Morphology, Age, and Growth of Triplophysa strauchii in Sayram Lake, Xinjiang, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Processing
2.2. Biological Determination
2.2.1. Traditional Morphological Measurement
2.2.2. Truss Morphometry Measurement
2.3. Sex Determination
2.4. Lapillus Processing and Age Estimation
2.5. Relationship Between Body Length and Body Weight
2.6. Growth Equation
3. Results
3.1. Traditional Morphology
3.1.1. Morphological Description
3.1.2. Measurable Traits
3.1.3. Principal Component Analysis
3.1.4. Discriminant Analysis
3.2. Truss Structure
3.3. Age Estimation
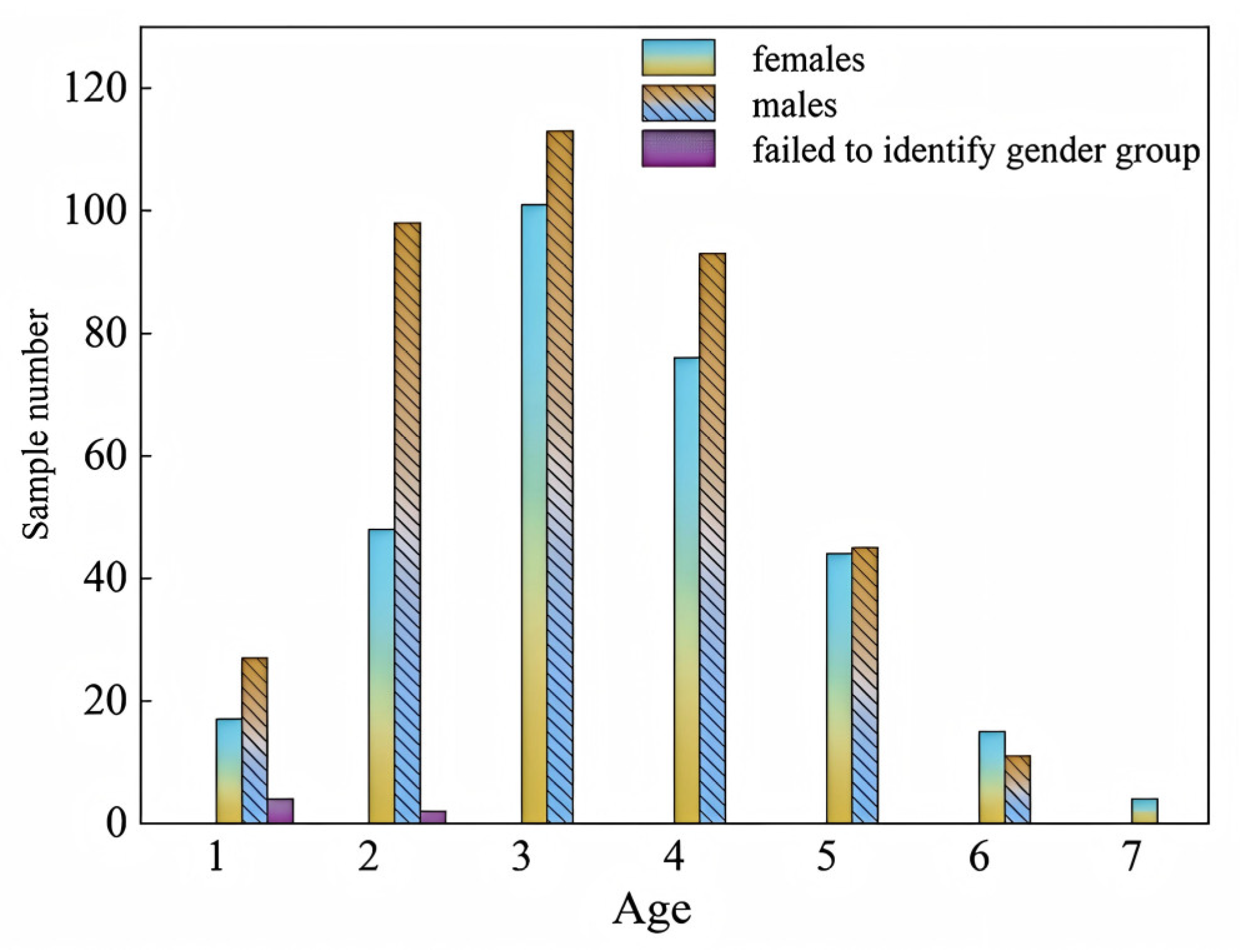
3.4. Population Structure Characteristics
3.5. Body Length–Weight Relationship
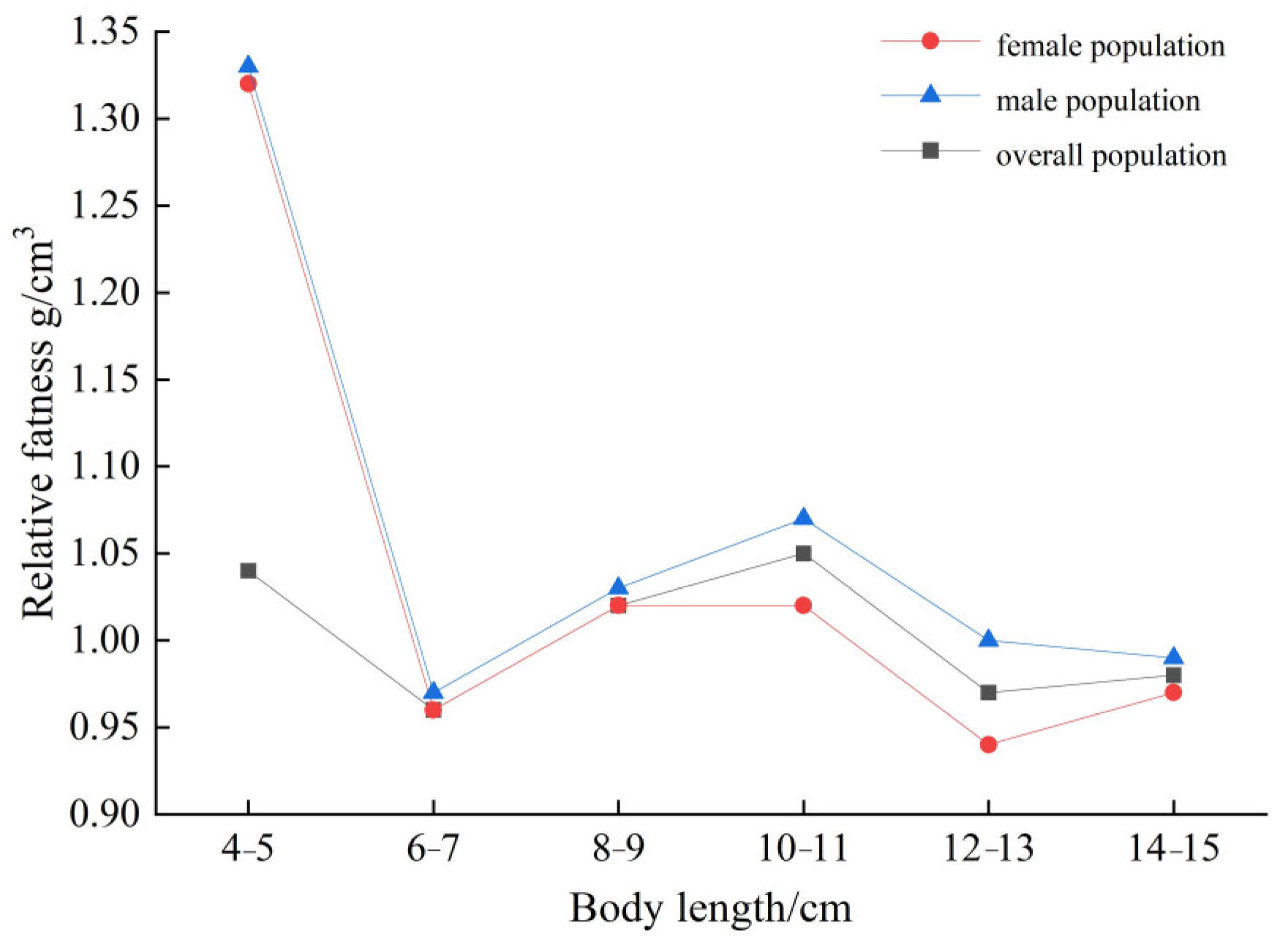
3.6. Degree of Fatness
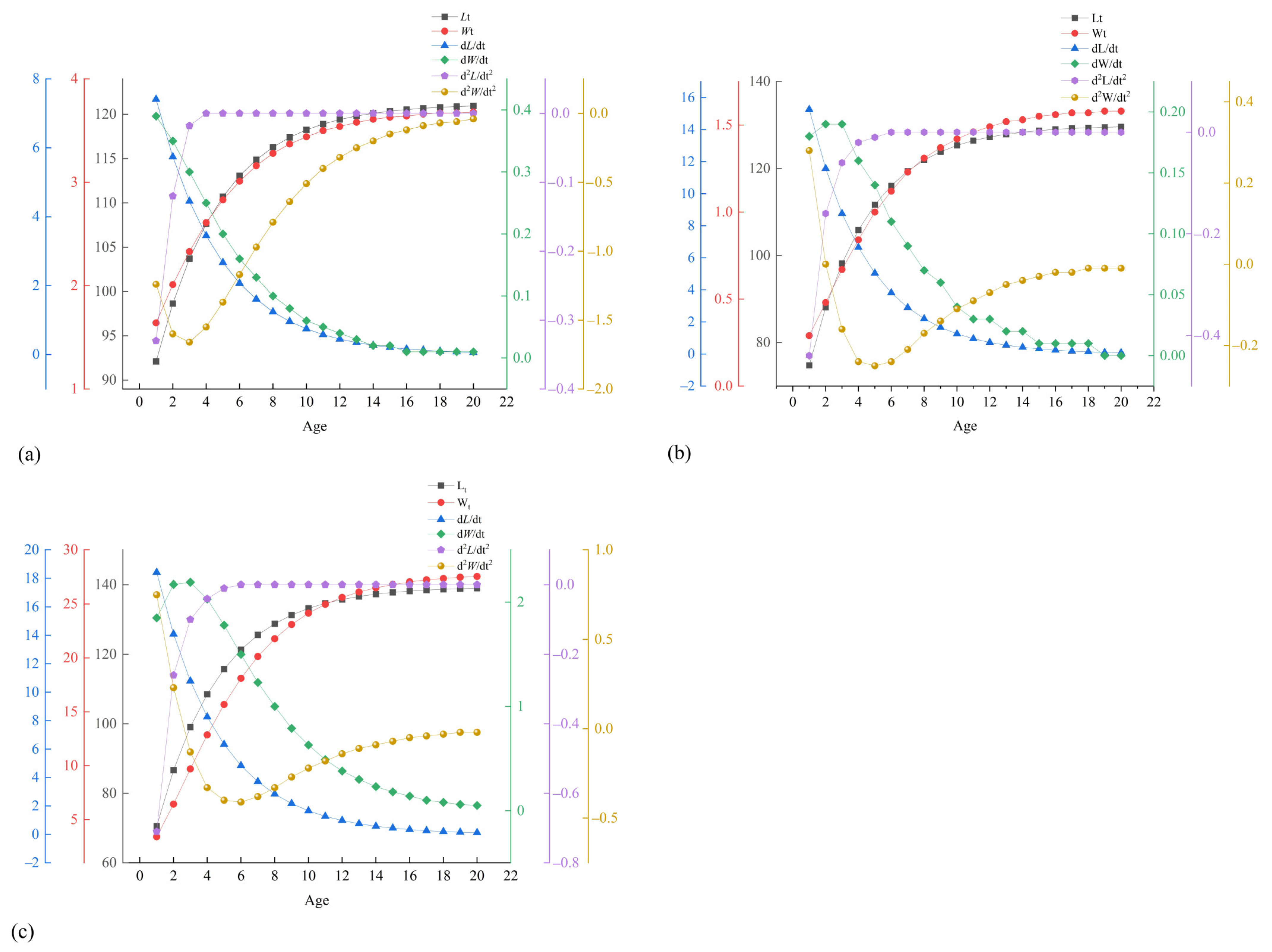
3.7. Growth Characteristics
4. Discussion
4.1. Individual Morphological Characteristics
4.2. Age and Growth Features
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Yu, Y.Y. Sayram Lake: The last tear of the Atlantic Ocean. Geogr. Teach. 2023, 15, 2+65. [Google Scholar]
- Han, J.R.; Zhang, H.; Zheng, W.S.; Chen, G.J.; Du, Z.J. Confluentibacter citreus sp. nov., isolated from lake sediment, and emended description of the genus Confluentibacter. Int. J. Syst. Evol. Microbiol. 2017, 67, 4008–4012. [Google Scholar] [PubMed]
- Liu, X.Y.; Li, R.C.; Shao, L. Exploration on the Causes and Treatment Methods of Natural Grassland Degradation in the Sayram Lake Area of Bole City. Xinjiang Anim. Husb. 2021, 4, 58–59+42. [Google Scholar]
- Fang, L.; Chen, L.; Liu, Y.; Tao, W.; Zhang, Z.; Liu, H.; Tang, Y. Planktonic and sedimentary bacterial diversity of Lake Sayram in summer. MicrobiologyOpen 2015, 4, 814–825. [Google Scholar]
- Guo, Y.; Cai, L.G.; Tuerxun; Zhang, R.M.; Liu, K.L.; Zhang, B.P. Biological study on Triplophysa strauchii in Sayram Lake. Chin. J. Fish. 2002, 2, 6–11. [Google Scholar]
- Belgrad, B.A.; Griffen, B.D. Predator-prey interactions mediated by prey personality and predator hunting mode. Proc. R. Soc. B Biol. Sci. 2016, 283, 20160408. [Google Scholar]
- Zdilla, K.M. Trophic cascades: Predators, prey and the changing dynamics of nature. Ecol. Econ. 2011, 70, 2534–2535. [Google Scholar]
- Ian Perry, R.; Ommer, R.E. Introduction: Coping with global change in marine social-ecological systems. Mar. Policy 2010, 34, 739–741. [Google Scholar]
- Bundy, A.; Chuenpagdee, R.; Cooley, S.; Glaeser, B.; McManus, L.T. Global change, ensuing vulnerabilities, and social responses in marine environments. Reg. Environ. Change 2016, 16, 273–276. [Google Scholar]
- Acquay, H.K. Implications of structural adjustment for Ghana’s marine fisheries policy. Fish. Res. 1992, 14, 59–70. [Google Scholar] [CrossRef]
