Effects of Yeast β-Glucan Supplementation on Calf Intestinal and Respiratory Health
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Growth Performance and Health Recording
2.3. The Oxidative Stress Level in Serum
2.4. Cytokines, Immunoglobulins, and Diamine Oxidase Expression
2.5. The Levels of Three Mucosal Defense Proteins in Rectal Contents
2.6. 16S rRNA Gene Sequencing
2.7. Statistical Analyses
3. Results
3.1. Pre-Stimulation with Yeast β-Glucan Induced Inflammatory Response and Oxidative Stress of Calves
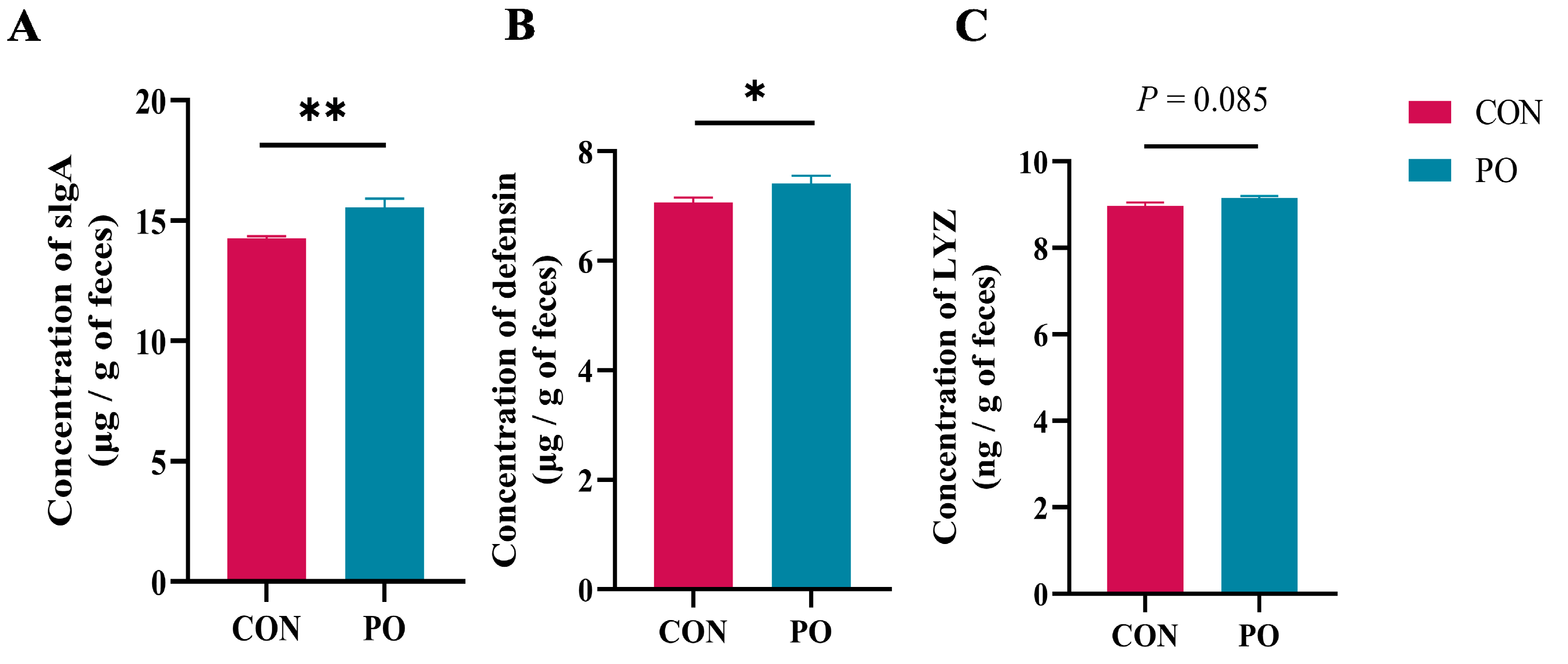
3.2. Pre-Stimulation with Yeast β-Glucan Enhanced Concentration of Several Defensive Proteins in Rectal Feces
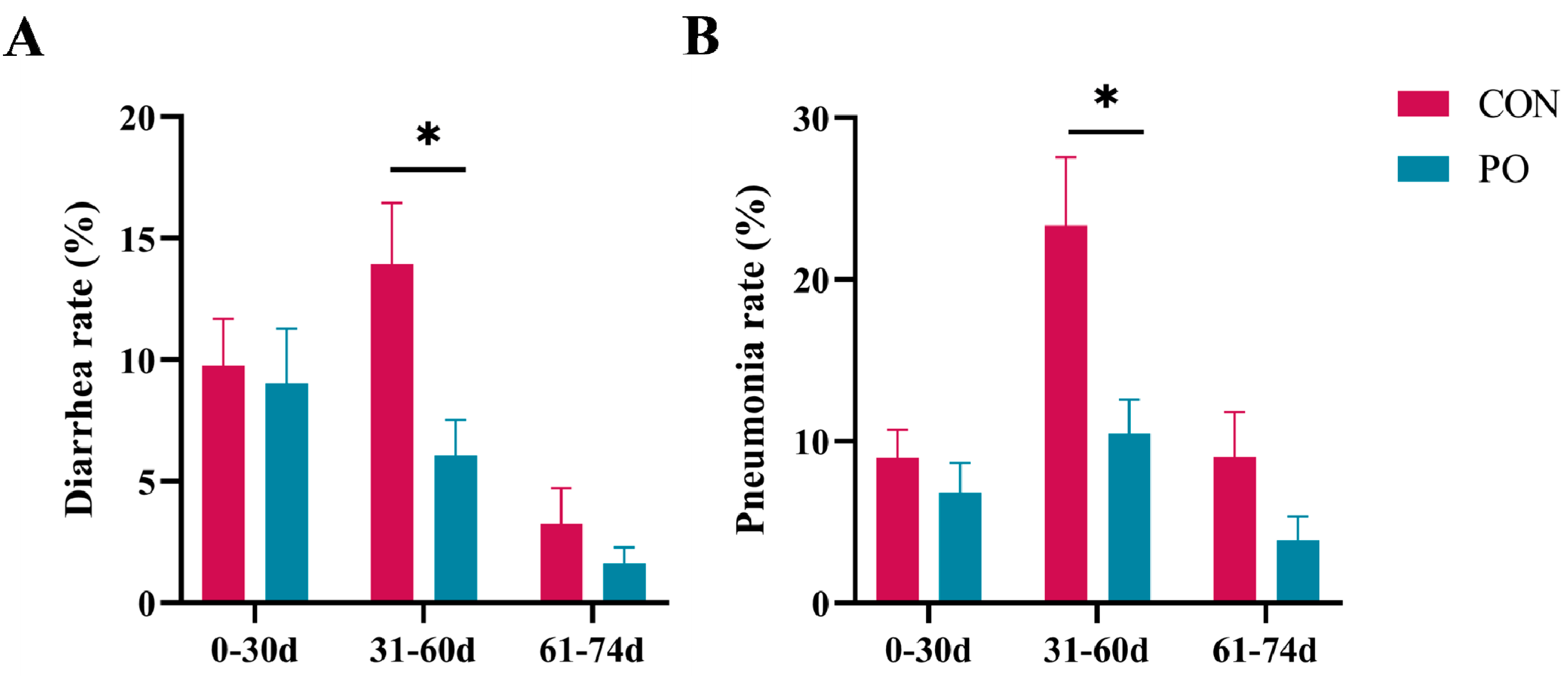
3.3. Pre-Stimulation with Yeast β-Glucan Decreased the Incidence of Diarrhea and Pneumonia in Calves
3.4. Pre-Stimulation Enhanced the Inflammatory Response and Alleviated Intestinal Damage of Calves at 30 Days Old
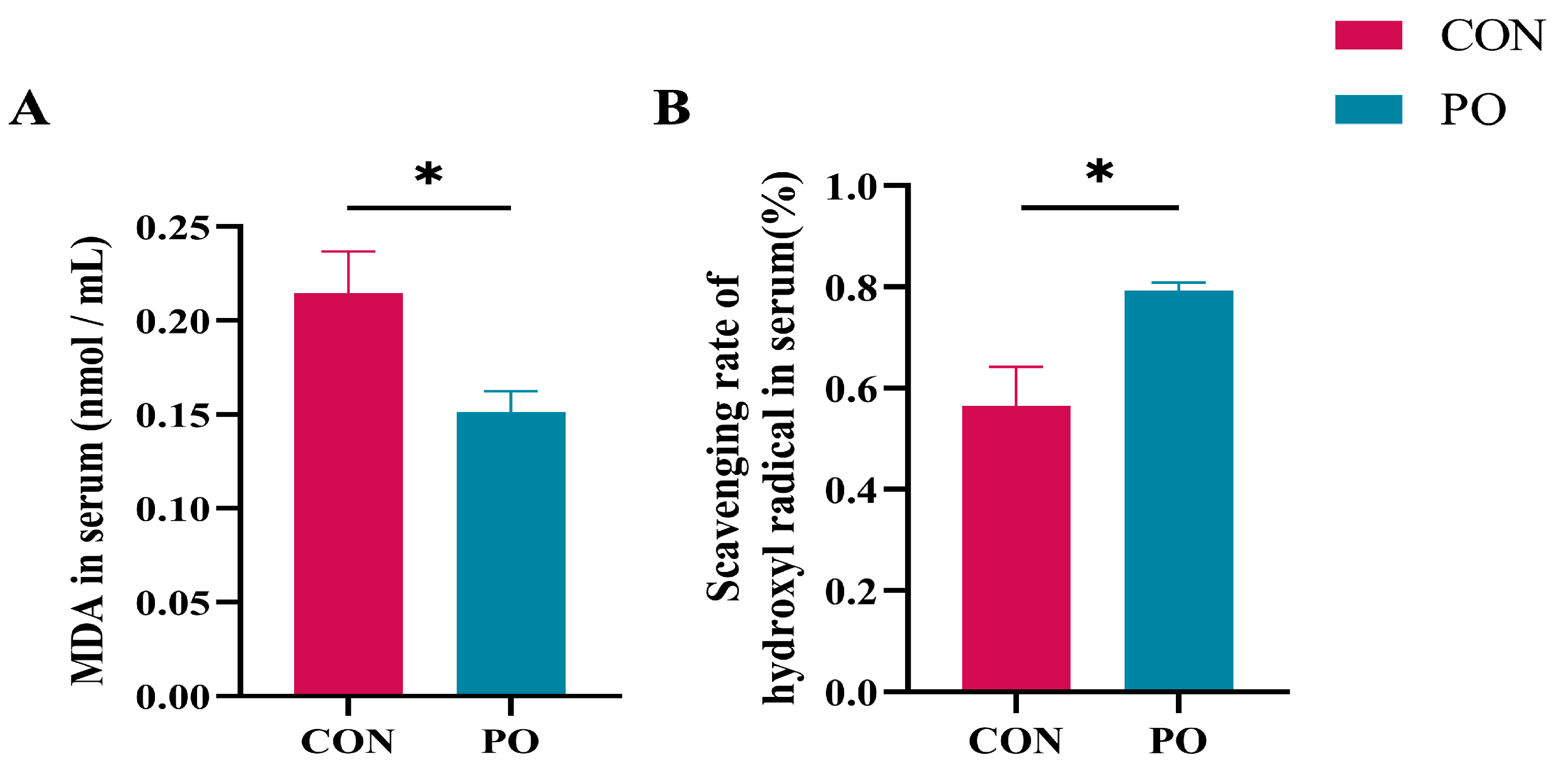
3.5. Pre-Stimulation with Yeast β-Glucan Alleviated the Oxidative Stress of Calves Later
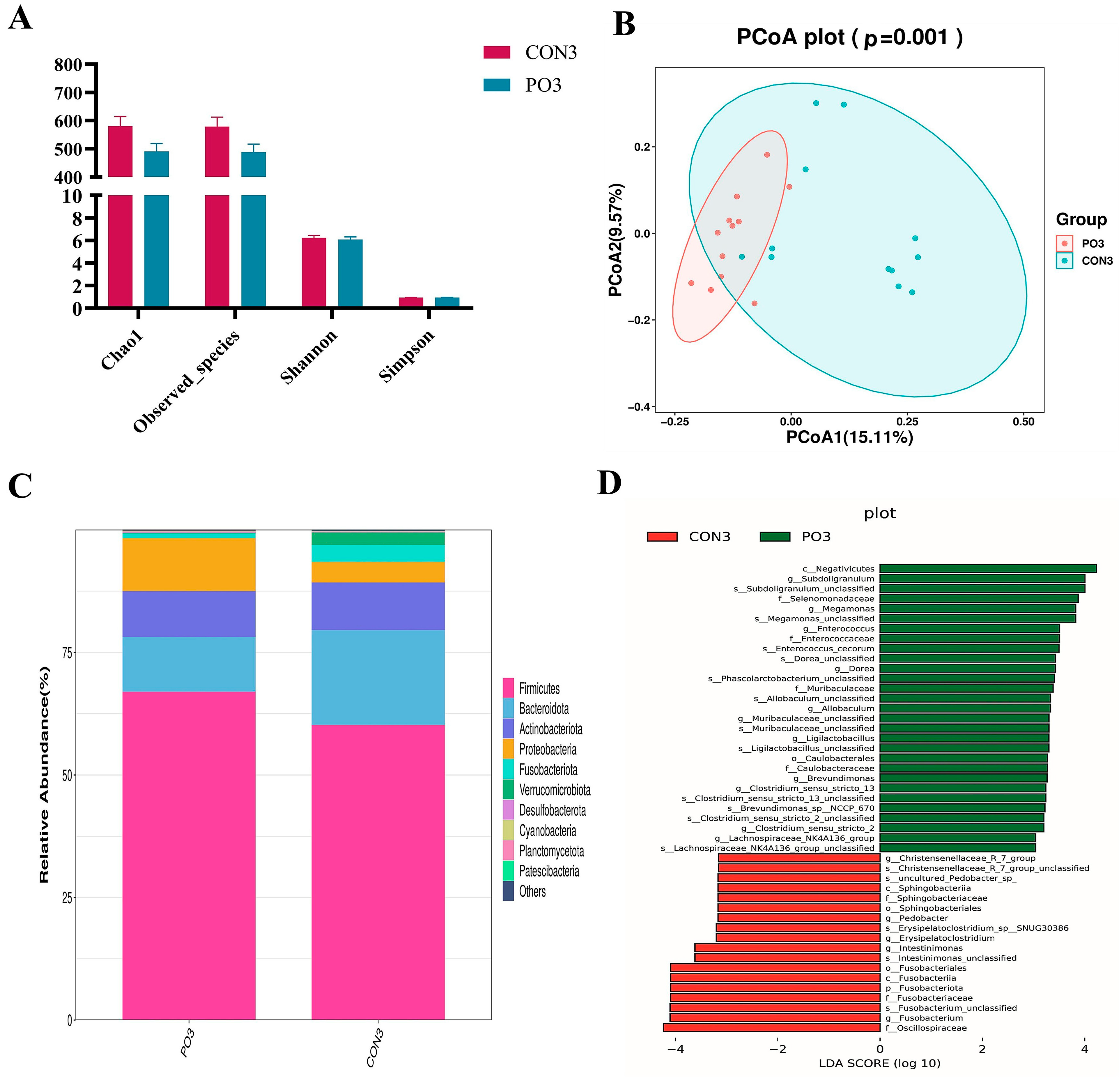
3.6. Pre-Stimulation with Yeast β-Glucan Altered the Rectal Bacterial Community of Calves at 30 Days Old
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diao, Q.Y.; Zhang, R.; Tu, Y. Current research progresses on calf rearing and nutrition in China. J. Integr. Agric. 2017, 16, 2805–2814. [Google Scholar] [CrossRef]
- Stefańska, B.; Gąsiorek, M.; Nowak, W. Short- and long-term effects of initial serum total protein, average starter feed intake during the last week of the preweaning period, and rearing body gain on primiparous dairy heifers’ performance. J. Dairy Sci. 2021, 104, 1645–1659. [Google Scholar] [CrossRef] [PubMed]
