The Persistence of Memory: Behavioral Analysis and Arm Usage of a Nine-Armed Octopus vulgaris
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
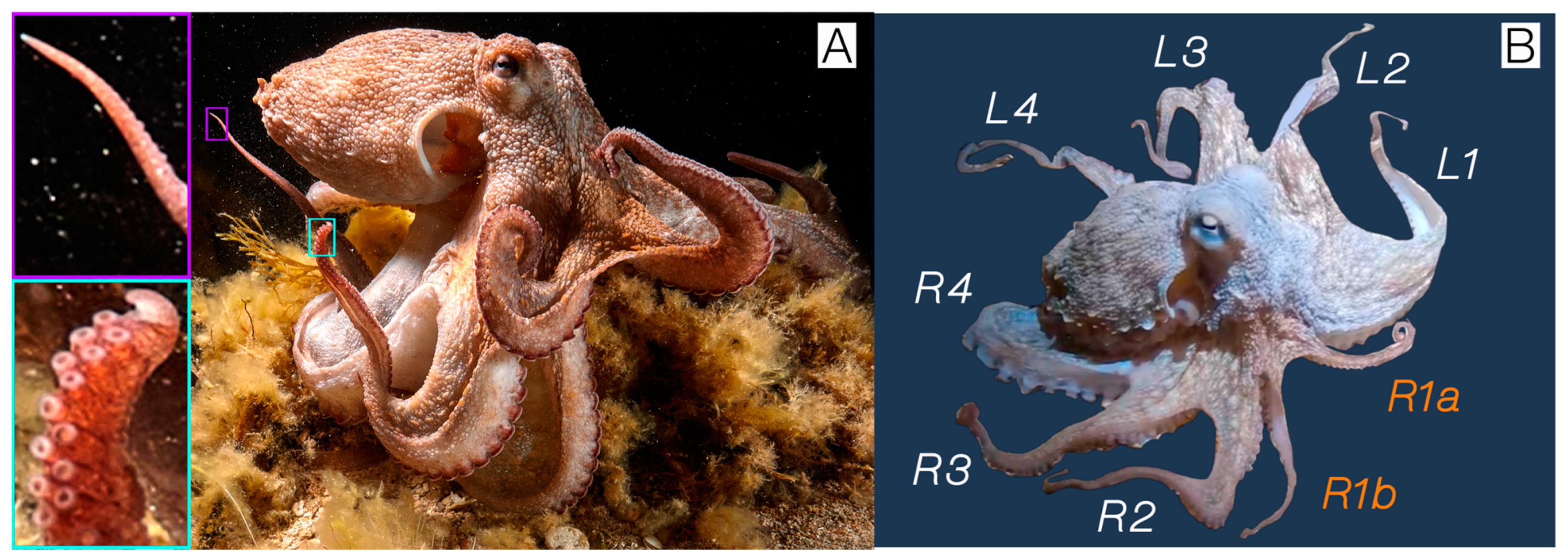
2.1. Study’s Subject
2.2. Study Site
2.3. Quantification of Behavioral Data
2.4. Data Manipulation and Exploration
2.5. Preliminary Analysis and Arm Groupings
2.6. Statistical Analysis
3. Results
3.1. Arm Usage and Their Behavioral Associations
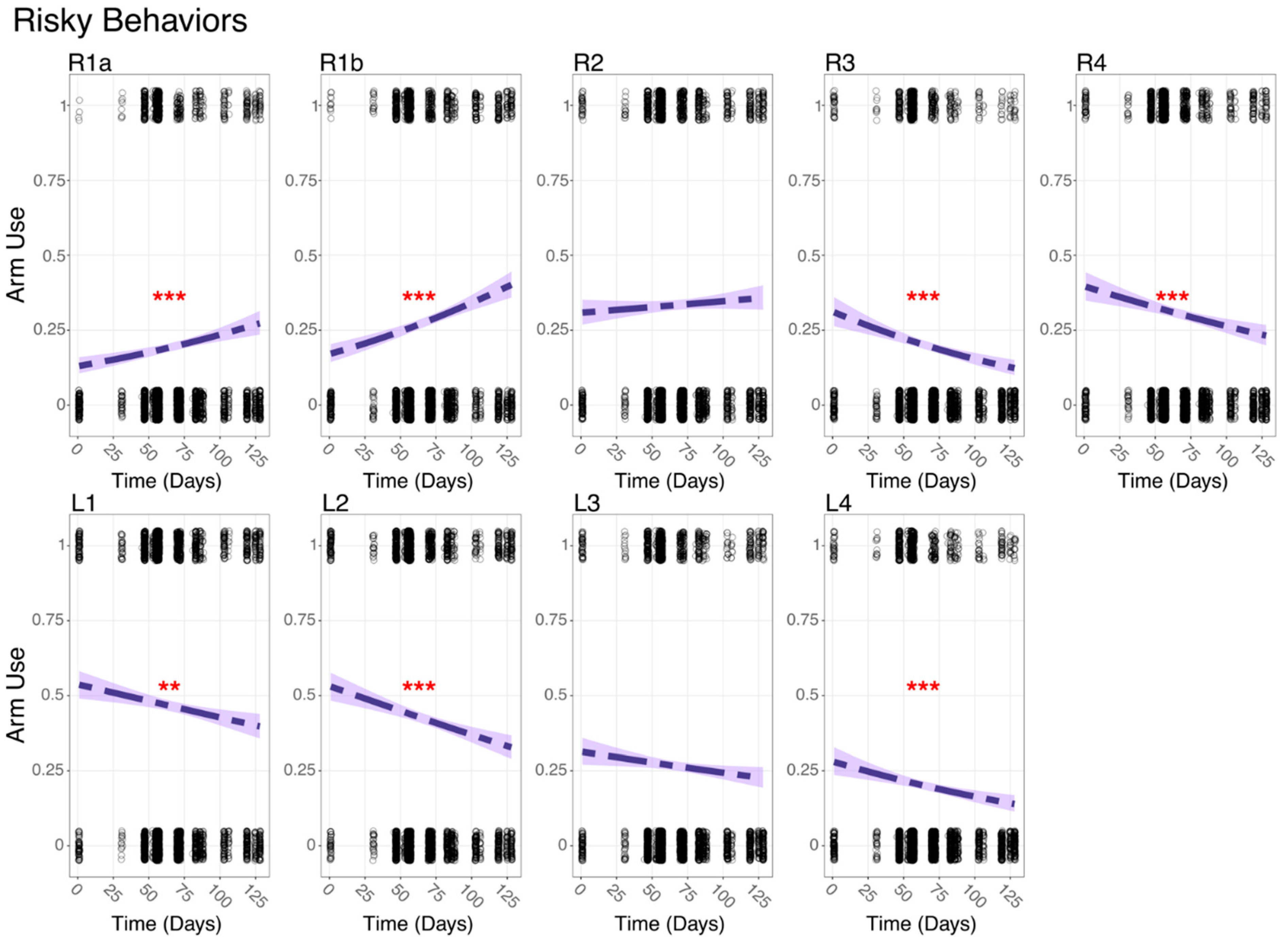
3.2. Risky and Safe Usage
3.3. Bifurcated Arms’ Specialized Use
3.4. Behavioral Change over Time
3.5. Behavioral Variations and Undescribed Behaviors
4. Discussion
4.1. Patterns of Arm Use
4.2. Persistence of Memory
4.3. Ecological and Neurological Significance
4.4. Future Lines of Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Imperadore, P.; Fiorito, G. Cephalopod tissue regeneration: Consolidating over a century of knowledge. Front. Physiol. 2018, 9, 593. [Google Scholar] [CrossRef]
- Lange, M. On the regeneration and finer structure of the arms of the cephalopods. J. Exp. Zool. 2005, 31, 1–57. [Google Scholar] [CrossRef]
- Voight, J. Movement, injuries and growth of members of a natural population of the Pacific pygmy octopus, Octopus digueti. J. Zool. 2009, 228, 247–264. [Google Scholar] [CrossRef]
- Alejo-Plata, C.; Valencia-Méndez, O. Arm abnormality in Octopus hubbsorum (Mollusca: Cephalopoda: Octopodidae). Am. Malacol. Bull. 2014, 32, 1–3. [Google Scholar] [CrossRef]
- Lee, P.N.; Callaerts, P.; De Couet, H.G.; Martindale, M.Q. Cephalopod Hox genes and the origin of morphological novelties. Nature 2003, 424, 1061–1065. [Google Scholar] [CrossRef]
- Gilbert, S.F. Developmental Biology, 10th ed.; Sinauer Associates, Inc.: Sunderland, MA, USA, 2014. [Google Scholar]
- Lodish, H.; Berk, A.; Kaiser, C.A.; Krieger, M.; Scott, M.P.; Bretscher, A.; Ploegh, H.; Matsudaira, P. Molecular Cell Biology, 5th ed.; W.H. Freeman and Co.: New York, NY, USA, 2003. [Google Scholar]
- Tarazona, O.A.; Lopez, D.H.; Slota, L.A.; Cohn, M.J. Evolution of limb development in cephalopod mollusks. eLife 2019, 8, e43828. [Google Scholar] [CrossRef]
- Grimaldi, A.; Tettamanti, G.; Acquati, F.; Bossi, E.; Guidali, M.L.; Banfi, S.; Monti, L.; Valvassori, R.; de Eguileor, M. A hedgehog homolog is involved in muscle formation and organization of Sepia officinalis (Mollusca) mantle. Dev. Dyn. 2008, 237, 659–671. [Google Scholar] [CrossRef] [PubMed]
