Computed Tomography Assessment of Healthy Elbow Joint Congruity in Dogs Being Affected by Pronation and Supination Angulation: A Cadaveric Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
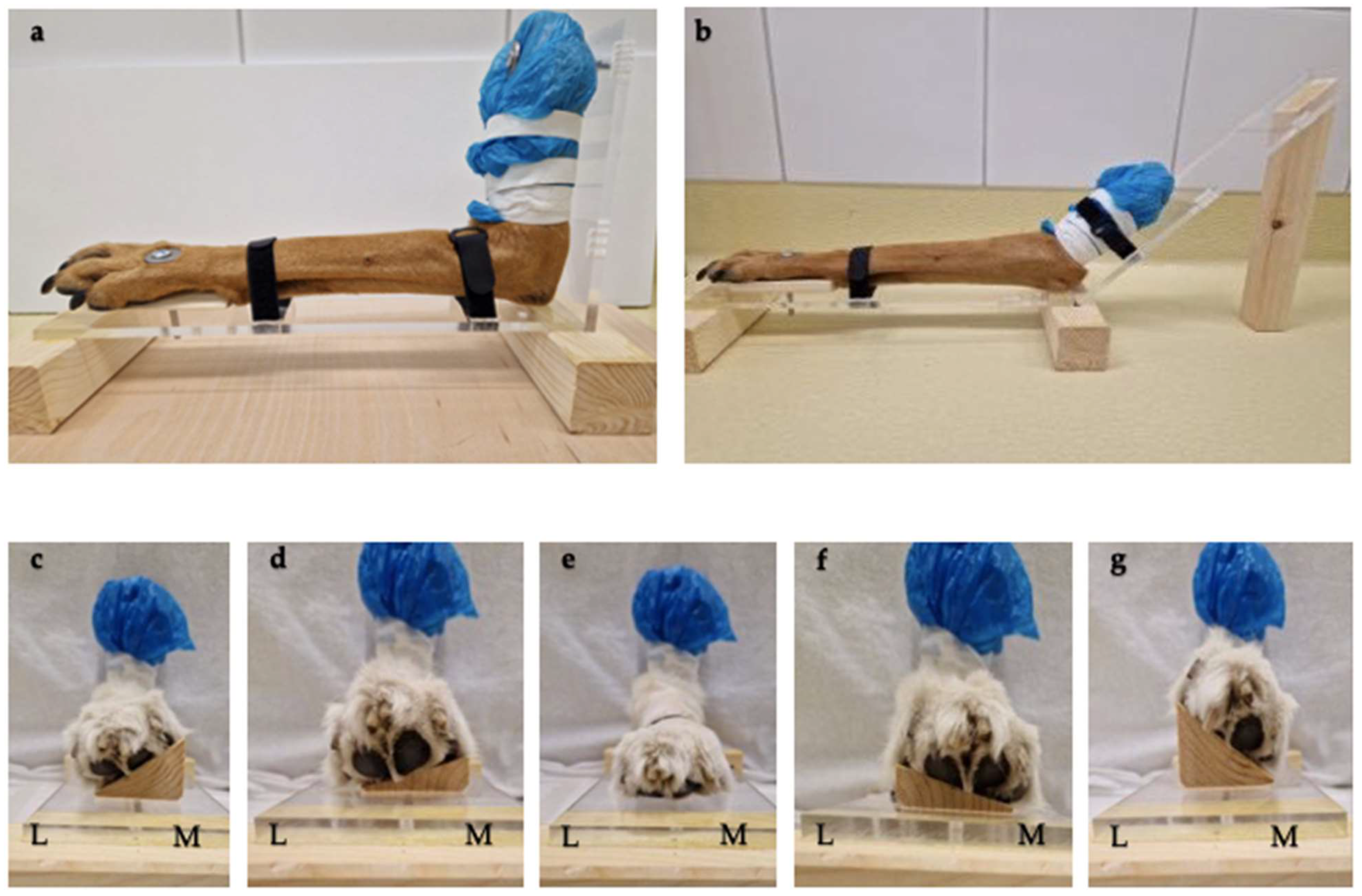
2.2. Positioning Aids
2.3. Image Acquisition
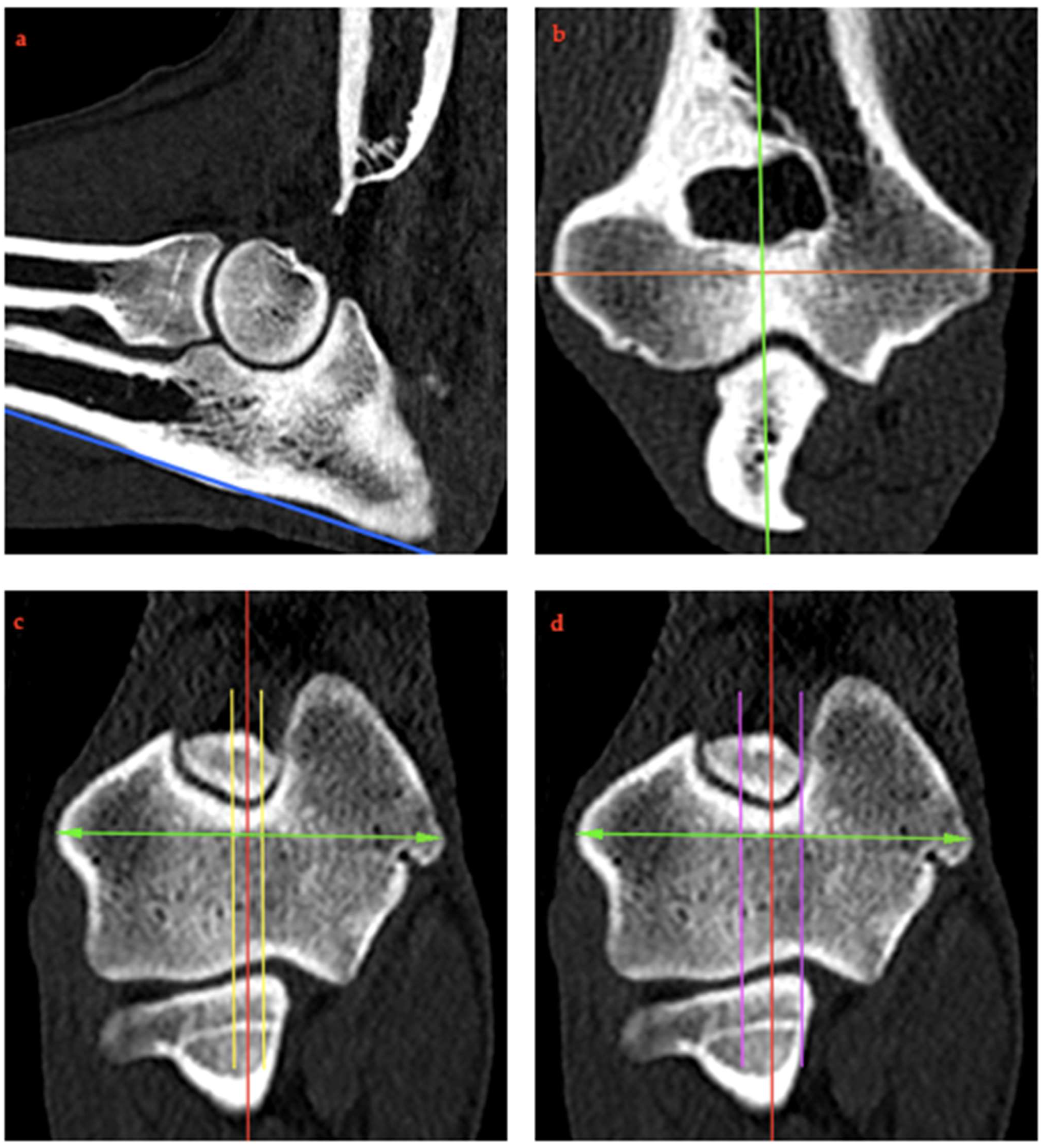
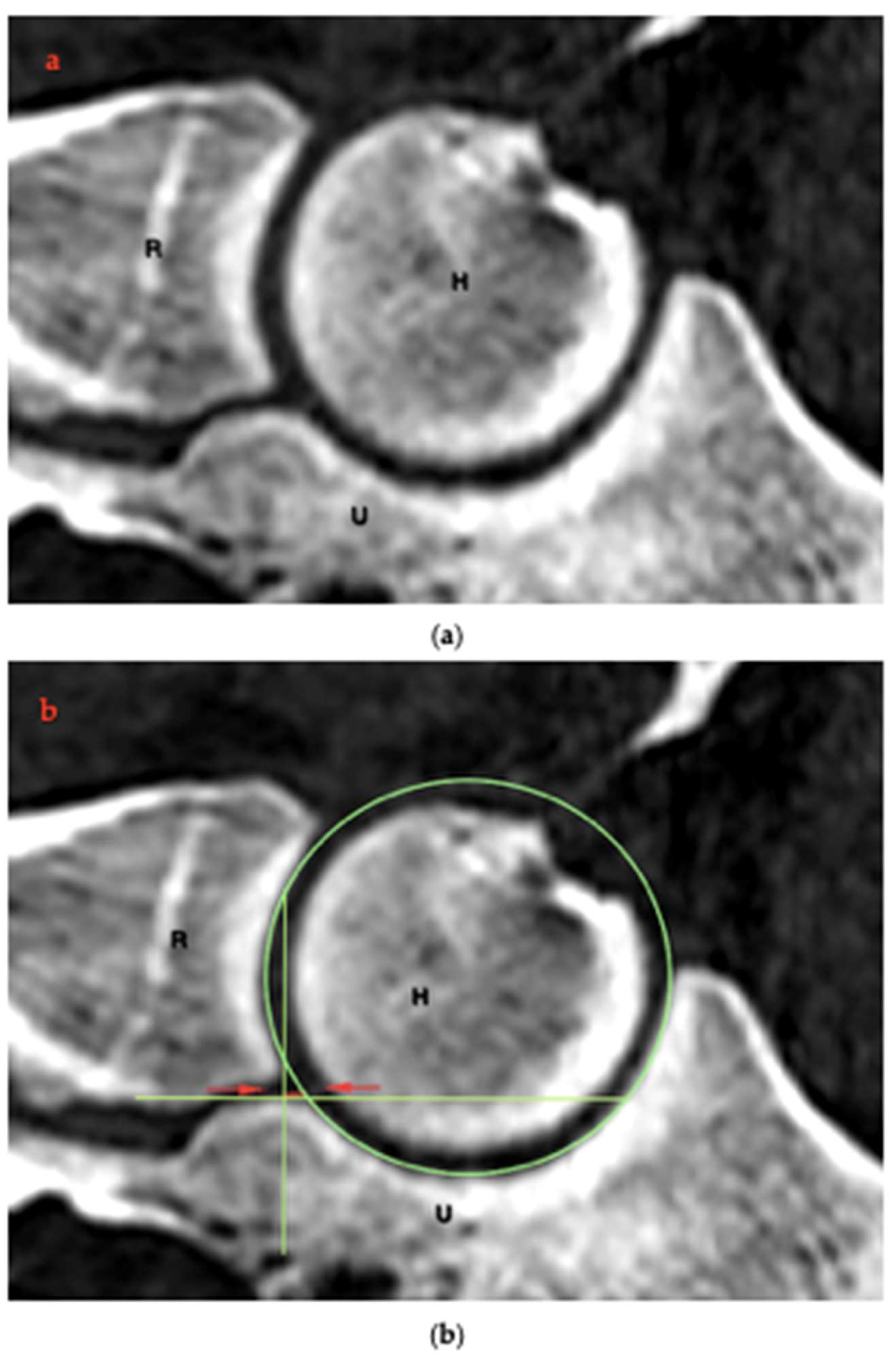
2.4. RUI Measurements
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wind, A. Etiology and pathogenesis of elbow dysplasia; a hypothesis. In Proceedings of the American Animal Hospital Association’s 57th Annual Meeting Proceedings, Knoxville, TN, USA, 9–10 February 2024; pp. 725–727. [Google Scholar]
- Krotscheck, U.; Böttcher, P.B.; Thompson, M.S.; Todhunter, R.J.; Mohammed, H.O. Cubital subchondral joint space width and CT osteoabsorptiometry in dogs with and without fragmented medial coronoid process. Vet. Surg. 2014, 43, 330–338. [Google Scholar] [CrossRef]
- Samoy, Y.; Van Ryssen, B.; Gielen, I.; Walschot, N.; van Bree, H. Review of the literature: Elbow incongruity in the dog. Vet. Comp. Orthop. Traumatol. 2006, 19, 1–8. [Google Scholar] [PubMed]
- Kramer, A.; Holsworth, I.G.; Wisner, E.R.; Kass, P.H.; Schulz, K.S. Computed tomographic evaluation of canine radioulnar incongruence in vivo. Vet. Surg. 2006, 35, 24–29. [Google Scholar] [CrossRef]
- Grøndalen, J.; Grøndalen, T. Arthrosis in the elbow joint of young rapidly growing dogs. V. A pathoanatomical investigation. Nord. Veterinaermedicine 1981, 33, 1–16. [Google Scholar]
- Kirberger, R.M.; Fourie, S.L. Elbow dysplasia in the dog: Pathophysiology, diagnosis and control. J. S Afr. Vet. Assoc. 1998, 69, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.P.; Wind, A.; Davidson, A.P. Bone dysplasias in the labrador retriever: A radiographic study. J. Am. Anim. Hosp. Assoc. 1999, 35, 332–340. [Google Scholar] [CrossRef]