- McGuirk, S.M. Disease management of dairy calves and heifers. Vet. Clin. N. Am. Food Anim. Pract. 2008, 24, 139–153. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.N.; Doelman, J.; Keppler, B.R.; Steele, M.A.; Martín-Tereso, J. Preweaning nutrient supply alters serum metabolomics profiles related to protein and energy metabolism and hepatic function in Holstein heifer calves. J. Dairy Sci. 2021, 104, 7711–7724. [Google Scholar] [CrossRef]
- Abuelo, A.; Havrlant, P.; Wood, N.; Hernandez-Jover, M. An investigation of dairy calf management practices, colostrum quality, failure of transfer of passive immunity, and occurrence of enteropathogens among Australian dairy farms. J. Dairy Sci. 2019, 102, 8352–8366. [Google Scholar] [CrossRef]
- Kamel, M.S.; Davidson, J.L.; Verma, M.S. Strategies for Bovine Respiratory Disease (BRD) Diagnosis and Prognosis: A Comprehensive Overview. Animals 2024, 14, 627. [Google Scholar] [CrossRef]
- Odagiri, K.; Yoshizawa, N.; Sakihara, H.; Umeda, K.; Rahman, S.; Nguyen, S.V.; Suzuki, T. Development of Genotype-Specific Anti-Bovine Rotavirus A Immunoglobulin Yolk Based on a Current Molecular Epidemiological Analysis of Bovine Rotaviruses A Collected in Japan during 2017–2020. Viruses 2020, 12, 1386. [Google Scholar] [CrossRef]
- Anand, S.; Mandal, S.; Patil, P.; Tomar, S.K. Pathogen-induced secretory diarrhea and its prevention. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1721–1739. [Google Scholar] [CrossRef]
- Lachowicz-Wolak, A.; Klimowicz-Bodys, M.D.; Płoneczka-Janeczko, K.; Bednarski, M.; Dyba, K.; Knap, P.; Rypuła, K. Simultaneous Presence of Antibodies against Five Respiratory Pathogens in Unvaccinated Dairy Calves from South-Western Poland. Animals 2024, 14, 2520. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef]
- Geckin, B.; Konstantin Föhse, F.; Domínguez-Andrés, J.; Netea, M.G. Trained immunity: Implications for vaccination. Curr. Opin. Immunol. 2022, 77, 102190. [Google Scholar] [PubMed]
- Lilly, E.A.; Ikeh, M.; Nash, E.E.; Fidel, P.L., Jr.; Noverr, M.C. Immune Protection against Lethal Fungal-Bacterial Intra-Abdominal Infections. mBio 2018, 9, e01472-17. [Google Scholar] [CrossRef] [PubMed]