- González, Á.F.; Guerra, Á. First observation of a double tentacle bifurcation in cephalopods. Mar. Biodivers. Rec. 2008, 1, e44. [Google Scholar] [CrossRef]
- Nakajima, R.; Shigeno, S.; Zullo, L.; De Sio, F.; Schmidt, M.R. Cephalopods between science, art, and engineering: A contemporary synthesis. Front. Commun. 2018, 3, 20. [Google Scholar] [CrossRef]
- O’Brien, C.E.; Roumbedakis, K.; Winkelmann, I.E. The current state of cephalopod science and perspectives on the most critical challenges ahead from three early-career researchers. Front. Physiol. 2018, 9, 700. [Google Scholar] [CrossRef]
- Friard, O.; Gamba, M. BORIS: A free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol. Evol. 2016, 7, 1325–1330. [Google Scholar] [CrossRef]
- Voss, K.M. Armed and dangerous: Patterns and drivers of octopus arm loss. Master’s Thesis, University of California Santa Cruz, Santa Cruz, CA, USA, 2022, unpublished. [Google Scholar]
- Borcard, D.; Gillet, F.; Legendre, P. Numerical Ecology with R; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.; O’Hara, B.; Simpson, G.; Solymos, P.; Stevens, H.; Wagner, H. Vegan: Community ecology package. R Package Version 2015, 2, 1–2. [Google Scholar]
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Peres-Neto, P.; Legendre, P.; Dray, S.; Borcard, D. Variation partitioning of species data matrices: Estimation and comparison of fractions. Ecology 2006, 87, 2614–2625. [Google Scholar] [CrossRef]
- Ezekiel, M. Methods of Correlation Analysis; Wiley: New York, NY, USA, 1930; 427p. [Google Scholar]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Huffard, C.L. Ethogram of Abdopus aculeatus (d’Orbigny, 1834) (Cephalopoda: Octopodidae): Can behavioural characters inform octopodid taxonomy and systematics? J. Molluscan Stud. 2007, 73, 185–193. [Google Scholar] [CrossRef]
- Mather, J.A. How do octopuses use their arms? J. Comp. Psychol. 1998, 112, 306–316. [Google Scholar] [CrossRef]
- Mather, J.A. Foraging, feeding and prey remains in middens of juvenile Octopus vulgaris (Mollusca: Cephalopoda). J. Zool. 1991, 224, 27–39. [Google Scholar] [CrossRef]
