Coprolite Multimodal Analysis: A Tool for Hyaenid Predator Identification
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Feces
2.1.2. Standards
2.2. Methods
2.2.1. Fourier Transform Infrared Spectrometry (FTIR)
2.2.2. Statistical Analyses: Methods
2.2.3. Scanning Electron Microscopy (SEM)
2.2.4. Energy-Dispersive Spectrometry (EDS)
3. Results
3.1. Morphology
3.2. Contents
3.3. Composition
3.3.1. Global Data
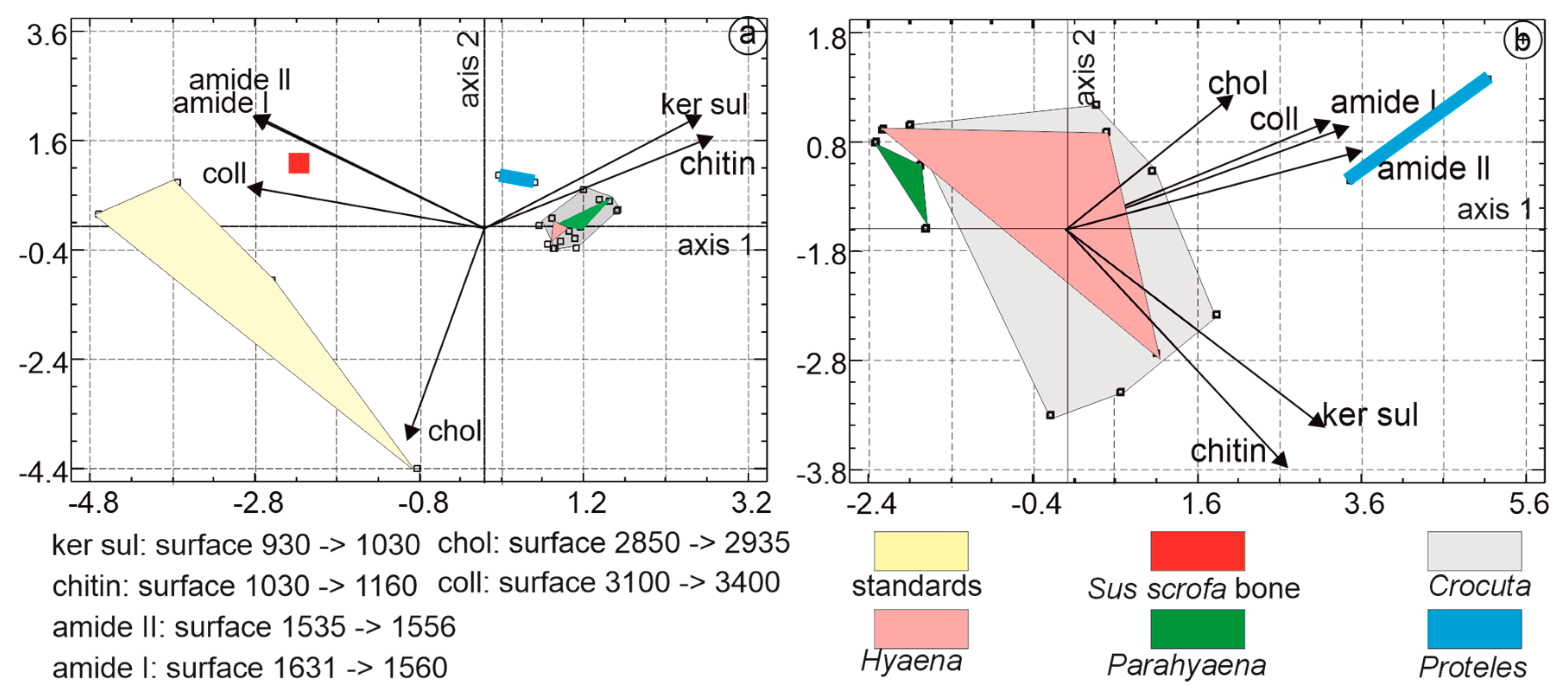
3.3.2. Statistical Analyses
Selection of the “Mineral” Bands
- -
- The surface between 560 and 600 cm−1, indicative of PO4 in apatite [53];
- -
- The surface between 860 and 880 cm−1, indicative of CO3;
- -
- The surface between 1409 and 1425 cm−1, indicative of CO3;
- -
- The surface between 1030 and 1010 cm−1, a crystallinity index (CI) [54];
- -
- The CO3/PO4 ratio was estimated using bands at 871 and 1017 cm−1;
- -
- The CO3/PO4b ratio was estimated using bands at 1415 and 575 cm−1 [55].
