and hyperosmolar States. J. Clin. Endocrinol. Metab. 2011, 96, 1046–1052. [Google Scholar] [CrossRef]
- Brownstein, M.J.; Russell, J.T.; Gainer, H. Synthesis, transport, and release of posterior pituitary hormones. Science 1980, 207, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, G.; Subburaju, S.; Young, S.; Chen, J. The parvocellular vasopressinergic system and responsiveness of the hypothalamic pituitary adrenal axis during chronic stress. Prog. Brain Res. 2008, 170, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Holwerda, D.A. A glycopeptide from the posterior lobe of pig pituitaries. I. Isolation and characterization. Eur. J. Biochem. 1972, 28, 334–339. [Google Scholar] [CrossRef]
- Morgenthaler, N.G.; Struck, J.; Alonso, C.; Bergmann, A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin. Chem. 2006, 52, 112–119. [Google Scholar] [CrossRef]
- Dupuy, A.M.; Chastang, E.; Cristol, J.P.; Jreige, R.; Lefebvre, S.; Sebbane, M. Analytical performances of the newly developed, fully automated Kryptor Copeptin assay: Which impact factor for myocardial infarction rules out in the emergency department? Clin. Lab. 2012, 58, 635–644. [Google Scholar]
- Dong, X.Q.; Huang, M.; Yang, S.B.; Yu, W.H.; Zhang, Z.Y. Copeptin is associated with mortality in patients with traumatic brain injury. J. Trauma 2011, 71, 1194–1198. [Google Scholar] [CrossRef] [PubMed]