- Fiorito, G.; Gherardi, F. Prey-handling behaviour of Octopus vulgaris (Mollusca, Cephalopoda) on bivalve preys. Behav. Process. 1999, 46, 75–88. [Google Scholar] [CrossRef]
- Bidel, F.; Bennett, N.C.; Wardill, T.J. Octopus bimaculoides’ arm recruitment and use during visually evoked prey capture. Curr. Biol. 2022, 32, 4727–4733.e3. [Google Scholar] [CrossRef]
- Villanueva, R.; Perricone, V.; Fiorito, G. Cephalopods as predators: A short journey among behavioral flexibilities, adaptations, and feeding habits. Front. Physiol. 2017, 8, 598. [Google Scholar] [CrossRef] [PubMed]
- Flash, T.; Zullo, L. Biomechanics, motor control and dynamic models of the soft limbs of the octopus and other cephalopods. J. Exp. Biol. 2023, 226 (Suppl. 1), jeb245295. [Google Scholar] [CrossRef]
- Sumbre, G.; Gutfreund, Y.; Fiorito, G.; Flash, T.; Hochner, B. Control of octopus arm extension by a peripheral motor program. Science 2001, 293, 1845–1848. [Google Scholar] [CrossRef] [PubMed]
- Sumbre, G.; Fiorito, G.; Flash, T.; Hochner, B. Octopuses use a human-like strategy to control precise point-to-point arm movements. Curr. Biol. 2006, 16, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.; Kuba, M.; Meisel, D.; Griebel, U.; Mather, J. Does Octopus vulgaris have preferred arms? J. Comp. Psychol. 2006, 120, 198–204. [Google Scholar] [CrossRef]
- Kennedy, E.B.L.; Buresch, K.C.; Boinapally, P.; Hanlon, R.T. Octopus arms exhibit exceptional flexibility. Sci. Rep. 2020, 10, 20872. [Google Scholar] [CrossRef]
- Levy, G.; Flash, T.; Hochner, B. Arm coordination in octopus crawling involves unique motor control strategies. Curr. Biol. 2015, 25, 1195–1200. [Google Scholar] [CrossRef]
- Scheel, D.; Godfrey-Smith, P.; Lawrence, M. Signal use by octopuses in agonistic interactions. Curr. Biol. 2016, 26, 377–382. [Google Scholar] [CrossRef]
- Voss, K.M.; Mehta, R.S. Asymmetry in the frequency and proportion of arm truncation in three sympatric California Octopus species. Zoology 2021, 147, 125940. [Google Scholar] [CrossRef]
- Huffard, C.L.; Caldwell, R.L.; Boneka, F. Mating behavior of Abdopus aculeatus (d’Orbigny, 1834) (Cephalopoda: Octopodidae) in the wild. Mar. Biol. 2008, 154, 353–362. [Google Scholar] [CrossRef]
- Weertman, W.L.; Scheel, D. Hold it close: Male octopus hold their hectocotylus closer to their body. Mar. Biol. 2024, 171, 95. [Google Scholar] [CrossRef]