- Buffen, K.; Oosting, M.; Quintin, J.; Ng, A.; Kleinnijenhuis, J.; Kumar, V.; van de Vosse, E.; Wijmenga, C.; van Crevel, R.; Oosterwijk, E.; et al. Autophagy controls BCG-induced trained immunity and the response to intravesical BCG therapy for bladder cancer. PLoS. Pathog. 2014, 10, e1004485. [Google Scholar]
- Zhou, X.Y.; Gao, R.; Hu, J.; Gao, D.P.; Liao, Y.L.; Yang, J.J. Trained Innate Immunity by Repeated Low-Dose Lipopolysaccharide Injections Displays Long-Term Neuroprotective Effects. Mediat. Inflamm. 2020, 2020, 8191079. [Google Scholar] [CrossRef]
- Petit, J.; Wiegertjes, G.F. Long-lived effects of administering β-glucans: Indications for trained immunity in fish. Dev. Comp. Immunol. 2016, 64, 93–102. [Google Scholar]
- Leonhardt, J.; Große, S.; Marx, C.; Siwczak, F.; Stengel, S.; Bruns, T.; Bauer, R.; Kiehntopf, M.; Williams, D.L.; Wang, Z.Q.; et al. Candida albicans β-Glucan Differentiates Human Monocytes into a Specific Subset of Macrophages. Front. Immunol. 2018, 9, 2818. [Google Scholar] [CrossRef]
- De Marco Castro, E.; Calder, P.C.; Roche, H.M. β-1,3/1,6-Glucans and Immunity: State of the Art and Future Directions. Mol. Nutr. Food Res. 2021, 65, e1901071. [Google Scholar]
- Librán-Pérez, M.; Costa, M.M.; Figueras, A.; Novoa, B. β-glucan administration induces metabolic changes and differential survival rates after bacterial or viral infection in turbot (Scophthalmus maximus). Fish Shellfish Immunol. 2018, 82, 173–182. [Google Scholar]
- Ciarlo, E.; Heinonen, T.; Théroude, C.; Asgari, F.; Le Roy, D.; Netea, M.G.; Roger, T. Trained Immunity Confers Broad-Spectrum Protection Against Bacterial Infections. J. Infect. Dis. 2020, 222, 1869–1881. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.S.; Yan, F.; Yang, W.Q.; Tang, G.F.; Cui, M.H.; Xu, X.R. Intraperitoneal injection of β-glucan during the suckling period improved the intestinal health of newly weaned rabbits by enhancing immune responses. Livest. Sci. 2023, 272, 105214. [Google Scholar]