- Xiao, S.J.; Mou, Z.B.; Yang, R.B.; Fan, D.D.; Liu, J.Q.; Zou, Y.; Zhu, S.L.; Zou, M.; Zhou, C.W.; Liu, H.P. Genome and population evolution and environmental adaptation of Glyptosternon maculatum on the Qinghai-Tibet Plateau. Zool. Res. 2021, 42, 502–513. [Google Scholar] [PubMed]
- Abdelnour, S.A.; Naiel, M.A.E.; Said, M.B.; Alnajeebi, A.M.; Nasr, F.A.; Al-Doaiss, A.A.; Mahasneh, Z.M.H.; Noreldin, A.E. Environmental epigenetics: Exploring phenotypic plasticity and transgenerational adaptation in fish. Environ. Res. 2024, 252 Pt 1, 118799. [Google Scholar]
- Greenway, R.; De-Kayne, R.; Brown, A.P.; Camarillo, H.; Delich, C.; McGowan, K.L.; Nelson, J.; Arias-Rodriguez, L.; Kelley, J.L.; Tobler, M. Integrative analyses of convergent adaptation in sympatric extremophile fishes. Curr. Biol. 2024, 34, 4968–4982.e7. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.; Dong, H. Effects of body shape on hydrodynamic interactions in a dense diamond fish school. Phys. Fluids 2024, 36, 031907. [Google Scholar] [CrossRef]
- Luo, Y.; Xiao, Q.; Shi, G.; Pan, G.; Chen, D. The effect of variable stiffness of tuna-like fish body and fin on swimming performance. Bioinspir. Biomim. 2020, 16, 016003. [Google Scholar]
- Morat, F.; Wicquart, J.; Schiettekatte, N.M.D.; de Sinéty, G.; Bienvenu, J.; Casey, J.M.; Brandl, S.J.; Vii, J.; Carlot, J.; Degregori, S.; et al. Individual back-calculated size-at-age based on otoliths from Pacific coral reef fish species. Sci. Data 2020, 7, 370. [Google Scholar] [CrossRef]
- Ding, C.; He, D.; Chen, Y.; Jia, Y.; Tao, J. Otolith microstructure analysis based on wild young fish and its application in confirming the first annual increment in Tibetan Gymnocypris selincuoensis. Fish. Res. 2020, 221, 105386. [Google Scholar]
- Morioka, S.M.; Machinandiarena, L.; Research, F. Comparison of daily increment formation pattern between sagittae and lapilli of ling (Genypterus blacodes) larvae and juveniles collected off Argentina. N. Zeal. J. Mar. 2001, 35, 111–119. [Google Scholar]
- Lockwood, S.J. The use of the von Bertalanffy growth equation to describe the seasonal growth of fish. ICES J. Mar. Sci. 1974, 35, 175–179. [Google Scholar]
- Živkov, M.T.; Trichkova, T.A.; Raikova-Petrova, G.N. Biological reasons for the unsuitability of growth parameters and indices for comparing fish growth. Environ. Biol. Fishes 1999, 54, 67–76. [Google Scholar]
- Feng, C.; Tong, C.; Zhang, R.; Li, G.; Wanghe, K.; Tang, Y.; Zhang, C.; Zhao, K. Biodiversity and distribution patterns of Triplophysa species in the northeastern margin of the Tibetan Plateau. Biodivers. Sci. 2017, 25, 53–61. [Google Scholar] [CrossRef][Green Version]