- Hernández-Urcera, J.; Garci, M.E.; Cabanellas-Reboredo, M. Bipedal locomotion by Octopus vulgaris. Mar. Biodivers. 2020, 50, 87. [Google Scholar] [CrossRef]
- Huffard, C.L.; Boneka, F.; Full, R.J. Underwater bipedal locomotion by octopuses in disguise. Science 2005, 307, 1927. [Google Scholar] [CrossRef]
- Gutnick, T.; Zullo, L.; Hochner, B.; Kuba, M.J. Use of peripheral sensory information for central nervous control of arm movement by Octopus vulgaris. Curr. Biol. 2020, 30, 4322–4327.e3. [Google Scholar] [CrossRef]
- Hochner, B. How nervous systems evolve in relation to their embodiment: What we can learn from octopuses and other molluscs. Brain Behav. Evol. 2013, 82, 19–30. [Google Scholar] [CrossRef]
- Zullo, L.; Sumbre, G.; Agnisola, C.; Flash, T.; Hochner, B. Nonsomatotopic organization of the higher motor centers in Octopus. Curr. Biol. 2009, 19, 1632–1636. [Google Scholar] [CrossRef] [PubMed]
- Zullo, L.; Eichenstein, H.; Maiole, F.; Hochner, B. Motor control pathways in the nervous system of Octopus vulgaris arm. J. Comp. Physiol. A 2019, 205, 271–279. [Google Scholar] [CrossRef]
- Budelmann, B.; Young, J. Brain pathways of the brachial nerves of Sepia and Loligo. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1987, 315, 345–352. [Google Scholar] [CrossRef]
- Crook, R.J. Conditioned place preference reveals tonic pain in octopus. bioRxiv 2020. [Google Scholar] [CrossRef]
- Crook, R.J. Behavioral and neurophysiological evidence suggests affective pain experience in octopus. iScience 2021, 24, 102229. [Google Scholar] [CrossRef]
- Côté, I.M.; Verde, A. Visual signals of the East Pacific red octopus (Octopus rubescens) during conspecific interactions. In Proceedings of the 38th American Academy of Underwater Sciences Symposium, Vancouver, BC, Canada, 8–11 October 2019. [Google Scholar]
- Messenger, J.B. Cephalopod chromatophores: Neurobiology and natural history. Biol. Rev. Camb. Philos. Soc. 2001, 76, 473–528. [Google Scholar] [CrossRef] [PubMed]
- Gutnick, T.; Byrne, R.A.; Hochner, B.; Kuba, M.J. Octopus vulgaris uses visual information to determine the location of its arm. Curr. Biol. 2011, 21, 460–462. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Kuba, M.J.; Meisel, D.V.; Griebel, U.; Mather, J.A. Octopus arm choice is strongly influenced by eye use. Behav. Brain Res. 2006, 172, 195–201. [Google Scholar] [CrossRef]