- Angulo, M.; Reyes-Becerril, M.; Cepeda-Palacios, R.; Angulo, C. Oral administration of Debaryomyces hansenii CBS8339-β-glucan induces trained immunity in newborn goats. Dev. Comp. Immunol. 2020, 105, 103597. [Google Scholar] [CrossRef] [PubMed]
- Angulo, M.; Angulo, C. Immunometabolic changes of β-glucan-trained immunity induction and inhibition on neonatal calf immune innate cells. Mol. Immunol. 2023, 159, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Medrano-Galarza, C.; LeBlanc, S.J.; Jones-Bitton, A.; DeVries, T.J.; Rushen, J.; Marie de Passillé, A.; Endres, M.I.; Haley, D.B. Associations between management practices and within-pen prevalence of calf diarrhea and respiratory disease on dairy farms using automated milk feeders. J. Dairy Sci. 2018, 101, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- McGuirk, S.M.; Peek, S.F. Timely diagnosis of dairy calf respiratory disease using a standardized scoring system. Anim. Health Res. Rev. 2014, 15, 145–147. [Google Scholar] [CrossRef]
- Zou, T.; Yang, J.; Guo, X.; He, Q.; Wang, Z.; You, J. Dietary seaweed-derived polysaccharides improve growth performance of weaned pigs through maintaining intestinal barrier function and modulating gut microbial populations. J. Anim. Sci. Biotechnol. 2021, 12, 28. [Google Scholar] [CrossRef]
- Maier, G.U.; Breitenbuecher, J.; Gomez, J.P.; Samah, F.; Fausak, E.; Van Noord, M. Vaccination for the Prevention of Neonatal Calf Diarrhea in Cow-Calf Operations: A Scoping Review. Vet. Anim. Sci. 2022, 15, 100238. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Saif, L.J. Bovine Coronavirus and the Associated Diseases. Front. Vet. Sci. 2021, 8, 643220. [Google Scholar] [CrossRef]
- Salzmann, M.; Haider, P.; Kaun, C.; Brekalo, M.; Hartmann, B.; Lengheimer, T.; Pichler, R.; Filip, T.; Derdak, S.; Podesser, B.; et al. Innate Immune Training with Bacterial Extracts Enhances Lung Macrophage Recruitment to Protect from Betacoronavirus Infection. J. Innate Immun. 2020, 14, 293–305. [Google Scholar] [CrossRef]