- Li, J.; Wang, Y.; Jin, H.; Li, W.; Yan, C.; Yan, P.; Zhang, X.; He, S.; Song, Z. Identification of Triplophysa species from the Qinghai-Tibetan Plateau (QTP) and its adjacent regions through DNA barcodes. Gene 2017, 605, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Song, S.; Huang, T.; Liu, H.; Liu, Z. Mitochondrial Genome and Phylogenetic Analysis of Triplophysa erythraea. Chin. J. Zool. 2024, 59, 588–596. [Google Scholar]
- Kanu, U.C.; Zhao, G.; Xie, P.; Li, Y.; Lei, D.; Niu, J.; Ma, X. The complete mtDNA genome of Triplophysa strauchii (Cypriniformes, Balitoridae, Cobitoidea): Genome charaterization and phylogenetic analysis. Mitochondrial DNA Part A 2016, 27, 2637–2638. [Google Scholar] [CrossRef]
- Ma, K.; Tong, G.; Zhang, Y.; Zhang, Q.; Yin, J.; Mou, Z.; Kuang, Y. Karyotype analysis and evolutionary status of Oxygymnocypris stewartii. J. Northwest A F Univ. 2021, 49, 28–33+42. [Google Scholar]
- Gai, S.; Li, J.; Shen, L.; Fang, D.; Wei, Q. Population characteristics and resource status of Coreius heterodon in the Yichang section in the middle reaches of the Yangtze River. J. Fish. China 2024, 48, 049315. [Google Scholar]
- Li, S.; Li, C.; Li, J. Analysis of morphological variations among strains of nile tilapia (Oreochromis niloticus). Acta Zool. Sin. 1998, 44, 450–457. [Google Scholar]
- Xie, J.Y.; Xia, Y.; Yan, Y.C.; Liang, W.; Ren, C. Reproductive cycle of Triplophysa stenura (Herzenstein, 1888) (Balitoridae: Nemacheilinae) from the Yarlung Tsangpo River in the Tibetan Plateau, China. J. Appl. Ichthyol. 2017, 33, 37–41. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Liu, H. Values of eight structures as age determination of Ptychobarbus dipogon, tibet autonomous region. Acta Hydrobiol. Sin. 2019, 43, 579–588. [Google Scholar]
- Wang, Y.; Fang, D.; Kuang, Z.; Tan, L.; Liang, Y.; Luo, H.; Qi, H.; Xu, D. Correlation between otolith structure and early growth of Gymnocypris przewalskii larvae and juveniles. J. Dalian Ocean Univ. 2024, 39, 20–28. [Google Scholar]
- Li, L.; Yang, X.; Yang, R.; Fan, Q.; Wei, K.; Jiang, H. Age structure and growth characteristics of Triplophysa orientalis in the middle of the Yarlung Tsangpo River, Tibet. J. Huazhong Agric. Univ. 2016, 35, 117–123. [Google Scholar]
- Tian, N.; Yang, R.; Tan, B.; Zeng, X.; He, L.; Xu, Z.; Zhu, Z.; Liu, H.; Yang, X. Age, growth, and reproductive characteristics of Triplophysa stewarti in Lake Chugutso, Tibet. J. Fish. Sci. China 2022, 29, 1013–1021. [Google Scholar]
- Lostrom, S.; Evans, J.P.; Grierson, P.F.; Collin, S.P.; Davies, P.M.; Kelley, J.L. Linking stream ecology with morphological variability in a native freshwater fish from semi-arid Australia. Ecol. Evol. 2015, 5, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Suriyampola, P.S.; Zúñiga-Vega, J.J.; Jayasundara, N.; Flores, J.; Lopez, M.; Bhat, A.; Martins, E.P. River zebrafish combine behavioral plasticity and generalized morphology with specialized sensory and metabolic physiology to survive in a challenging environment. Sci. Rep. 2023, 13, 16398. [Google Scholar] [CrossRef]