- Mason, D.R.; Schulz, K.S.; Fujita, Y.; Kass, P.H.; Stover, S.M. Measurement of humeroradial and humeroulnar transarticular joint forces in the canine elbow joint after humeral wedge and humeral slide osteotomies. Vet. Surg. 2008, 37, 63–70. [Google Scholar] [CrossRef]
- Michelsen, J. Canine elbow dysplasia: Aetiopathogenesis and current treatment recommendations. Vet. J. 2013, 196, 12–19. [Google Scholar]
- Kubiak-Nowak, D.; Kiełbowicz, Z.; Prządka, P.; Borawski, W.; Kubiak, K.; Jankowski, M.; Spużak, J.; Glińska-Suchocka, K. Medial coronoid process disease in the course of elbow dysplasia in dogs. Med. Weter. 2024, 80, 264–270. [Google Scholar] [CrossRef]
- Wind, A. Elbow incongruity and developmental elbow diseases in the dog. J. Am. Anim. Hosp. Assoc. 1986, 22, 725–730. [Google Scholar]
- Hazewinkel, H. Elbow dysplasia; definitions and clinical diagnoses. In Proceedings of the 23rd Annual Meeting International Elbow Working Group, Dublin, Ireland, 20 August 2008; pp. 8–12. [Google Scholar]
- Eljack, H.; Böttcher, P. Relationship between axial radioulnar incongruence with cartilage damage in dogs with medial coronoid disease. Vet. Surg. 2015, 44, 174–179. [Google Scholar] [CrossRef] [PubMed]
- McConkey, M.J.; Valenzano, D.M.; Wei, A.; Li, T.; Thompson, M.S.; Mohammed, H.O.; van der Meulen, M.C.; Krotscheck, U. Effect of the Proximal Abducting Ulnar Osteotomy on Intra-Articular Pressure Distribution and Contact Mechanics of Congruent and Incongruent Canine Elbows Ex Vivo. Vet. Surg. 2016, 45, 347–355. [Google Scholar] [CrossRef]