- Rodhouse, P.G.K.; Pierce, G.J.; Nichols, O.C.; Sauer, W.H.H.; Arkhipkin, A.I.; Laptikhovsky, V.V.; Lipiński, M.R.; Ramos, J.E.; Gras, M.; Kidokoro, H.; et al. Chapter Two—Environmental Effects on Cephalopod Population Dynamics: Implications for Management of Fisheries. In Advances in Marine Biology; Advances in Cephalopod Science: Biology, Ecology, Cultivation and Fisheries; Vidal, E.A.G., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 67, pp. 99–233. [Google Scholar] [CrossRef]
- Birk, M.A.; Liscovitch-Brauer, N.; Dominguez, M.J.; McNeme, S.; Yue, Y.; Hoff, J.D.; Twersky, I.; Verhey, K.J.; Sutton, R.B.; Eisenberg, E.; et al. Temperature-Dependent RNA Editing in Octopus Extensively Recodes the Neural Proteome. Cell 2023, 186, 2544–2555.e13. [Google Scholar] [CrossRef] [PubMed]
- Doubleday, Z.A.; Prowse, T.A.A.; Arkhipkin, A.; Pierce, G.J.; Semmens, J.; Steer, M.; Leporati, S.C.; Lourenço, S.; Quetglas, A.; Sauer, W.; et al. Global Proliferation of Cephalopods. Curr. Biol. 2016, 26, R406–R407. [Google Scholar] [CrossRef]
- Papadopoulo, K.; Hillinger, A.; Mucientes, G.; Roura, Á.; Villegas-Ríos, D.; Irisarri, J.; González, Á.F.; Alonso-Fernández, A. First Insights into the Spatial Behaviour of Octopus Vulgaris in the Wild Using Acoustic Telemetry. Anim. Biotelem. 2024, 12, 16. [Google Scholar] [CrossRef]
- Gutnick, T.; Neef, A.; Cherninskyi, A.; Ziadi-Künzli, F.; Di Cosmo, A.; Lipp, H.-P.; Kuba, M.J. Recording Electrical Activity from the Brain of Behaving Octopus. Curr. Biol. 2023, 33, 1171–1178.e4. [Google Scholar] [CrossRef]
- Packard, A.; Hochberg, F.G.; Nixon, M.; Messenger, J. Skin Patterning in Octopus and Other Genera. In The Biology of Cephalopods; Academic Press: London, UK, 1977. [Google Scholar]
- Packard, A.; Sanders, G. What the Octopus Shows to the World. Endeavour 1969, 28, 92–99. [Google Scholar]
- Packard, A.; Sanders, G.D. Body Patterns of Octopus vulgaris and Maturation of the Response to Disturbance. Anim. Behav. 1971, 19, 780–790. [Google Scholar] [CrossRef]
- Cowdry, E.V. The Colour Changes of Octopus vulgaris LMK [Microform]. University Library: Toronto, Canada, 1911. Available online: http://archive.org/details/cihm_71205 (accessed on 18 February 2025).