- Hara, T.; Ohtomo, Y.; Endo, A.; Niijima, S.; Yasui, M.; Shimizu, T. Evaluation of Urinary Aquaporin 2 and Plasma Copeptin as Biomarkers of Effectiveness of Desmopressin Acetate for the Treatment of Monosymptomatic Nocturnal Enuresis. J. Urol. 2017, 198, 921–927. [Google Scholar] [CrossRef]
- Sailer, C.O.; Refardt, J.; Blum, C.A.; Schnyder, I.; Molina-Tijeras, J.A.; Fenske, W.; Christ-Crain, M. Validity of different copeptin assays in the differential diagnosis of the polyuria-polydipsia syndrome. Sci. Rep. 2021, 11, 10104. [Google Scholar] [CrossRef]
- Zimodro, J.M.; Gasecka, A.; Jaguszewski, M.; Amanowicz, S.; Szkiela, M.; Denegri, A.; Pruc, M.; Duchnowski, P.; Peacock, F.W.; Rafique, Z.; et al. Role of copeptin in diagnosis and outcome prediction in patients with heart failure: A systematic review and meta-analysis. Biomarkers 2022, 27, 720–726. [Google Scholar] [CrossRef]
- Golembiewska, E.; Qureshi, A.R.; Dai, L.; Lindholm, B.; Heimbürger, O.; Söderberg, M.; Brismar, T.B.; Ripsweden, J.; Barany, P.; Johnson, R.J.; et al. Copeptin is independently associated with vascular calcification in chronic kidney disease stage 5. BMC Nephrol. 2020, 21, 43. [Google Scholar] [CrossRef]
- Dobsa, L.; Edozien, K.C. Copeptin and its potential role in diagnosis and prognosis of various diseases. Biochem. Med. 2013, 23, 172–190. [Google Scholar] [CrossRef] [PubMed]
- El Amrousy, D.; Abdelhai, D.; Nassar, M. Predictive Value of Plasma Copeptin Level in Children with Acute Heart Failure. Pediatr. Cardiol. 2022, 43, 1737–1742. [Google Scholar] [CrossRef] [PubMed]