- Gidmark, N.J.; Pos, K.; Matheson, B.; Ponce, E.; Westneat, M.W. Functional Morphology and Biomechanics of Feeding in Fishes. In Feeding in Vertebrates: Evolution, Morphology, Behavior, Biomechanics; Bels, V., Whishaw, I.Q., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 297–332. [Google Scholar]
- Lloyd, E.; Olive, C.; Stahl, B.A.; Jaggard, J.B.; Amaral, P.; Duboué, E.R.; Keene, A.C. Evolutionary shift towards lateral line dependent prey capture behavior in the blind Mexican cavefish. Dev. Biol. 2018, 441, 328–337. [Google Scholar] [CrossRef]
- De Almeida-Val, V.M.F.; Chippari Gomes, A.R.; Lopes, N.P. Metabolic and Physiological Adjustments to Low Oxygen and High Temperature in Fishes of the Amazon. In Fish Physiology; Academic Press: Cambridge, MA, USA, 2005; Volume 21, pp. 443–500. [Google Scholar]
- Prokofiev, A.M. Morphological characteristics and intraspecific structure of Triplophysa orientalis (Balitoridae: Nemacheilinae). J. Ichthyol. 2016, 56, 200–207. [Google Scholar] [CrossRef]
- Matthews, D.G.; Albertson, R.C. Effect of craniofacial genotype on the relationship between morphology and feeding performance in cichlid fishes. Evolution 2017, 71, 2050–2061. [Google Scholar] [CrossRef]
- Baker, J.; Venditti, C. Rapid Change in Mammalian Eye Shape Is Explained by Activity Pattern. Curr. Biol. 2019, 29, 1082–1088.e3. [Google Scholar] [CrossRef]
- Gibb, A.C.; Dickson, K.A.; Lauder, G.V. Tail kinematics of the chub mackerel Scomber japonicus: Testing the homocercal tail model of fish propulsion. J. Exp. Biol. 1999, 202 Pt 18, 2433–2447. [Google Scholar] [CrossRef]
- Ohlberger, J.; Staaks, G.; Hölker, F. Swimming efficiency and the influence of morphology on swimming costs in fishes. J. Comp. Physiol. B 2006, 176, 17–25. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, A.; De-Pablos-Heredero, C.; González, M.; Rodriguez, J.; Barba, C.; García, A. Usefulness of Discriminant Analysis in the Morphometric Differentiation of Six Native Freshwater Species from Ecuador. Animals 2021, 11, 111. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.; Meng, W.; Si, S.; Chu, M.; Wang, Z. Multivariate analysis of Harpadon nehereus populations from coastal areas of China based on morphological characters. J. Fish. Sci. China 2020, 27, 1234–1242. [Google Scholar]
- Jiang, Y. Morphology and Genetic Diversity Analysis of Harpadon nehereus. Master’s Thesis, Zhejiang Ocean University, Zhoushan, China, 2021. [Google Scholar]