- Gu, H.; Zeng, X.; Peng, L.; Xiang, C.; Zhou, Y.; Zhang, X.; Zhang, J.; Wang, N.; Guo, G.; Li, Y.; et al. Vaccination induces rapid protection against bacterial pneumonia via training alveolar macrophage in mice. eLife 2021, 10, e69951. [Google Scholar]
- Bastos, R.G.; Alzan, H.F.; Rathinasamy, V.A.; Cooke, B.M.; Dellagostin, O.A.; Barletta, R.G.; Suarez, C.E. Harnessing Mycobacterium bovis BCG Trained Immunity to Control Human and Bovine Babesiosis. Vaccines 2022, 10, 123. [Google Scholar] [CrossRef]
- Chando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained immunity—Basic concepts and contributions to immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.C.; Quintin, J.; Cramer, R.A.; Shepardson, K.M.; Saeed, S.; Kumar, V.; Giamarellos-Bourboulis, E.J.; Martens, J.H.; Rao, N.A.; Aghajanirefah, A.; et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science 2014, 345, 1250684. [Google Scholar] [CrossRef] [PubMed]
- Horneck Johnston, C.J.H.; Ledwith, A.E.; Lundahl, M.L.E.; Charles-Messance, H.; Hackett, E.E.; O’Shaughnessy, S.D.; Clegg, J.; Prendeville, H.; McGrath, J.P.; Walsh, A.M.; et al. Recognition of yeast β-glucan particles triggers immunometabolic signaling required for trained immunity. iScience 2024, 27, 109030. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.P.; Papasian, C.J.; Ritter, R.C. The effects of cold stress on neonatal calves. I. Clinical condition and pathological lesions. Can. J. Comp. Med. 1980, 44, 11–18. [Google Scholar]
- Hulbert, L.E.; Moisá, S.J. Stress, immunity, and the management of calves. J. Dairy Sci. 2023, 99, 3199–3216. [Google Scholar] [CrossRef]
- Nabenishi, H.; Yamazaki, A. Effects of temperature-humidity index on health and growth performance in Japanese black calves. Trop. Anim. Health Prod. 2017, 49, 397–402. [Google Scholar] [CrossRef]
