Morphological and Histological Analysis of the Gastrointestinal Systems in Triplophysa strauchii and Triplophysa tenuis: Insights into Digestive Adaptations
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Morphological Observation
2.3. Histological Analysis
2.4. Data Processing
3. Results
3.1. Morphology of the Digestive System
3.2. Histology of the Digestive System
3.2.1. Oropharyngeal Cavity
3.2.2. Esophagus
3.2.3. Stomach
3.2.4. Intestine
3.2.5. Hepatopancreas
4. Discussion
4.1. The Correlation Between the Morphology of the Digestive System and Feeding Habits
4.2. Histological Characteristics and Digestive Functions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, C.; Hu, B.; Ma, W.; Yang, C.; Zhao, W.; Li, B.; Meng, X.; Yang, Y.; Nie, G. A new fish record of the genus Triplophysa in Henan Province—Triplophysa dalaica. J. Henan Norm. Univ. (Nat. Sci. Ed.) 2020, 48, 113–124. [Google Scholar]
- Sheraliev, B.; Peng, Z. Triplophysa ferganaensis, a new loach species from Fergana Valley in Central Asia (Teleostei: Nemacheilidae). J. Fish Biol. 2021, 99, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gu, Q.; Zhou, C.; Tang, Y.; Husemann, M.; Meng, X.; Zhang, J.-X.; Nie, G.-X.; Li, X.-J. Molecular phylogeny and biogeography of Triplophysa stone loaches in the Central Chinese Mountains. Biol. J. Linn. Soc. 2020, 130, 563–577. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Feng, C.; Zhao, K.; Song, Z.; Zhang, Y.; Yang, L.; He, S. Mitogenomic perspectives on the origin of Tibetan loaches and their adaptation to high altitude. Sci. Rep. 2016, 6, 29690. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Jin, H.; Li, W.; Yan, C.; Yan, P.; Zhang, X.; He, S.; Song, Z. Identification of Triplophysa species from the Qinghai-Tibetan Plateau (QTP) and its adjacent regions through DNA barcodes. Gene 2017, 605, 12–19. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, L.G.; Tuerxun; Zhang, R.M.; Liu, K.L.; Zhang, B.P. Biological study on Triplophysa strauchii in Sayram Lake. Chin. J. Fish. 2002, 2, 6–11. [Google Scholar]
- Wang, Z.; Gao, S.; Hamid, S.M.; Xiao, Q.; Zhu, W.; Nie, Z.; Wei, J. Morphological Difference Analysis of Triplophysa strauchii from Different Geographical Populations in Xinjiang, China. Water 2025, 17, 467. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, H.; Nie, Z.; Wei, J.; Zhang, D. Feeding habits of Triplophysa tenuis in Xinjiang based on fatty acid and stable carbon and nitrogen isotopic analysis. South China Fish. Sci. 2024, 20, 92–101. [Google Scholar]