- Danielson, K.C.; Fitzpatrick, N.; Muir, P.; Manley, P.A. Histomorphometry of fragmented medial coronoid process in dogs: A comparison of affected and normal coronoid processes. Vet. Surg. 2006, 35, 501–509. [Google Scholar] [CrossRef]
- Gemmill, T.J.; Clements, D.N. Fragmented coronoid process in the dog: Is there a role for incongruency? J. Small Anim. Pract. 2007, 48, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Preston, C.A.; Schulz, K.S.; Taylor, K.T.; Kass, P.H.; Hagan, C.E.; Stover, S.M. In vitro experimental study of the effect of radial shortening and ulnar ostectomy on contact patterns in the elbow joint of dogs. Am. J. Vet. Res. 2001, 62, 1548–1556. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, N.; Garcia, T.C.; Daryani, A.; Bertran, J.; Watari, S.; Hayashi, K. Micro-CT Structural Analysis of the Canine Medial Coronoid Disease. Vet. Surg. 2016, 45, 336–346. [Google Scholar] [CrossRef]
- Gemmill, T.J.; Mellor, D.J.; Clements, D.N.; Clarke, S.P.; Farrell, M.; Bennett, D.; Carmichael, S. Evaluation of elbow incongruency using reconstructed CT in dogs suffering fragmented coronoid process. J. Small Anim. Pract. 2005, 46, 327–333. [Google Scholar] [CrossRef]
- Guillou, R.; Déjardin, L.; Bey, M.; McDonald, C. Three dimensional kinematics of the normal canine elbow at the walk and trot. In Proceedings of the 2011 American College of Veterinary Surgeons Veterinary Symposium November, Chicago, IL, USA, 3–5 November 2011; pp. 3–5. [Google Scholar]
- Rohwedder, T.; Böttcher, P. Relation of Computed Tomography-Based Static Axial Radioulnar Incongruence Measurements under General Anaesthesia and Dynamic, In Vivo RUI during the Walk in Canine Elbow Joints with and without Medial Coronoid Process Disease. Vet. Comp. Orthop. Traumatol. 2021, 34, 386–393. [Google Scholar] [CrossRef]