- Borrelli, L.; Gherardi, F.; Fiorito, G. A Catalogue of Body Patterning in Cephalopoda; Stazione Zoologica A. Dohrn; Firenze University Press: Firenze, Italy, 2006; 626p. [Google Scholar]
- Mather, J.A.; Mather, L. Skin Colours and Patterns of Juvenile Octopus vulgaris (Mollusca, Cephalopoda) in Bermuda. Vie Milieu 1994, 44, 267–272. [Google Scholar]
- Hanlon, R.T.; Messenger, J.B. Cephalopod Behaviour, 2nd ed.; Cambridge University Press: Cambridge, UK, 2018. [Google Scholar] [CrossRef]
- Mather, J.A.; Alupay, J.S. An Ethogram for Benthic Octopods (Cephalopoda: Octopodidae). J. Comp. Psychol. 2016, 130, 109–127. [Google Scholar] [CrossRef] [PubMed]


| Video Number | Recording Date (dd-mm-yy) | Day Correlation (d) | Video Duration (h:min:s) | Percent Analyzed (%) |
|---|---|---|---|---|
| 1 | 13-12-21 | 1 | 0:16:18 | 96.9 |
| 2 | 12-01-22 | 31 | 0:19:51 | 90.9 |
| 3 | 26-01-22 | 45 | 0:5:42 | 91.1 |
| 4 | 28-01-22 | 47 | 0:32:47 | 98.0 |
| 5 | 30-01-22 | 49 | 0:3:48 | 97.4 |
| 6 | 03-02-22 | 53 | 0:23:44 | 76.0 |
| 7 | 05-02-22 | 55 | 0:28:24 | 95.7 |
| 8 | 06-02-22 | 56 | 0:44:36 | 98.8 |
| 9 | 07-02-22 | 57 | 0:32:39 | 95.1 |
| 10 | 20-02-22 | 70 | 0:28:25 | 89.1 |
| 11 | 21-02-22 | 71 | 0:33:27 | 76.6 |
| 12 | 22-02-22 | 72 | 0:41:54 | 79.9 |
| 14 | 27-02-22 | 77 | 0:4:26 | 88.1 |
| 15 | 03-03-22 | 81 | 0:4:31 | 86.4 |
| 16 | 05-03-22 | 83 | 0:40:34 | 93.8 |
| 17 | 08-03-22 | 86 | 0:42:43 | 96.5 |
| 18 | 10-03-22 | 88 | 0:42:06 | 38.1 |
| 19 | 25-03-22 | 103 | 0:25:41 | 96.2 |
| 20 | 28-03-22 | 106 | 0:30:22 | 77.5 |
| 21 | 10-04-22 | 119 | 0:44:46 | 98.8 |
| 22 | 16-04-22 | 125 | 0:41:51 | 93.4 |
| 23 | 19-04-22 | 128 | 0:39:36 | 91.4 |
| Total | - | 128 | 10.46:85 | - |
| Mean | - | 72.8 | 0:28:33 | 88.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soule, S.E.; Cabanellas-Reboredo, M.; González, Á.F.; Juijn, H.; Hernández-Urcera, J. The Persistence of Memory: Behavioral Analysis and Arm Usage of a Nine-Armed Octopus vulgaris. Animals 2025, 15, 1034. https://doi.org/10.3390/ani15071034
Soule SE, Cabanellas-Reboredo M, González ÁF, Juijn H, Hernández-Urcera J. The Persistence of Memory: Behavioral Analysis and Arm Usage of a Nine-Armed Octopus vulgaris. Animals. 2025; 15(7):1034. https://doi.org/10.3390/ani15071034
Chicago/Turabian StyleSoule, Sam Ellington, Miguel Cabanellas-Reboredo, Ángel F. González, Hidde Juijn, and Jorge Hernández-Urcera. 2025. "The Persistence of Memory: Behavioral Analysis and Arm Usage of a Nine-Armed Octopus vulgaris" Animals 15, no. 7: 1034. https://doi.org/10.3390/ani15071034
APA StyleSoule, S. E., Cabanellas-Reboredo, M., González, Á. F., Juijn, H., & Hernández-Urcera, J. (2025). The Persistence of Memory: Behavioral Analysis and Arm Usage of a Nine-Armed Octopus vulgaris. Animals, 15(7), 1034. https://doi.org/10.3390/ani15071034