- Zhang, L.R.; Ji, B.W.; Nie, Z.L.; Wei, J. Age Structure and Growth Characteristics of Loach Triplophysa tenuis in Muzati River, Xinjiang. Chin. J. Fish. 2024, 37, 30–37. [Google Scholar]
- Khalil, N.; Allah, H.M.; Mousa, M.A. The effect of maternal thyroxine injection on growth, survival and development of the digestive system of Nile tilapia, Oreochromis niloticus, larvae. Adv. Biosci. Biotechnol. 2011, 2, 320–329. [Google Scholar] [CrossRef][Green Version]
- Yang, S.; Du, J.; Duan, Y.L.; Xiao, Q.; Li, N.Q.; Lin, Q.; Zhao, L.L.; Du, Z.J.; Zhou, J.; Du, J. Differences in the digestive enzyme activity, intestinal mucosa and microbial community in loach cultivated in two separate environments. BMC Microbiol. 2018, 18, 113. [Google Scholar] [CrossRef] [PubMed]
- Purushothaman, K.; Ho Jia Wen, R.; Bin Mohamed, M.H.; Rwei Qing, S.D.T.; Heng Wuan, L.; Liang, B.; Thanh Vu, N.; Voigtmann, M.; McLean Press, C.; Loo, G.; et al. Comparative Nutritional and Histological Analysis of Malabar Red Snapper (Lutjanus malabaricus) and Asian Seabass (Lates calcarifer). Anim. Open Access J. 2024, 14, 1803. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y. Sayram Lake: The last tear of the Atlantic Ocean. Geogr. Teach. 2023, 15, 65. [Google Scholar]
- Zhao, Q.; Zhao, C.; Qin, Y.; Chang, Y.; Wang, J. Response of the hydrological processes to climate change in the Muzati Riverbasin with high glacierization, southern slope of the Tianshan Mountains. J. Glaciol. Geocryol. 2020, 42, 1285–1298. [Google Scholar]
- Seibel, H.; Weirup, L.; Schulz, C. Fish Welfare—Between Regulations, Scientific Facts and Human Perception. Food Ethics 2020, 5, 4. [Google Scholar] [CrossRef]
- Ma, K.; Tong, G.; Zhang, Y.; Zhang, Q.; Yin, J.; Mou, Z.; Kuang, Y. Karyotype analysis and evolutionary status of Oxygymnocypris stewartii. J. Northwest A F Univ. (Nat. Sci. Ed.) 2021, 49, 28–33+42. [Google Scholar]
- Albrecht, M.P.; Ferreira, M.F.N.; Caramaschi, E.P. Anatomical features and histology of the digestive tract of two related neotropical omnivorous fishes (Characiformes; Anostomidae). J. Fish Biol. 2001, 58, 419–430. [Google Scholar] [CrossRef]
- Palladino, A.; De Felice, E.; Attanasio, C.; Barone, C.M.A.; Crasto, A.; D’Angelo, L.; Giaquinto, D.; Lambiase, C.; Scocco, P.; Serrapica, F.; et al. A Morphological and Ultrastructural Study of the Anterior Digestive Tract of Adult Nile Tilapia Oreochromis niloticus. Animals 2023, 13, 420. [Google Scholar] [CrossRef]