- Rohwedder, T.; Fischer, M.; Böttcher, P. In vivo fluoroscopic kinematography of dynamic radio-ulnar incongruence in dogs. Open Vet. J. 2017, 7, 221–228. [Google Scholar] [CrossRef][Green Version]
- Alves-Pimenta, S.; Ginja, M.M.; Colaço, J.; Fernandes, A.M.; Melo-Pinto, P.; Colaço, B. Curvature Radius Measurements From the Ulnar Trochlear Notch in Large Dogs. Anat. Rec. 2015, 298, 1748–1753. [Google Scholar] [CrossRef]
- Alves-Pimenta, S.; Ginja, M.M.; Colaço, B. Role of Elbow Incongruity in Canine Elbow Dysplasia: Advances in Diagnostics and Biomechanics. Vet. Comp. Orthop. Traumatol. 2019, 32, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Blond, L.; Dupuis, J.; Beauregard, G.; Breton, L.; Moreau, M. Sensitivity and specificity of radiographic detection of canine elbow incongruence in an in vitro model. Vet. Radiol. Ultrasound 2005, 46, 210–216. [Google Scholar] [CrossRef]
- Burton, N.J.; Dobney, J.A.; Owen, M.R.; Colborne, G.R. Joint angle, moment and power compensations in dogs with fragmented medial coronoid process. Vet. Comp. Orthop. Traumatol. 2008, 21, 110–118. [Google Scholar] [CrossRef]
- Burton, N.J.; Warren-Smith, C.M.; Roper, D.P.; Parsons, K.J. CT assessment of the influence of dynamic loading on physiological incongruency of the canine elbow. J. Small Anim. Pract. 2013, 54, 291–298. [Google Scholar] [CrossRef]
- Skinner, O.T.; Warren-Smith, C.M.; Burton, N.J.; Parsons, K.J. Computed tomographic evaluation of elbow congruity during arthroscopy in a canine cadaveric model. Vet. Comp. Orthop. Traumatol. 2015, 28, 19–24. [Google Scholar] [CrossRef] [PubMed]
- House, M.R.; Marino, D.J.; Lesser, M.L. Effect of limb position on elbow congruity with CT evaluation. Vet. Surg. 2009, 38, 154–160. [Google Scholar] [CrossRef]