- Beamish, R.J.; McFarlane, G.A. The Forgotten Requirement for Age Validation in Fisheries Biology. Trans. Am. Fish. Soc. 1983, 112, 735–743. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; Song, Z. Age and Growth of Triplophysa markehenensis from the Markehe River in Upper Reaches of the Dadu River. Chin. J. Zool. 2010, 45, 11–20. [Google Scholar]
- Xu, X.W.; Shao, C.W.; Xu, H.; Zhou, Q.; You, F.; Wang, N.; Li, W.L.; Li, M.; Chen, S.L. Draft genomes of female and male turbot Scophthalmus maximus. Sci. Data 2020, 7, 90. [Google Scholar]
- He, W.; Gao, H.; Zhou, C.; Wang, W.; Liu, Y. A Review of the Age, Growth Characteristics, and Population Resources of Ptychobarbus dipogon in the Middle and Upper Reaches of the Yarlung Zangbo River. Water 2023, 15, 1713. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, B.; Zhang, R.M.; Cai, L.G. Tuerxun, Evaluation of the effect of introduction and transplantation of high whitefish in Sailim Lake. J. Hydroecology 2003, 3, 30–32. [Google Scholar]
- Fraser, D.F.; Gilliam, J.F. Nonlethal Impacts of Predator Invasion: Facultative Suppression of Growth and Reproduction. Ecology 1992, 73, 959–970. [Google Scholar] [CrossRef]
- Poole, J.R.; Bajer, P.G. A small native predator reduces reproductive success of a large invasive fish as revealed by whole-lake experiments. PLoS ONE 2019, 14, e0214009. [Google Scholar]
- Cubillos, L.A.; Claramunt, G.; Castro, L.R. Simulation of fishery-induced changes on the reproductive cycle of common sardine, Strangomera bentincki, off central southern Chile. Fish. Res. 2014, 160, 103–111. [Google Scholar]
- Renner-Martin, K.; Brunner, N.; Kühleitner, M.; Nowak, W.G.; Scheicher, K. Model for the Mass-Growth of Wild-Caught Fish. Open J. Model. Simul. 2019, 7, 19–40. [Google Scholar] [CrossRef][Green Version]
- Murray, A.J. Energy metabolism and the high-altitude environment. Exp. Physiol. 2016, 101, 23–27. [Google Scholar] [PubMed]
- Passow, C.N.; Greenway, R.; Arias-Rodriguez, L.; Jeyasingh, P.D.; Tobler, M. Reduction of Energetic Demands through Modification of Body Size and Routine Metabolic Rates in Extremophile Fish. Physiol. Biochem. Zool. 2015, 88, 371–383. [Google Scholar] [PubMed]
- Pauly, D.; Moreau, J. Comparison of overall growth performance of tilapia in open waters and aquaculture. In Proceedings of the International Symposium on Tilapia in Aquaculture, Bangkok, Thailand, 16–20 March 1987. [Google Scholar]
- Killam, K.A.; Parsons, G.R. Age and Growth of the Blacktip Shark, Carcharhinus limbatus, near Tampa Bay, Florida. Fish. Bull. 1989, 87, 845–857. [Google Scholar]
- Andrews, J.W.; Murai, T.; Gibbons, G. The Influence of Dissolved Oxygen on the Growth of Channel Catfish. Trans. Am. Fish. Soc. 1973, 102, 835–838. [Google Scholar]
- Bœuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar]