- Moawad, U.K.; Awaad, A.S.; Tawfiek, M.G. Histomorphological, histochemical, and ultrastructural studies on the stomach of the adult African catfish (Clarias gariepinus). J. Microsc. Ultrastruct. 2017, 5, 155–166. [Google Scholar]
- Gidmark, N.J.; Pos, K.; Matheson, B.; Ponce, E.; Westneat, M.W. Functional Morphology and Biomechanics of Feeding in Fishes. In Feeding in Vertebrates: Evolution, Morphology, Behavior, Biomechanics; Bels, V., Whishaw, I.Q., Eds.; Springer: Cham, Switzerland, 2019; pp. 297–332. [Google Scholar]
- Satyam, K.; Thiruchitrambalam, G. Chapter 7—Habitat Ecology and Diversity of Rocky Shore Fauna. In Biodiversity and Climate Change Adaptation in Tropical Islands; Sivaperuman, C., Velmurugan, A., Singh, A.K., Jaisankar, I., Eds.; Academic Press: London, UK, 2018; pp. 187–215. [Google Scholar]
- Schwalbe, M.A.B.; Webb, J.F. Sensory basis for detection of benthic prey in two Lake Malawi cichlids. Zoology 2014, 117, 112–121. [Google Scholar] [CrossRef]
- Ferry, L.A. Functional Morphology: ‘Point and Shoot’ Prey Capture in Fishes. Curr. Biol. 2015, 25, R982–R984. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Xing, S.; Wang, X.; Duan, S. Analysis on the diet composition and rRNA gene ITS interval of Trilophysa brevviuda in Tibet. Plateau Sci. Res. 2019, 3, 9–14. [Google Scholar]
- Purushothaman, K.; Lau, D.; Saju, J.M.; Musthaq, S.S.; Lunny, D.P.; Vij, S.; Orbán, L. Morpho-histological characterisation of the alimentary canal of an important food fish, Asian seabass (Lates calcarifer). PeerJ 2016, 4, e2377. [Google Scholar] [CrossRef] [PubMed]
- Moraes, G.; de Almeida, L.C. Chapter 11—Nutrition and functional aspects of digestion in fish. In Biology and Physiology of Freshwater Neotropical Fish; Baldisserotto, B., Urbinati, E.C., Cyrino, J.E.P., Eds.; Academic Press: London, UK, 2020; pp. 251–271. [Google Scholar]
- Chaudhuri, A.; Mukherjee, S.; Homechaudhuri, S. Dietary preference and digestive physiology of plankti-benthivorous fishes inhabiting mudflats of Indian Sundarban estuaries. Trop. Zool. 2016, 29, 53–72. [Google Scholar] [CrossRef]
- Davis, A.M.; Unmack, P.J.; Pusey, B.J.; Pearson, R.G.; Morgan, D.L. Ontogenetic development of intestinal length and relationships to diet in an Australasian fish family (Terapontidae). BMC Evol. Biol. 2013, 13, 53. [Google Scholar] [CrossRef]
- Sokolova, I. Bioenergetics in environmental adaptation and stress tolerance of aquatic ectotherms: Linking physiology and ecology in a multi-stressor landscape. J. Exp. Biol. 2021, 224 (Suppl. S1), jeb236802. [Google Scholar] [CrossRef]