- Murphy, S.T.; Lewis, D.D.; Shiroma, J.T.; Neuwirth, L.A.; Parker, R.B.; Kubilis, P.S. Effect of radiographic positioning on interpretation of cubital joint congruity in dogs. Am. J. Vet. Res. 1998, 59, 1351–1357. [Google Scholar] [CrossRef]
- Wagner, K.; Griffon, D.J.; Thomas, M.W.; Schaeffer, D.J.; Schulz, K.; Samii, V.F.; Necas, A. Radiographic, computed tomographic, and arthroscopic evaluation of experimental radio-ulnar incongruence in the dog. Vet. Surg. 2007, 36, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Dickomeit, M.J.; Böttcher, P.; Hecht, S.; Liebich, H.G.; Maierl, J. Topographic and age-dependent distribution of subchondral bone density in the elbow joints of clinically normal dogs. Am. J. Vet. Res. 2011, 72, 491–499. [Google Scholar] [CrossRef]
- Lau, S.F.; Hazewinkel, H.A.; Voorhout, G. Radiographic and computed tomographic assessment of the development of the antebrachia and elbow joints in Labrador Retrievers with and without medial coronoid disease. Vet. Comp. Orthop. Traumatol. 2015, 28, 186–192. [Google Scholar] [CrossRef]
- Probst, A.; Modler, F.; Künzel, W.; Mlynarik, V.; Trattnig, S. Demonstration of the articular cartilage of the canine ulnar trochlear notch using high-field magnetic resonance imaging. Vet. J. 2008, 177, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wucherer, K.L.; Ober, C.P.; Conzemius, M.G. The use of delayed gadolinium enhanced magnetic resonance imaging of cartilage and T2 mapping to evaluate articular cartilage in the normal canine elbow. Vet. Radiol. Ultrasound 2012, 53, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Gendler, A.; Keuler, N.S.; Schaefer, S.L. Computed tomographic arthrography of the normal canine elbow. Vet. Radiol. Ultrasound 2015, 56, 144–152. [Google Scholar] [CrossRef]