- Darroch, H.; Astin, J.W.; Hall, C.J. Towards a new model of trained immunity: Exposure to bacteria and β-glucan protects larval zebrafish against subsequent infections. Dev. Comp. Immunol. 2022, 132, 104400. [Google Scholar] [CrossRef]
- Angulo, M.; Reyes-Becerril, M.; Tovar-Ramírez, D.; Ascencio, F.; Angulo, C. Debaryomyces hansenii CBS 8339 β-glucan enhances immune responses and down-stream gene signaling pathways in goat peripheral blood leukocytes. Dev. Comp. Immunol. 2018, 88, 173–182. [Google Scholar] [CrossRef]
- Cui, M.; Tang, G.; Yan, F.; Wang, S.; Wang, X.; Yao, J.; Xu, X. Oral administration of heat-inactivated Escherichia coli during suckling alleviated Salmonella typhimurium-derived intestinal injury after rat weaning. Front. Immunol. 2023, 14, 1119747. [Google Scholar] [CrossRef]
- Serafini, N.; Jarade, A.; Surace, L.; Goncalves, P.; Sismeiro, O.; Varet, H.; Legendre, R.; Coppee, J.Y.; Disson, O.; Durum, S.K.; et al. Trained ILC3 responses promote intestinal defense. Science 2022, 375, 859–863. [Google Scholar] [CrossRef]
- Zhou, H.; Lu, X.; Huang, J.; Jordan, P.; Ma, S.; Xu, L.; Hu, F.; Gui, H.; Zhao, H.; Bai, Z.; et al. Induction of Trained Immunity Protects Neonatal Mice Against Microbial Sepsis by Boosting Both the Inflammatory Response and Antimicrobial Activity. J. Inflamm. Res. 2022, 15, 3829–3845. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Ye, T.; Wang, M.; Wang, M.; Li, Y.; Ma, L.; Yang, Y.; Wang, Y.; Zhao, X.; Liu, L.; et al. Traditional Chinese Medicine Formula Kang Shuai Lao Pian Improves Obesity, Gut Dysbiosis, and Fecal Metabolic Disorders in High-Fat Diet-Fed Mice. Front. Pharmacol. 2020, 11, 297. [Google Scholar]