- Zha, J.; Zou, S.; Hao, J.; Liu, X.; Delaplace, G.; Jeantet, R.; Dupont, D.; Wu, P.; Dong Chen, X.; Xiao, J. The role of circular folds in mixing intensification in the small intestine: A numerical study. Chem. Eng. Sci. 2021, 229, 116079. [Google Scholar] [CrossRef]
- Jiao, F.; Zhang, L.; Limbu, S.M.; Yin, H.; Xie, Y.; Yang, Z.; Shang, Z.; Kong, L.; Rong, H. A comparison of digestive strategies for fishes with different feeding habits: Digestive enzyme activities, intestinal morphology, and gut microbiota. Ecol. Evol. 2023, 13, e10499. [Google Scholar] [CrossRef]
- Honda, Y.; Takagi, W.; Wong, M.K.S.; Ogawa, N.; Tokunaga, K.; Kofuji, K.; Hyodo, S. Morphological and functional development of the spiral intestine in cloudy catshark (Scyliorhinus torazame). J. Exp. Biol. 2020, 223 Pt 13, jeb225557. [Google Scholar] [CrossRef]
- Liu, N. Analysis of the Diet of Triplophysa rosa (Teleostei, Cypriniformes). Master’s Thesis, Southwest University, Chongqing, China, 2020. [Google Scholar]
- Li, C. The Primary Studies on the Structure of Digestive System in Botia superciliaris. Master’s Thesis, Southwest University, Chongqing, China, 2009. [Google Scholar]
- Tebbett, S.B.; Goatley, C.H.R.; Huertas, V.; Mihalitsis, M.; Bellwood, D.R. A functional evaluation of feeding in the surgeonfish Ctenochaetus striatus: The role of soft tissues. R. Soc. Open Sci. 2018, 5, 171111. [Google Scholar] [CrossRef]
- Dash, S.; Das, S.K.; Samal, J.; Thatoi, H.N. Epidermal mucus, a major determinant in fish health: A review. Iran. J. Vet. Res. 2018, 19, 72–81. [Google Scholar] [PubMed]
- Hansen, A.; Ghosal, R.; Caprio, J.; Claus, A.W.; Sorensen, P.W. Anatomical and physiological studies of bigheaded carps demonstrate that the epibranchial organ functions as a pharyngeal taste organ. J. Exp. Biol. 2014, 217 Pt 21, 3945–3954. [Google Scholar] [CrossRef] [PubMed]
- Krauss, R.S.; Chihara, D.; Romer, A.I. Embracing change: Striated-for-smooth muscle replacement in esophagus development. Skelet. Muscle 2016, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Alabssawy, A.N.; Khalaf-Allah, H.M.M.; Gafar, A.A. Anatomical and histological adaptations of digestive tract in relation to food and feeding habits of lizardfish, Synodus variegatus (Lacepède, 1803). Egypt. J. Aquat. Res. 2019, 45, 159–165. [Google Scholar] [CrossRef]
- Johansson, M.E.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef]