- Quoniam, C.; Hay, L.; Roll, J.P.; Harlay, F. Age effects on reflex and postural responses to propriomuscular inputs generated by tendon vibration. J. Gerontol. Biol. Sci. Med. Sci. 1995, 50, B155–B165. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E. The Heart as a Target of Vasopressin and Other Cardiovascular Peptides in Health and Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 4414. [Google Scholar] [CrossRef] [PubMed]
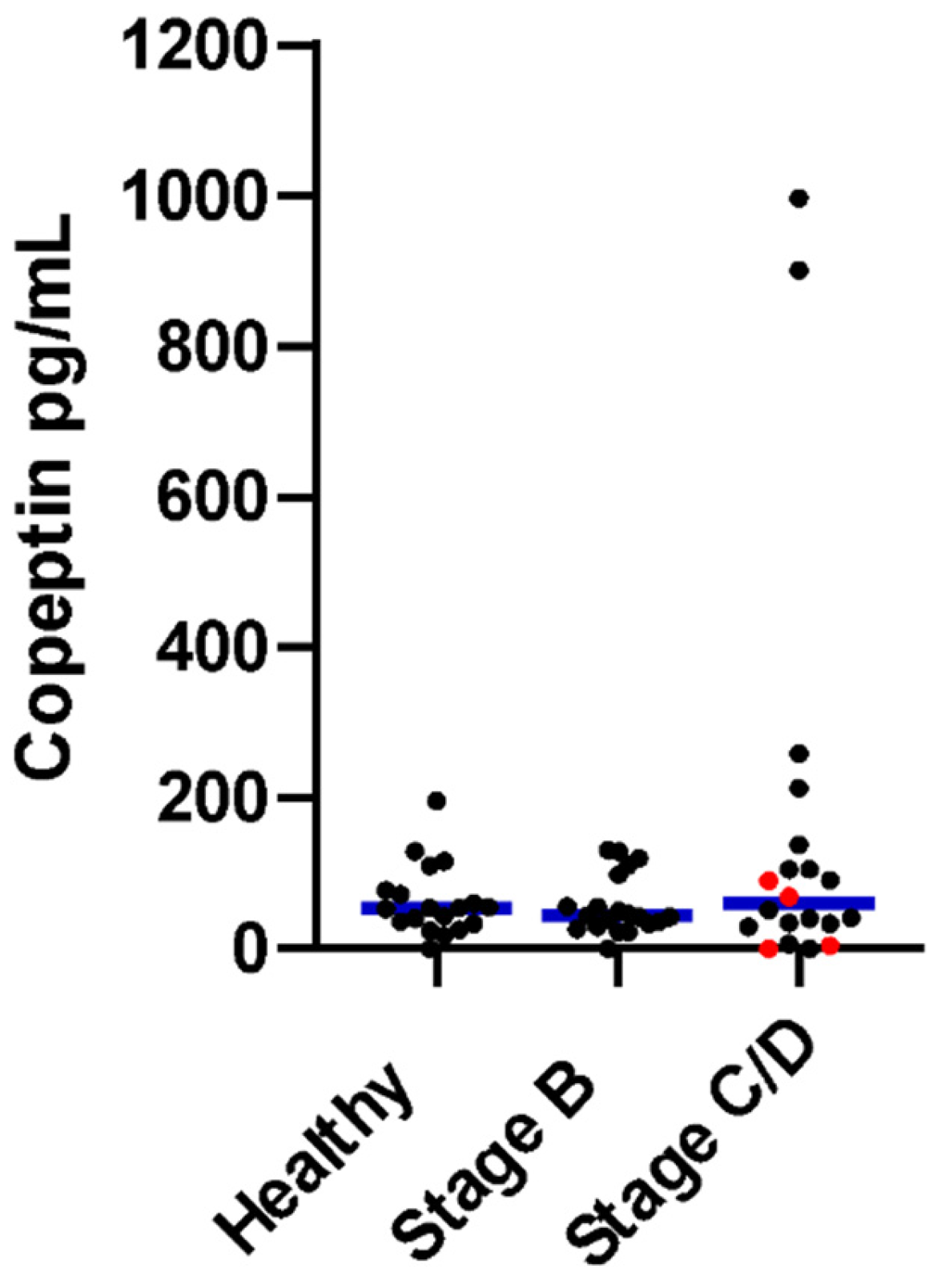
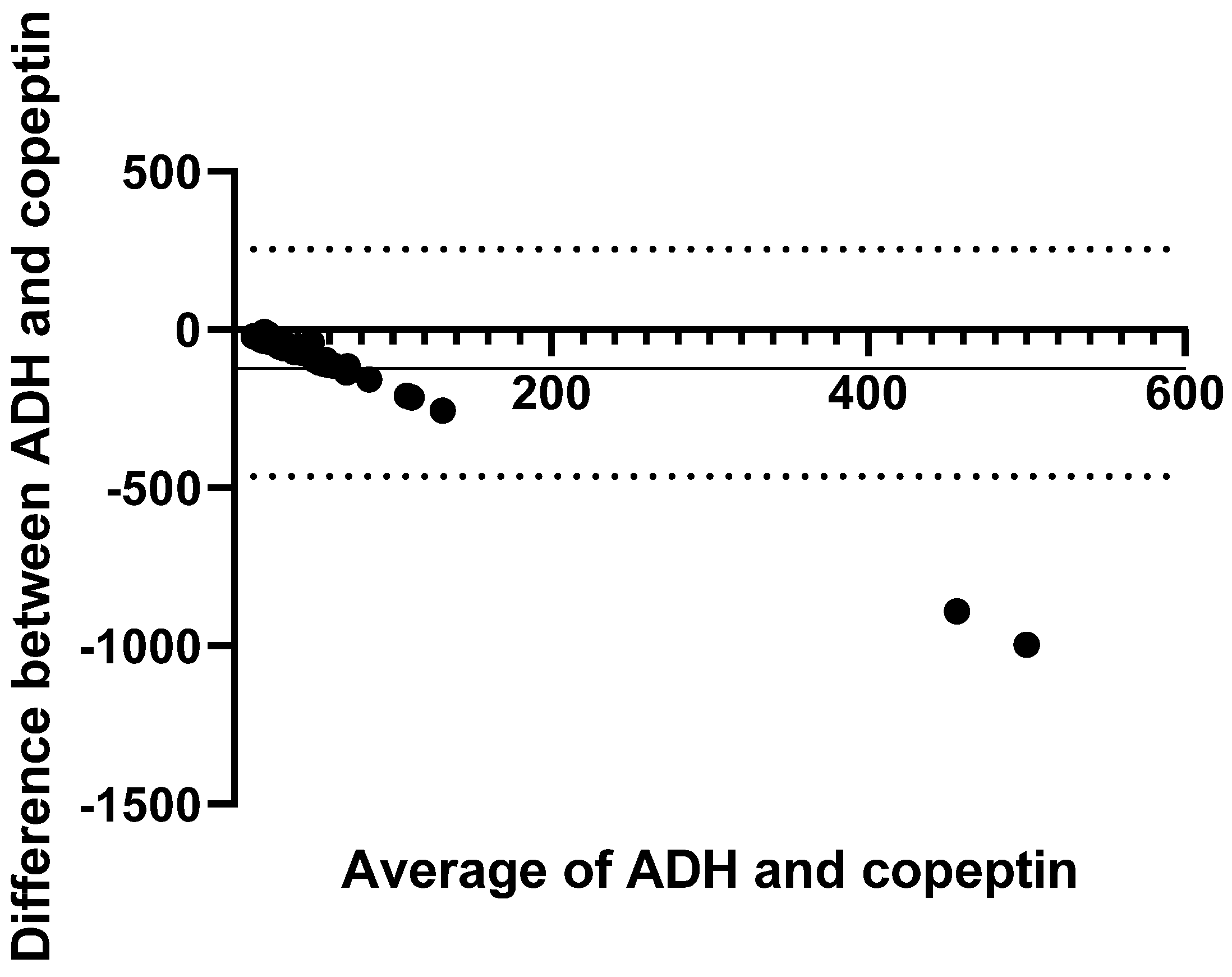
| Variable | Healthy | Preclinical (Stage B) | CHF (Stage C/D) | p Value |
|---|---|---|---|---|
| Age (months) | 62 (24–148) | 124 (82–191) * | 144 (37–182) * | <0.001 |
| Weight (kg) | 15.7 (3.4–32.6) | 8.6 (3.2–32.9) | 8.6 (4.2–71.5) | 0.10 |
| Sex | 0 FI, 11 FS, 0 MI, 8 MC | 0 FI, 8 FS, 1 MI, 11 MC | 0 FI, 8 FS, 3 MI, 9 MC | 0.46 |
| [Na+] (reference range 141.9–150.6 mEq/L) | 146.4 (141.1–148.6) | 146.8 (145.1–151.6) | 145.4 (138.7–153.7) | 0.19 |
| [Cl−] (reference range 107.8–117.1 mEq/L) | 113.8 (109.4–117.6) # | 111.3 (105.4–115.7) * | 108.0 (97.9–112.5) *# | <0.001 |
| Corrected [Cl−] (mEq/L) | 113.7 (109.4–117.6) # | 110.6 (104.4–113.8) * | 107.3 (100.1–113.9) *# | <0.001 |
| Amount of [Cl−] correction (mEq/L) | −0.10 (−1.9–4.1) | −0.40 (−4.1–0.9) | 0.70 (−5.4–5.6) | 0.17 |
| Antidiuretic hormone (pg/mL) | 3.4 (0.1–6.2) | 6.5 (1.8–33.8)* | 6.7 (2.0–28.1) * | 0.007 |