- Hayden, B.; Pulcini, D.; Kelly-Quinn, M.; O’Grady, M.; Caffrey, J.; McGrath, A.; Mariani, S. Hybridisation between two cyprinid fishes in a novel habitat: Genetics, morphology and life-history traits. BMC Evol. Biol. 2010, 10, 169. [Google Scholar]
- Redfern, J.C.; Foggo, A.; Mieszkowska, N. Handling the heat: Responses of two congeneric limpet species to environmental temperature differences. J. Exp. Mar. Biol. Ecol. 2021, 536, 151500. [Google Scholar]
- White, E.P.; Ernest, S.K.M.; Kerkhoff, A.J.; Enquist, B.J. Relationships between body size and abundance in ecology. Trends Ecol. Evol. 2007, 22, 323–330. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, X.B.; Zhao, N.H.; Wei, J.; Shen, J.Z. Morphology and Age Determination of Loach Triplophysa stoliczkae in Kizil River, Xinjiang Uyghur Autonomous Region. Chin. J. Fish. 2021, 34, 8–15. [Google Scholar]
- Zhao, J.F.; Qiu, L.H.; Zhou, Q.; Zhou, X.Y.; Shen, J.Z. Age structure and growth characteristics of Triplophysa yarkandensis in the Qarqan River, Xinjiang. J. Huazhong Agric. Univ. 2023, 42, 221–228. [Google Scholar]
- Zhang, L.R.; Ji, B.W.; Nie, Z.L.; Wei, J. Age Structure and Growth Characteristics of Loach Triplophysa tenuis in Muzati River, Xinjiang. Chin. J. Fish. 2024, 37, 30–37. [Google Scholar]
- Zhang, R.; Wang, J.L.; Li, P.L.; Liu, J.C.; Liu, Y.B.; Wang, T.; Liu, K.; Zhang, J.; Zhao, P. Model Analysis and Comparative Study on Growth Characteristics of Triplophysa scleroptera. Chin. Agric. Sci. Bull. 2024, 40, 158–164. [Google Scholar]
- Yao, N.; Ma, L.; Jin, S.S.; Chen, S.A. Growth characteristics of Triplophysa siluroides (Herzenstein) in beichuanhe Basin of Qinghai. J. Inn. Mong. Agric. Univ. 2019, 40, 5–10. [Google Scholar]
- Ani, J.S.; Manyala, J.O.; Masese, F.O.; Fitzsimmons, K. Effect of stocking density on growth performance of monosex Nile Tilapia (Oreochromis niloticus) in the aquaponic system integrated with lettuce (Lactuca sativa). Aquac. Fish. 2022, 7, 328–335. [Google Scholar] [CrossRef]
- Li, Y.; Feng, M.; Huang, L.; Zhang, P.; Wang, H.; Zhang, J.; Tian, Y.; Xu, J. Weight–Length Relationship Analysis Revealing the Impacts of Multiple Factors on Body Shape of Fish in China. Fishes 2023, 8, 269. [Google Scholar] [CrossRef]
- Bateman, A.J. Intra-sexual selection in Drosophila. Heredity 1948, 2 Pt. 3, 349–368. [Google Scholar] [CrossRef]
- Kamler, E. Parent–egg–progeny Relationships in Teleost Fishes: An Energetics Perspective. Rev. Fish Biol. Fish. 2005, 15, 399–421. [Google Scholar] [CrossRef]
- Alonzo, S.H.; Heckman, K.L. The unexpected but understandable dynamics of mating, paternity and paternal care in the ocellated wrasse. Proc. R. Soc. B Biol. Sci. 2010, 277, 115–122. [Google Scholar] [CrossRef]