- Benfante, V.; Stefano, A.; Comelli, A.; Giaccone, P.; Cammarata, F.P.; Richiusa, S.; Scopelliti, F.; Pometti, M.; Ficarra, M.; Cosentino, S.; et al. A New Preclinical Decision Support System Based on PET Radiomics: A Preliminary Study on the Evaluation of an Innovative (64)Cu-Labeled Chelator in Mouse Models. J. Imaging 2022, 8, 92. [Google Scholar] [CrossRef]
- Werner, H.; Winkels, P.; Grevel, V.; Oechtering, G.; Böttcher, P. Sensitivity and specificity of arthroscopic estimation of positive and negative radio-ulnar incongruence in dogs. An in vitro study. Vet. Comp. Orthop. Traumatol. 2009, 22, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Janthur, I.; Meyer-Lindenberg, A.; Fehr, M. Arthroscopic diagnosis of canine elbow joint diseases. Tierärztliche Praxis. Ausgabe K, Kleintiere/Heimtiere 2000, 28, 11–18. [Google Scholar]
- Meyer-Lindenberg, A.; Langhann, A.; Fehr, M.; Nolte, I. Arthrotomy versus arthroscopy in the treatment of the fragmented medial coronoid process of the ulna (FCP) in 421 dogs. Vet. Comp. Orthop. Traumatol. 2003, 16, 204–210. [Google Scholar]



| RUI | Flexion | Compared Groups | Mean (%) | p-Value |
|---|---|---|---|---|
| BMC | 90° | 0° and 15° Pronation | 1.36 | 0.492 |
| 0° and 35° Pronation | 8.18 | <0.001 | ||
| 0° and 15° Supination | 5.51 | <0.001 | ||
| 0° and 35° Supination | 12.62 | <0.001 | ||
| 135° | 0° and 15° Pronation | 2.25 | 0.065 | |
| 0° and 35° Pronation | 7.27 | <0.001 | ||
| 0° and 15° Supination | 5.51 | <0.001 | ||
| 0° and 35° Supination | 11.51 | <0.001 | ||
| AMC | 90° | 0° and 15° Pronation | 16.00 | <0.001 |
| 0° and 35° Pronation | 30.97 | <0.001 | ||
| 0° and 15° Supination | 9.02 | 0.080 | ||
| 0° and 35° Supination | 24.90 | <0.001 | ||
| 135° | 0° and 15° Pronation | 18.14 | <0.001 | |