- Ofelio, C.; Cohen, S.; Adriaens, D.; Radaelli, G.; Díaz, A.O. Histochemistry of goblet cells and micro-computed tomography to study the digestive system in the long-snouted seahorse Hippocampus guttulatus. Aquaculture 2019, 502, 400–409. [Google Scholar] [CrossRef]
- Xiong, H.; Yao, Y.; Wang, Z. Study on the structure of the digestive tract in Triplophysa bleekeri. Artif. Intell. Sci. Eng. 2012, 37, 113–120. [Google Scholar]
- Rimoldi, S.; Antonini, M.; Gasco, L.; Moroni, F.; Terova, G. Intestinal microbial communities of rainbow trout (Oncorhynchus mykiss) may be improved by feeding a Hermetia illucens meal/low-fishmeal diet. Fish Physiol. Biochem. 2021, 47, 365–380. [Google Scholar] [CrossRef]
- Wang, E.; Zhou, Y.; Liang, Y.; Ling, F.; Xue, X.; He, X.; Zhai, X.; Xue, Y.; Zhou, C.; Tang, G.; et al. Rice flowering improves the muscle nutrient, intestinal microbiota diversity, and liver metabolism profiles of tilapia (Oreochromis niloticus) in rice-fish symbiosis. Microbiome 2022, 10, 231. [Google Scholar] [CrossRef]
- Nasruddin, N.S.; Azmai, M.N.; Ismail, A.; Saad, M.Z.; Daud, H.M.; Zulkifli, S.Z. Histological features of the gastrointestinal tract of wild Indonesian shortfin eel, Anguilla bicolor bicolor (McClelland, 1844), captured in Peninsular Malaysia. Sci. World J. 2014, 2014, 312670. [Google Scholar] [CrossRef]
- Akter, M.N.; Sutriana, A.; Talpur, A.D.; Hashim, R. Dietary supplementation with mannan oligosaccharide influences growth, digestive enzymes, gut morphology, and microbiota in juvenile striped catfish, Pangasianodon hypophthalmus. Aquac. Int. 2016, 24, 127–144. [Google Scholar] [CrossRef]
- Torrecillas, S.; Montero, D.; Caballero, M.J.; Robaina, L.; Zamorano, M.J.; Sweetman, J.; Izquierdo, M. Effects of dietary concentrated mannan oligosaccharides supplementation on growth, gut mucosal immune system and liver lipid metabolism of European sea bass (Dicentrarchus labrax) juveniles. Fish Shellfish Immunol. 2015, 42, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Pergolizzi, S.; Savoca, S.; Fumia, A.; Mangano, A.; Albano, M.; Messina, E.; Aragona, M.; Lo Cascio, P.; Capillo, G.; et al. Detecting Intestinal Goblet Cells of the Broadgilled Hagfish Eptatretus cirrhatus (Forster, 1801): A Confocal Microscopy Evaluation. Biology 2022, 11, 1366. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.W.; Kim, S.K.; Kim, D.J.; Lee, B.I.; Park, S.J.; Hwang, H.G.; Jun, J.C.; Myeong, J.I.; Lee, C.H.; Lee, Y.D. Digestive Physiological Characteristics of the Gobiidae:–Characteristics of CCK-producing Cells and Mucus-secreting Goblet Cells of Stomach Fish and Stomachless Fish. Dev. Reprod. 2016, 20, 207–217. [Google Scholar] [CrossRef]