- Salam, M.A.; Das, T.R.; Paul, S.I.; Islam, F.; Baidya, A.; Rahman, M.L.; Shaha, D.C.; Mazumder, S.K. Dietary chitosan positively influences the immunity and reproductive performances of mature silver barb (Barbonymus gonionotus). Aquac. Rep. 2024, 36, 102155. [Google Scholar] [CrossRef]
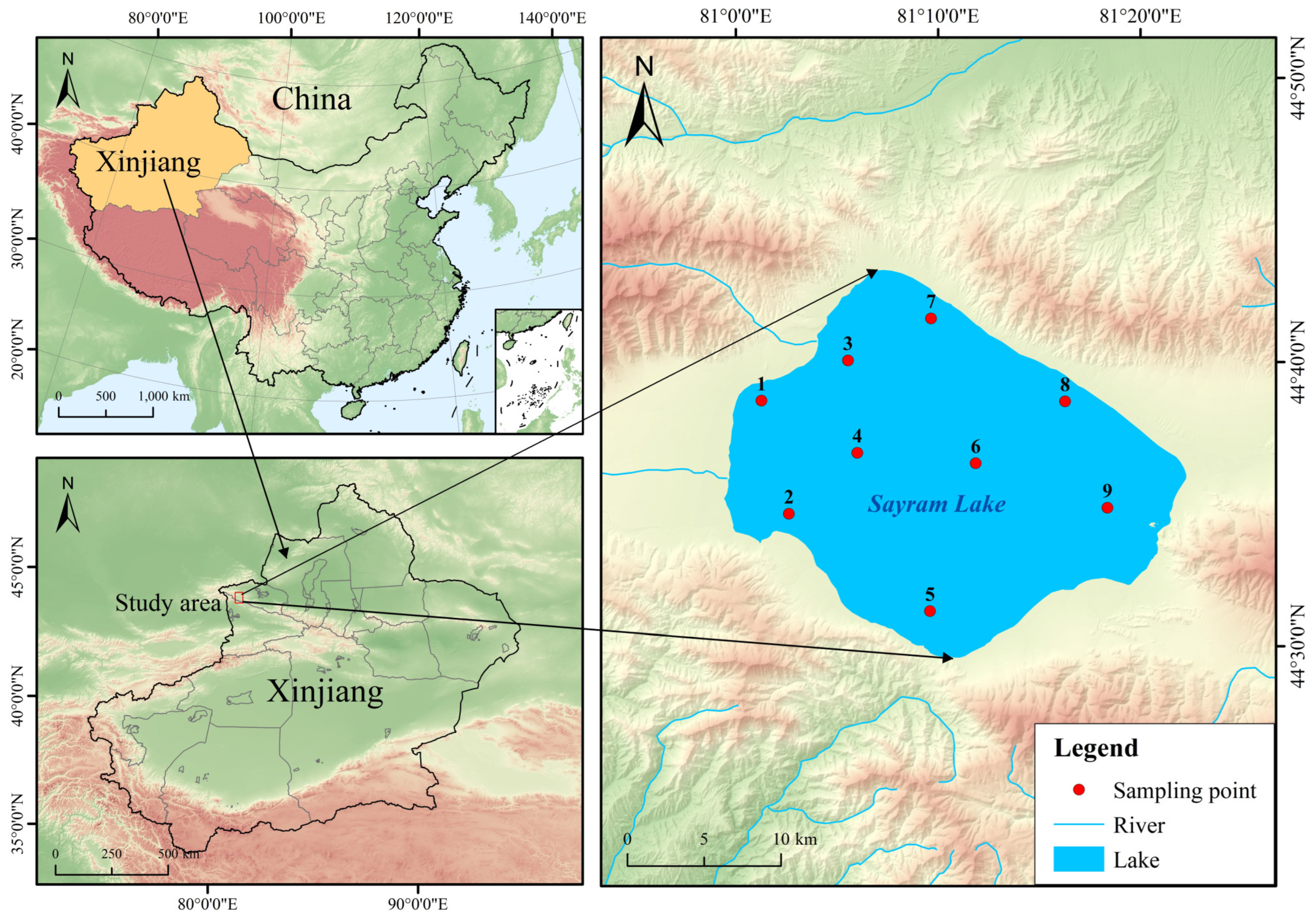

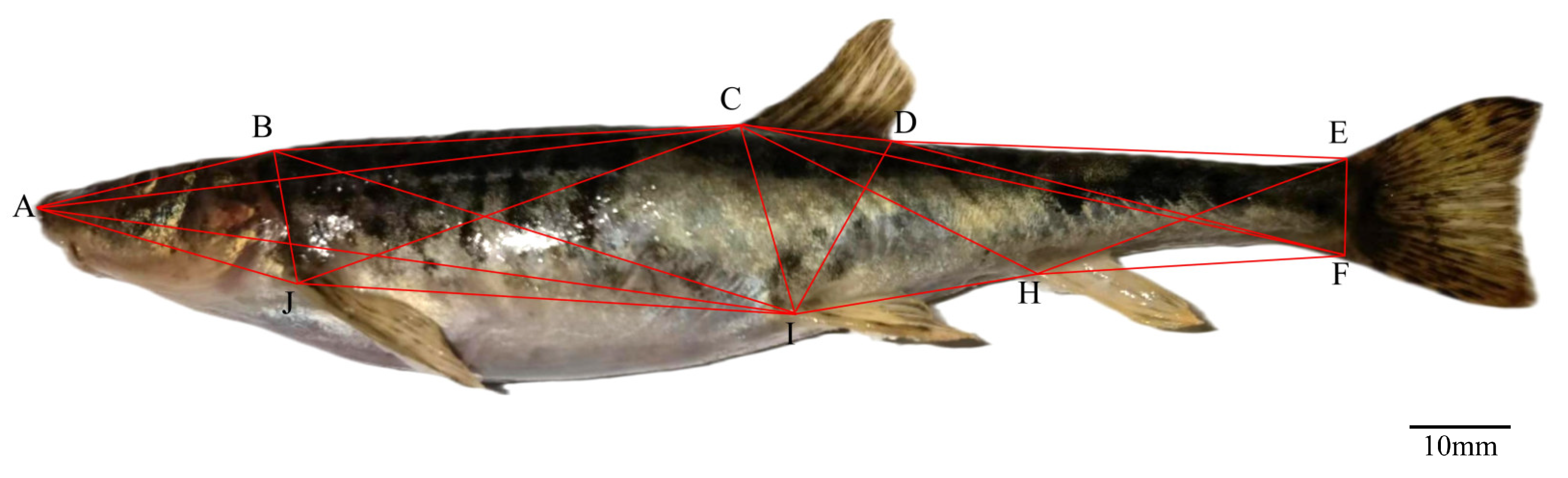

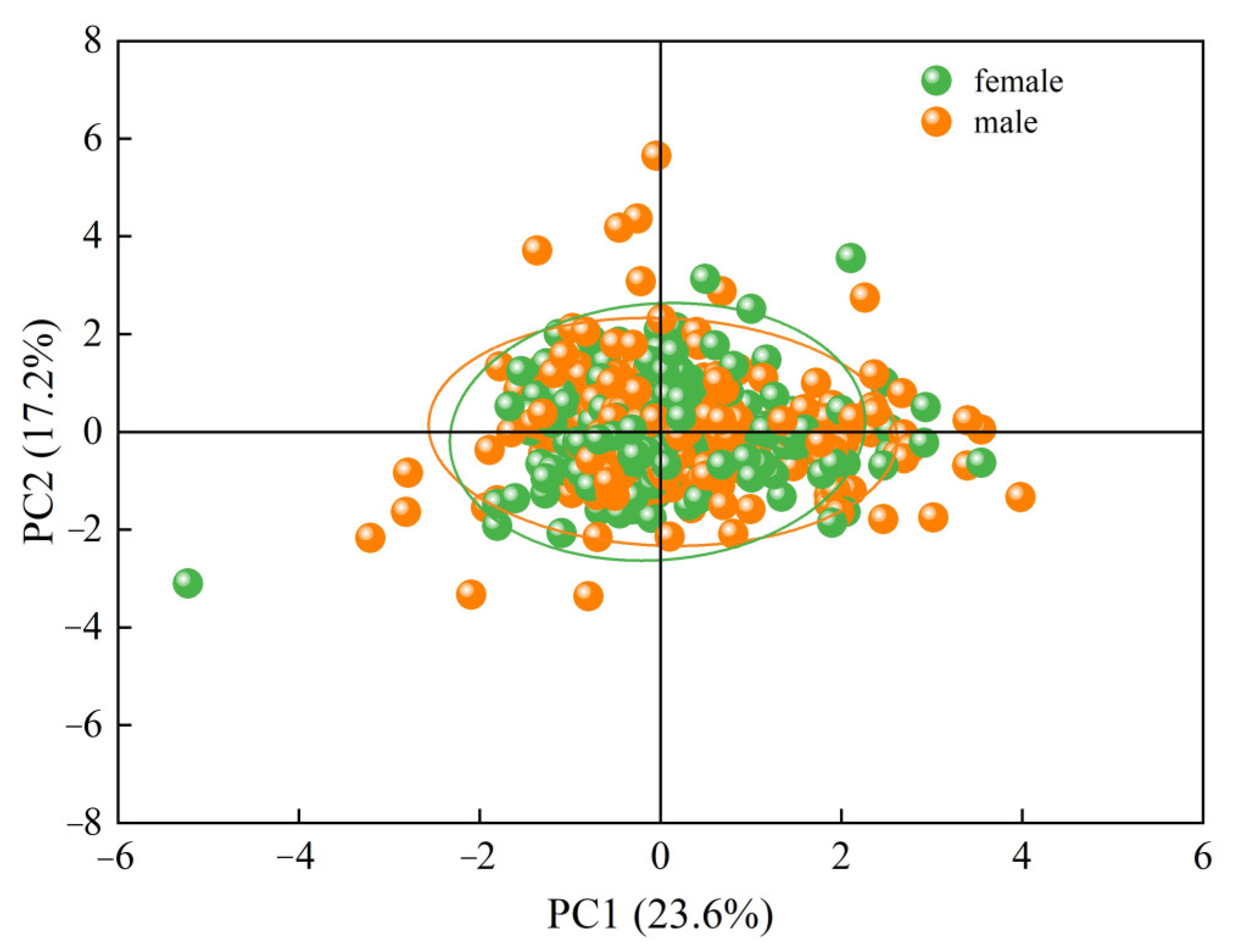
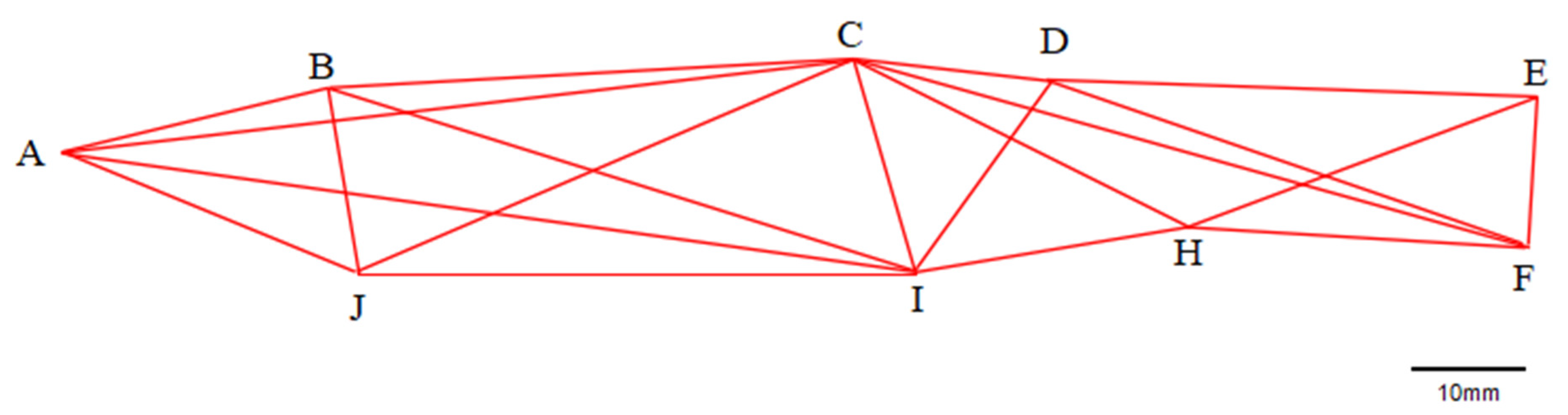



| Traits | Range | Mean ± SD 1 |
|---|---|---|
| Total length | 51.35~185.77 | 116.50 ± 22.68 |
| Body length | 43.50~142.14 | 97.79 ± 19.07 |
| Body width | 3.74~23.43 | 11.85 ± 3.22 |
| Body depth | 3.83~24.33 | 13.66 ± 3.57 |
| Head length | 3.40~30.13 | 19.68 ± 3.92 |
| Snout length | 1.44~13.84 | 7.81 ± 2.10 |
| Eye diameter | 0.93~6.29 | 3.17 ± 0.75 |
| Eye spacing | 1.90~11.29 | 6.19 ± 1.60 |
| Caudal peduncle length | 9.81~43.37 | 21.57 ± 4.95 |
| Caudal peduncle height | 2.12~14.08 | 6.43 ± 1.82 |
| Indicator | Male | Female | ||
|---|---|---|---|---|
| Range | Mean ± SD 1 | Range | Mean ± SD 1 | |
| Total length/body length | 0.38~1.74 | 1.19 ± 0.05 | 0.90~2.54 | 1.19 ± 0.79 |
| Body length/body width | 4.71~16.08 | 8.50 ± 1.39 | 3.86~15.29 | 8.58 ± 1.62 |
| Body length/body depth | 4.81~13.45 | 7.30 ± 1.20 | 3.89~13.11 | 7.37 ± 1.20 |
| Body depth/body width | 0.70~2.09 | 1.18 ± 0.22 | 0.59~2.13 | 1.18 ± 0.22 |
| Body length/head length | 3.99~36.59 | 5.08 ± 1.82 | 4.01~12.46 | 5.04 ± 0.88 |
| Body length/caudal peduncle length | 2.78~16.36 | 4.63 ± 0.89 | 2.09~8.94 | 4.64 ± 0.67 |
| Body length/eye diameter | 17.33~77.40 | 32.24 ± 7.27 | 10.53~117.19 | 32.14 ± 8.64 |
| Body length/eye spacing | 7.66~25.37 | 16.14 ± 2.55 | 9.85~32.32 | 16.26 ± 2.80 |
| Head length/snout length | 0.30~6.53 | 2.60 ± 0.70 | 0.96~8.40 | 2.68 ± 0.88 |
| Head length/eye diameter | 1.07~14.60 | 6.47 ± 1.49 | 2.04~23.75 | 6.48 ± 1.83 |
| Head length/caudal peduncle length | 0.16~3.30 | 0.93 ± 0.20 | 0.35~2.20 | 0.94 ± 0.16 |
| Head length/eye spacing | 0.49~4.97 | 3.24 ± 0.58 | 0.98~6.33 | 3.28 ± 0.65 |
| Caudal peduncle length/caudal peduncle height | 1.11~5.26 | 3.42 ± 0.64 | 1.42~6.18 | 3.46 ± 0.74 |
| Character | PC1 | PC2 | PC3 |
|---|---|---|---|
| Total length/body length | −0.131 | −0.024 | 0.160 |
| Body length/body width | 0.136 | −0.076 | 0.853 |
| Body length/body depth | 0.379 | −0.224 | −0.122 |
| Body depth/body width | −0.195 | 0.118 | 0.898 |
| Body length/head length | −0.322 | −0.218 | −0.048 |
| Body length/caudal peduncle length | 0.164 | 0.854 | −0.037 |
| Body length/eye diameter | 0.715 | −0.223 | −0.067 |
| Body length/eye spacing | 0.667 | −0.124 | 0.192 |
| Head length/snout length | 0.627 | 0.065 | −0.159 |
| Head length/eye diameter | 0.82 | −0.112 | −0.033 |
| Head length/caudal peduncle length | 0.323 | 0.894 | 0.006 |