- Ichikawa-Seki, M.; Motooka, D.; Kinami, A.; Murakoshi, F.; Takahashi, Y.; Aita, J.; Hayashi, K.; Tashibu, A.; Nakamura, S.; Iida, T.; et al. Specific increase of Fusobacterium in the faecal microbiota of neonatal calves infected with Cryptosporidium parvum. Sci. Rep. 2019, 9, 12517. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.Y.; Kim, S.; Kwon, M.S.; Lee, J.; Yu, D.H.; Song, R.H.; Choi, H.J.; Park, J. Rotavirus-mediated alteration of gut microbiota and its correlation with physiological characteristics in neonatal calves. J. Microbiol. 2019, 57, 113–121. [Google Scholar] [CrossRef]
- Ma, T.; Villot, C.; Renaud, D.; Skidmore, A.; Chevaux, E.; Steele, M.; Guan, L.L. Linking perturbations to temporal changes in diversity, stability, and compositions of neonatal calf gut microbiota: Prediction of diarrhea. ISME J. 2020, 14, 2223–2235. [Google Scholar]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Pietrzak, B.; Tomela, K.; Olejnik-Schmidt, A.; Mackiewicz, A.; Schmidt, M. Secretory IgA in Intestinal Mucosal Secretions as an Adaptive Barrier against Microbial Cells. Int. J. Mol. Sci. 2020, 21, 9254. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar]
- Luo, J.; Zeng, D.; Cheng, L.; Mao, X.; Yu, J.; Yu, B.; Chen, D. Dietary β-glucan supplementation improves growth performance, carcass traits and meat quality of finishing pigs. Anim. Nutr. 2019, 5, 380–385. [Google Scholar] [CrossRef]
- Ding, B.; Zheng, J.; Wang, X.; Zhang, L.; Sun, D.; Xing, Q.; Pirone, A.; Fronte, B. Effects of dietary yeast beta-1,3-1,6-glucan on growth performance, intestinal morphology and chosen immunity parameters changes in Haidong chicks. Asian-Australas. J. Anim. Sci. 2019, 32, 1558–1564. [Google Scholar]


| Items | Experimental Groups | SEM | p-Value | |
|---|---|---|---|---|
| CON | PO | |||
| IL-6, ng/L | 0.212 | 0.518 | 0.088 | 0.008 |
| IL-1β, ng/L | 2.149 | 2.996 | 0.154 | 0.003 |
| TNF-α, ng/L | 4.037 | 4.002 | 0.361 | 0.698 |
| IgG, μg/mL | 99.002 | 118.057 | 6.367 | 0.138 |
| IgM, μg/mL | 2.937 | 3.523 | 0.182 | 0.110 |
| Scavengiing rate of hydroxyl radical, % | 0.739 | 0.965 | 0.039 | 0.002 |
| MDA, nmol/mL | 0.241 | 0.523 | 0.030 | <0.001 |