- Degregori, S.; Schiettekatte, N.M.D.; Casey, J.M.; Brandl, S.J.; Mercière, A.; Amato, K.R.; Mazel, F.; Parravicini, V.; Barber, P.H. Host diet drives gut microbiome convergence between coral reef fishes and mammals. Mol. Ecol. 2024, 33, e17520. [Google Scholar] [CrossRef]
- Luo, L.; Cheng, L.; Liu, A.; Chen, S.; Liu, J. Study on the eating ecological strategy of Triplophysa yarkandensis in the Yarkant River. J. Tarim Univ. 2023, 35, 81–88. [Google Scholar]
- Kaur, I.; Tiwari, R.; Naidu, V.G.M.; Ramakrishna, S.; Tripathi, D.M.; Kaur, S. Bile Acids as Metabolic Inducers of Hepatocyte Proliferation and Liver Regeneration. Regen. Eng. Transl. Med. 2022, 8, 200–209. [Google Scholar] [CrossRef]
- Schulze, R.J.; Schott, M.B.; Casey, C.A.; Tuma, P.L.; McNiven, M.A. The cell biology of the hepatocyte: A membrane trafficking machine. J. Cell Biol. 2019, 218, 2096–2112. [Google Scholar] [CrossRef]
- Shao-ping, L. Growth characteristics and feed habit of Triplophysa stenura in Nujiang River. Freshw. Fish. 2010, 40, 26–33. [Google Scholar]
- Hu, J.; Liu, M.; He, D. Phylogeography of Triplophysa stenura (Nemacheilidae): Responded to the Mid-Pleistocene Climate Transition in the Qinghai-Tibetan Plateau. Zool. Stud. 2020, 59, e67. [Google Scholar]
- El-Bakary, N.E.R.; El-Gammal, H.L. Comparative Histological, Histochemical and Ultrastructural Studies on the Liver of Flathead Grey Mullet (Mugil cephalus) and Sea Bream (Sparus aurata); CABI Digital Library: Wallingford, UK, 2010. [Google Scholar]
- Prince, V.E.; Anderson, R.M.; Dalgin, G. Chapter Seven—Zebrafish Pancreas Development and Regeneration: Fishing for Diabetes Therapies. In Current Topics in Developmental Biology; Sadler, K.C., Ed.; Academic Press: London, UK, 2017; Volume 124, pp. 235–276. [Google Scholar]
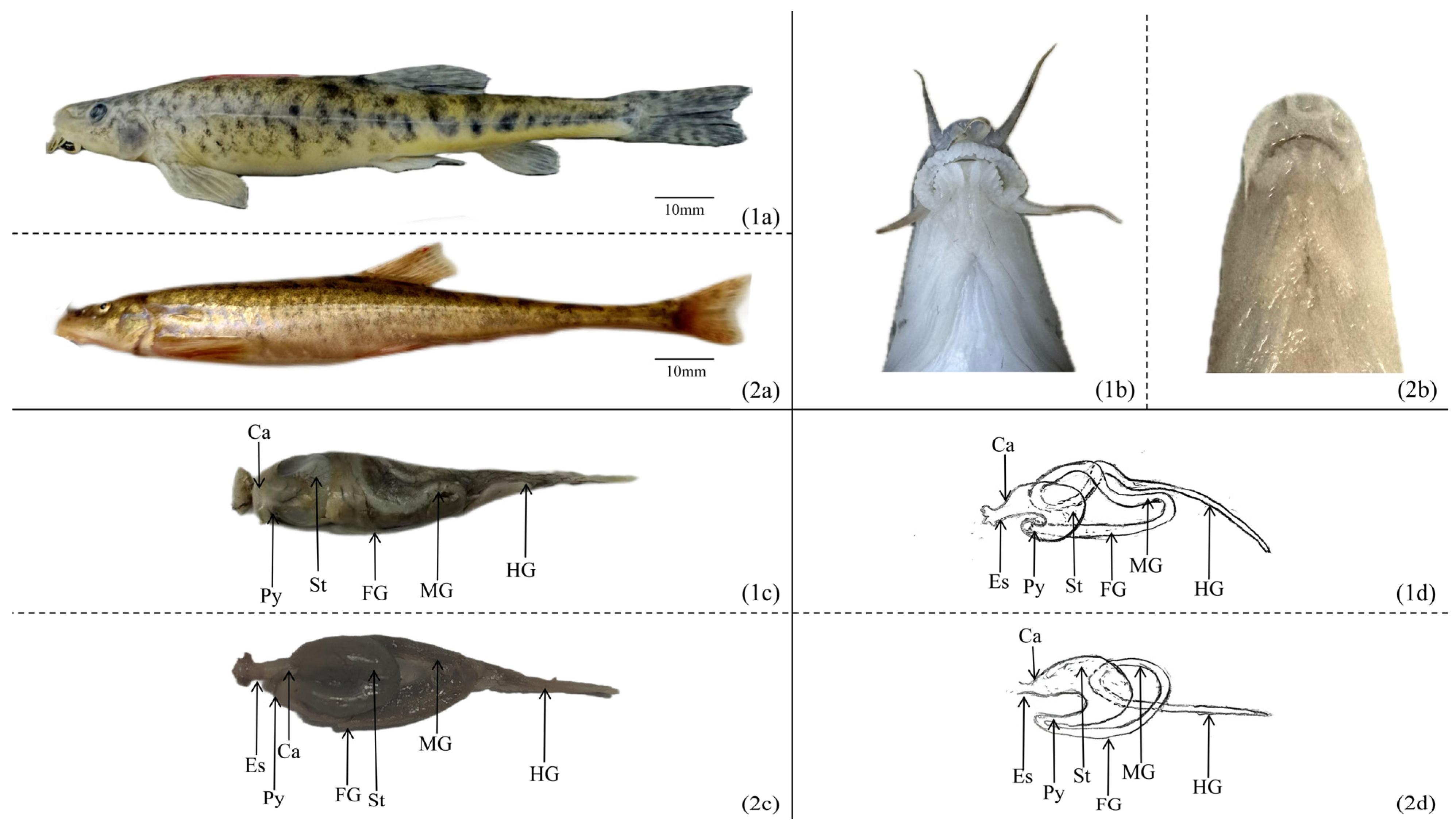
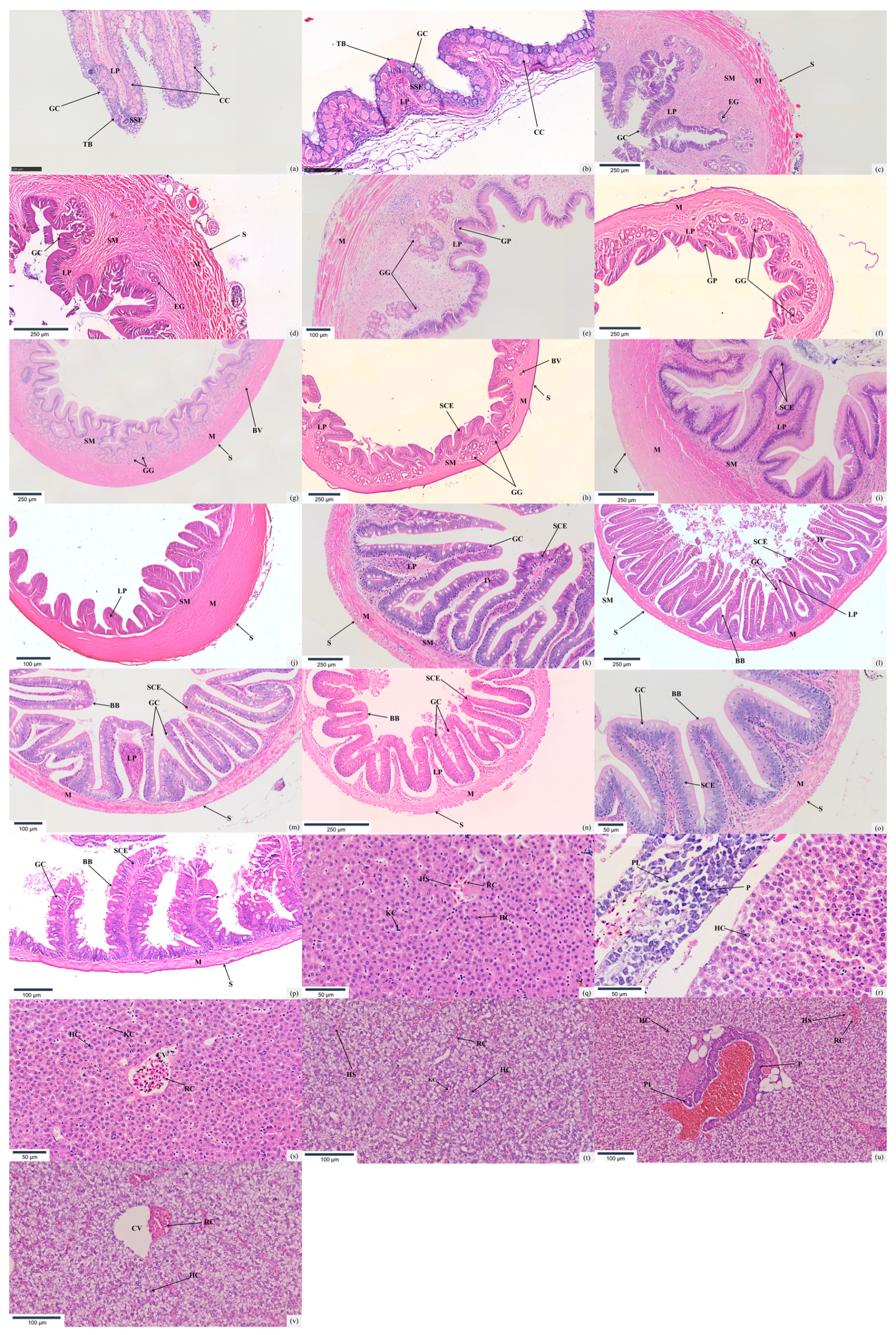
| Morphological Index | Species | Esophagus | Cardia | Stomach | Pylorus | Foregut | Midgut | Hindgut |
|---|---|---|---|---|---|---|---|---|
| Spiral valve height/μm | T. strauchii | 301.11 ± 96.06 | 250.65 ± 58.04 | 260.45 ± 40.02 | 639.51 ± 62.10 a | 408.87 ± 31.87 | 311.74 ± 55.86 a | 141.26 ± 50.04 a |
| T. tenuis | 291.59 ± 30.16 | 275.07 ± 29.35 | 277.54 ± 27.50 | 278.13 ± 57.40 b | 387.47 ± 36.69 | 248.30 ± 30.90 b | 218.24 ± 56.61 b | |
| Mucosal fold width/μm | T. strauchii | 115.66 ± 12.80 | 116.25 ± 26.08 a | 201.92 ± 20.95 a | 88.59 ± 9.90 a | 75.95 ± 26.94 a | 64.08 ± 7.04 | 92.71 ± 23.66 |