| Head length/eye spacing | 0.756 | 0.031 | 0.203 |
| Caudal peduncle length/caudal peduncle height | 0.161 | −0.711 | 0.056 |
| Location | Range | Mean ± SD 1 |
|---|---|---|
| A–B | 5.91~26.94 | 18.13 ± 2.57 |
| B–C | 12.97~61.89 | 35.35 ± 7.83 |
| C–D | 3.87~20.61 | 10.62 ± 2.65 |
| D–E | 9.94~69.19 | 35.62 ± 7.89 |
| E–F | 2.28~12.81 | 6.94 ± 1.85 |
| F–H | 5.50~50.63 | 24.90 ± 6.09 |
| H–I | 6.56~42.57 | 19.00 ± 4.89 |
| I–J | 11.82~63.70 | 33.58 ± 7.61 |
| A–J | 7.19~64.00 | 21.99 ± 5.27 |
| A–C | 22.80~76.12 | 52.21 ± 10.75 |
| A–I | 21.81~79.98 | 54.47 ± 11.09 |
| B–J | 4.18~20.71 | 11.13 ± 2.92 |
| B–I | 12.53~59.44 | 38.50 ± 8.85 |
| C–J | 12.90~59.17 | 33.75 ± 7.54 |
| C–I | 2.29~26.09 | 13.22 ± 3.42 |
| C–H | 4.99~45.71 | 23.05 ± 6.08 |
| C–F | 20.05~82.69 | 45.49 ± 9.74 |
| D–I | 4.78~27.37 | 13.46 ± 3.59 |
| D–H | 4.39~27.68 | 14.49 ± 4.01 |
| D–F | 7.68~55.53 | 35.50 ± 7.72 |
| E–H | 11.33~57.78 | 27.18 ± 6.26 |
| Fish Species | Data Source | Body Length/mm | Body Weight/g | b | ti (♀) | ti (♂) | ti | φ | k |
|---|---|---|---|---|---|---|---|---|---|
| T. strauchii | This study | 43.50~142.14 | 0.60~36.71 | 3.073 | 2.304 | 1.994 | 2.563 | 3.668 | 0.267 |
| T. stoliczkae | [64] | 27.86~66.43 | 4.00~60.32 | 3.234 | |||||
| T. yarkandensis | [65] | 38~290 | 0.80~271.60 | 2.856 | 8.50 | 5.90 | 4.228 | ||
| T. stewarti | [32] | 34.70~143 | 0.40~28.70 | 3.012 | 3.65 | ||||
| T. orientalis | [31] | 49~130 | 2.10~23.50 | 2.930 | 8.19 | 5.83 | 0.183 | ||
| T. markehenensis | [47] | 34.30~145 | 34.3~145 | 2.952 | 6.25 | ||||
| T. tenuis | [66] | 37.02~129.64 | 0.10~15.15 | 2.794 | 2.57 | ||||
| T. scleroptera | [67] | 47.80~178.10 | 0.97~47.20 | 2.989 | 10.03 | 0.097 | |||
| T. siluroides | [68] | 20.10~83.90 | 0.68~5.51 | 2.699 | 2.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Hao, H.; Wei, J.; Wu, H.; Hamid, S.M.; Lv, R.; Lu, H.; Nie, Z. Morphology, Age, and Growth of Triplophysa strauchii in Sayram Lake, Xinjiang, China. Animals 2025, 15, 1039. https://doi.org/10.3390/ani15071039
Wang Z, Hao H, Wei J, Wu H, Hamid SM, Lv R, Lu H, Nie Z. Morphology, Age, and Growth of Triplophysa strauchii in Sayram Lake, Xinjiang, China. Animals. 2025; 15(7):1039. https://doi.org/10.3390/ani15071039
Chicago/Turabian StyleWang, Zhengwei, Huimin Hao, Jie Wei, Hao Wu, Syeda Maira Hamid, Ruixian Lv, Huale Lu, and Zhulan Nie. 2025. "Morphology, Age, and Growth of Triplophysa strauchii in Sayram Lake, Xinjiang, China" Animals 15, no. 7: 1039. https://doi.org/10.3390/ani15071039
APA StyleWang, Z., Hao, H., Wei, J., Wu, H., Hamid, S. M., Lv, R., Lu, H., & Nie, Z. (2025). Morphology, Age, and Growth of Triplophysa strauchii in Sayram Lake, Xinjiang, China. Animals, 15(7), 1039. https://doi.org/10.3390/ani15071039