| DAO, nmol/min/mL | 5.916 | 5.937 | 0.315 | 0.974 |
| Items | Experimental Groups | SEM | p-Value | |
|---|---|---|---|---|
| CON | PO | |||
| 0–30 d | ||||
| Milk replacer intake, L/d | 8.524 | 8.618 | 0.056 | 0.408 |
| Calf-starter intake, kg/d | 0.035 | 0.039 | 0.006 | 0.727 |
| Total DM intake, kg/d | 1.254 | 1.270 | 0.010 | 0.475 |
| ADG, kg/d | 0.983 | 0.944 | 0.037 | 0.602 |
| FCR | 1.352 | 1.433 | 0.026 | 0.506 |
| 31–60 d | ||||
| Milk replacer intake, L/d | 9.262 | 9.476 | 0.057 | 0.057 |
| Calf-starter intake, kg/d | 0.126 | 0.105 | 0.011 | 0.323 |
| Total DM intake, kg/d | 1.451 | 1.460 | 0.012 | 0.708 |
| ADG, kg/d | 0.982 | 1.028 | 0.035 | 0.511 |
| FCR | 1.527 | 1.496 | 0.035 | 0.765 |
| 0–60 d | ||||
| Milk replacer intake, L/d | 8.893 | 8.922 | 0.079 | 0.518 |
| Calf-starter intake, kg/d | 0165 | 0.143 | 0.015 | 0.494 |
| Total DM intake, kg/d | 1.436 | 1.440 | 0.016 | 0.927 |
| ADG, kg/d | 0.982 | 1.001 | 0.009 | 0.324 |
| FCR | 1.465 | 1.439 | 0.015 | 0.413 |
| 0–180 d | ||||
| ADG, kg/d | 1.042 | 1.086 | 0.012 | 0.077 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Yan, F.; Xiong, M.; Dong, J.; Yang, W.; Xu, X. Effects of Yeast β-Glucan Supplementation on Calf Intestinal and Respiratory Health. Animals 2025, 15, 997. https://doi.org/10.3390/ani15070997
Wang J, Yan F, Xiong M, Dong J, Yang W, Xu X. Effects of Yeast β-Glucan Supplementation on Calf Intestinal and Respiratory Health. Animals. 2025; 15(7):997. https://doi.org/10.3390/ani15070997
Chicago/Turabian StyleWang, Jiamin, Fang Yan, Meng Xiong, Jieru Dong, Wenqian Yang, and Xiurong Xu. 2025. "Effects of Yeast β-Glucan Supplementation on Calf Intestinal and Respiratory Health" Animals 15, no. 7: 997. https://doi.org/10.3390/ani15070997
APA StyleWang, J., Yan, F., Xiong, M., Dong, J., Yang, W., & Xu, X. (2025). Effects of Yeast β-Glucan Supplementation on Calf Intestinal and Respiratory Health. Animals, 15(7), 997. https://doi.org/10.3390/ani15070997