| 0° and 35° Pronation | 31.31 | <0.001 | ||
| 0° and 15° Supination | 10.75 | 0.027 | ||
| 0° and 35° Supination | 28.67 | <0.001 |
| Flexion | Compared Groups | Mean (%) | p-Value |
|---|---|---|---|
| 135° | 0% and 5% Medial | 4.78 | <0.001 |
| 0% and 10% Medial | 9.67 | <0.001 | |
| 0% and 5% Lateral | 3.68 | <0.001 | |
| 0% and 10% Lateral | 8.57 | <0.001 | |
| 90° | 0% and 5% Medial | 4.77 | <0.001 |
| 0% and 10% Medial | 9.35 | <0.001 | |
| 0% and 5% Lateral | 4.05 | <0.001 | |
| 0% and 10% Lateral | 8.82 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Possiel, V.C.K.; Huels, N.H.; Wang-Leandro, A.; Volk, H.A.; Harms, O. Computed Tomography Assessment of Healthy Elbow Joint Congruity in Dogs Being Affected by Pronation and Supination Angulation: A Cadaveric Study. Animals 2025, 15, 921. https://doi.org/10.3390/ani15070921
Possiel VCK, Huels NH, Wang-Leandro A, Volk HA, Harms O. Computed Tomography Assessment of Healthy Elbow Joint Congruity in Dogs Being Affected by Pronation and Supination Angulation: A Cadaveric Study. Animals. 2025; 15(7):921. https://doi.org/10.3390/ani15070921
Chicago/Turabian StylePossiel, Vivienne Chantal Katharina, Nikolaus Hubertus Huels, Adriano Wang-Leandro, Holger Andreas Volk, and Oliver Harms. 2025. "Computed Tomography Assessment of Healthy Elbow Joint Congruity in Dogs Being Affected by Pronation and Supination Angulation: A Cadaveric Study" Animals 15, no. 7: 921. https://doi.org/10.3390/ani15070921
APA StylePossiel, V. C. K., Huels, N. H., Wang-Leandro, A., Volk, H. A., & Harms, O. (2025). Computed Tomography Assessment of Healthy Elbow Joint Congruity in Dogs Being Affected by Pronation and Supination Angulation: A Cadaveric Study. Animals, 15(7), 921. https://doi.org/10.3390/ani15070921