| T. tenuis | 119.54 ± 33.33 | 108.37 ± 20.20 b | 150.47 ± 29.51 b | 136.60 ± 17.98 b | 97.31 ± 19.63 b | 87.70 ± 11.41 | 102.13 ± 9.82 | |
| Sub mucosa thick/μm | T. strauchii | 261.66 ± 71.30 | 189.69 ± 46.23 | 205.10 ± 88.27 | 11.53 ± 4.10 | 9.64 ± 1.77 | 12.76 ± 1.63 | 16.45 ± 4.94 |
| T. tenuis | 251.66 ± 80.42 | 182.69 ± 49.90 | 193.10 ± 82.72 | 10.43 ± 3.03 | 9.34 ± 1.95 | 11.26 ± 1.71 | 14.95 ± 4.17 | |
| Muscle layer thickness/μm | T. strauchii | 170.09 ± 13.90 a | 88.98 ± 8.91 a | 252.18 ± 75.34 a | 49.53 ± 4.63 a | 49.17 ± 12.09 | 56.89 ± 11.82 a | 25.96 ± 2.60 |
| T. tenuis | 106.22 ± 6.49 b | 64.57 ± 11.90 b | 74.42 ± 12.22 b | 104.64 ± 7.68 b | 51.96 ± 7.55 | 39.02 ± 5.88 b | 28.39 ± 4.09 | |
| Goblet cell (cell number/100 μm) | T. strauchii | 30.10 ± 2.85 a | - | - | - | 151.40 ± 25.56 a | 159.1 ± 8.43 a | 178.50 ± 10.95 a |
| T. tenuis | 13.80 ± 2.62 b | - | - | - | 100.4 ± 12.17 b | 109.90 ± 7.95 b | 117.80 ± 9.62 b | |
| Intestinal villi number/mm2 | T. strauchii | - | - | - | - | 18.40 ± 2.07 a | 18.90 ± 3.07 | 12.70 ± 1.25 a |
| T. tenuis | - | - | - | - | 24.60 ± 2.17 b | 20.10 ± 3.14 | 15.00 ± 1.76 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhang, L.; Wei, J.; Hao, H.; Hamid, S.M.; Gao, S.; Li, W.; Nie, Z. Morphological and Histological Analysis of the Gastrointestinal Systems in Triplophysa strauchii and Triplophysa tenuis: Insights into Digestive Adaptations. Animals 2025, 15, 1095. https://doi.org/10.3390/ani15081095
Wang Z, Zhang L, Wei J, Hao H, Hamid SM, Gao S, Li W, Nie Z. Morphological and Histological Analysis of the Gastrointestinal Systems in Triplophysa strauchii and Triplophysa tenuis: Insights into Digestive Adaptations. Animals. 2025; 15(8):1095. https://doi.org/10.3390/ani15081095
Chicago/Turabian StyleWang, Zhengwei, Lirong Zhang, Jie Wei, Huimin Hao, Syeda Maira Hamid, Shixin Gao, Wenjun Li, and Zhulan Nie. 2025. "Morphological and Histological Analysis of the Gastrointestinal Systems in Triplophysa strauchii and Triplophysa tenuis: Insights into Digestive Adaptations" Animals 15, no. 8: 1095. https://doi.org/10.3390/ani15081095
APA StyleWang, Z., Zhang, L., Wei, J., Hao, H., Hamid, S. M., Gao, S., Li, W., & Nie, Z. (2025). Morphological and Histological Analysis of the Gastrointestinal Systems in Triplophysa strauchii and Triplophysa tenuis: Insights into Digestive Adaptations. Animals, 15(8), 1095. https://doi.org/10.3390/ani15081095