| Copeptin hormone (pg/mL) | 54.9 (0.5–196.1) | 43.6 (0.5–131.4) | 60.5 (0.5–997.8) | 0.76 |
| Variable | β (95% CI) | Adjusted p |
|---|---|---|
| Age (months) | −4.52 (−7.12 to −1.92) | 0.002 |
| Loop diuretic dosage | −19.40 (−49.55 to 10.76) | 0.19 |
| [Cl−] | −6.31 (−41.84 to 29.22) | 0.71 |
| calOsm | −3.65 (−16.37 to 9.06) | 0.55 |
| ADH | −9.07 (−14.55 to 32.69) | 0.42 |
| RAAS inhibitor use | −158.6 (−407.8 to 90.51) | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavigne, C.; Adin, D.B.; Hanner, C.; Cooper, A.; Castro, R.A.; Harris, A.N. Copeptin Hormone Concentrations in Dogs with Heart Disease and Relationship with Antidiuretic Hormone. Animals 2025, 15, 1013. https://doi.org/10.3390/ani15071013
Lavigne C, Adin DB, Hanner C, Cooper A, Castro RA, Harris AN. Copeptin Hormone Concentrations in Dogs with Heart Disease and Relationship with Antidiuretic Hormone. Animals. 2025; 15(7):1013. https://doi.org/10.3390/ani15071013
Chicago/Turabian StyleLavigne, Corine, Darcy B. Adin, Courtney Hanner, Alexis Cooper, Rebeca A. Castro, and Autumn N. Harris. 2025. "Copeptin Hormone Concentrations in Dogs with Heart Disease and Relationship with Antidiuretic Hormone" Animals 15, no. 7: 1013. https://doi.org/10.3390/ani15071013
APA StyleLavigne, C., Adin, D. B., Hanner, C., Cooper, A., Castro, R. A., & Harris, A. N. (2025). Copeptin Hormone Concentrations in Dogs with Heart Disease and Relationship with Antidiuretic Hormone. Animals, 15(7), 1013. https://doi.org/10.3390/ani15071013