Selection of the “Organic” Bands
“Mineral” and “Organic” Bands
4. Discussion
4.1. Collecting and Extracting Coprolite Samples
4.2. Observing and Analyzing Coprolite Samples
4.3. Comparison with Other Methods
4.4. Cross-Validations of the Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boskey, A.L.; Posner, A.S. Bone structure, composition, and mineralization. Orthop. Clin. N. Am. 1984, 15, 597–612. [Google Scholar] [CrossRef]
- Pate, F.L. Bone chemistry and diet. J. Archaeol. Meth. Theory 1984, 1, 161–209. [Google Scholar] [CrossRef]
- Brugal, J.-P.; Sanz, M. Aperçu des études sur les coprolithes. Approches morphométriques et contenus osseux et dentaires. In Fumier, Bouses et Guano: Ordures ou or Brun? 44è Rencontre Internat. d’Archéologie et d’Histoire de Nice Côte d’Azur, APDCA (éd); APDCA: Nice, France, 2025; in press. [Google Scholar]
- Pineda, A.; Saladié, P.; Expósito, I.; Rodríguez-Hidalgo, A.; Cáceres, I.; Huguet, R.; Rosas, A.; López-Polín, L.; Estalrrich, A.; García-Tabernero, A.; et al. Characterizing hyena coprolites from two latrines of the Iberian Peninsula during the Early Pleistocene: Gran Dolina (Sierra de Atapuerca, Burgos) and la Mina (Barranc de la Boella, Tarragona). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 480, 1–17. [Google Scholar] [CrossRef]
- Rodriguez, C.F.; Rego, A.M.; Cortizas, A.M. Characterization and Depositional Evolution of Hyaena (Crocuta crocuta) Coprolites from La Valifia Cave (Northwest Sain). J. Archaeol. Sci. 1995, 22, 597–607. [Google Scholar] [CrossRef]
- Chame, M. Terrestrial mammal feces: A morphometric summary and description. Mem. Inst. Oswaldo Cruz 2003, 98 (Suppl. I), 71–94. [Google Scholar] [CrossRef]
- Qvarnström, M.; Niedzwiedzki, G.; Tafforeau, P.; Zigaite, Z.; Ahlberg, P.E. Synchrotron phase-contrast microtomography of coprolites generates novel palaeobiological data. Sci. Rep. 2017, 7, 2723. [Google Scholar] [CrossRef]
- Larkin, N.R.; Alexander, J.; Lewis, M.D. Using experimental studies of recent faecal material to examine Hyaena coprolites from the West Runton Freshwater Bed, Norfolk, U.K. J. Archaeol. Sci. 2000, 27, 19–31. [Google Scholar] [CrossRef]
- Ogara, W.O.; Gitahi, N.G.; Andanje, S.A.; Oguge, N.; Nduati, D.W.; Mainga, A.O. Determination of carnivores prey base by scat analysis in Samburu community group ranches in Kenya. Afr. J. Environ. Sci. Technol. 2010, 4, 540–546. [Google Scholar]
- Balestrieri, A.; Remonti, L.; Prigioni, C. Assessing carnivore diet by faecal samples and stomach contents: A case study with Alpine red foxes. Open Life Sci. 2011, 6, 283–292. [Google Scholar] [CrossRef]
- Gill, F.L.; Bull, I.D. Lipid analysis of vertebrate coprolites. New Mex. Mus. Nat. Hist. Sci. Bull. 2012, 57, 93–98. [Google Scholar]
- Akrim, F.; Mahmood, T.; Max, T.; Sajid Nadeem, M.; Qasim, S.; Andleeb, S. Assessment of bias in morphological identification of carnivore scats confirmed with molecular scatology in north-eastern Himalayan region of Pakistan. PeerJ 2018, 6, e5262. [Google Scholar] [CrossRef] [PubMed]
- Monterroso, P.; Godinho, R.; Oliveira, T.; Ferreras, P.; Kelly, M.J.; Morin, D.J.; Waits, L.P.; Alves, P.C.; Mills, L.S. Feeding ecological knowledge: The underutilised power of faecal DNA approaches for carnivore diet analysis. Mammal Rev. 2019, 49, 97–112. [Google Scholar] [CrossRef]
- Mwebi, O.; Nguta, E.; Onduso, V.; Nyakundi, B.; Jiang, X.-L.; Kioko, E.N. Small mammal diversity of Mt. Kenya based on carnivore fecal and surface bone remains. Zool. Res. 2019, 40, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Herrera, C.M. Frugivory and seed dispersal by carnivorous mammals, and associated fruit characteristics, in undisturbed Mediterranean habitats. Oikos 1989, 55, 250–262. [Google Scholar] [CrossRef]
- Taylor, A.; Hutson, J.M.; Bryant, V.M.; Jenkins, D.L. Dietary items in Early to Late Holocene human coprolites from Paisley Caves, Oregon, USA. Palynology 2019, 44, 12–23. [Google Scholar] [CrossRef]
- Sanz, M.; Daura, J.; Égüez, N.; Brugal, J.-P. Not only hyenids: A multi-scale analysis of Upper Pleistocene carnivore coprolites in Cova del Coll Verdaguer (NE Iberian Peninsula). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 443, 249–262. [Google Scholar] [CrossRef]
- Campmas, E.; Michel, P.; Costamagno, S.; Abdeljalil El Hajraoui, M.; Nespoulet, R. Which predators are responsible for faunal accumulations at the Late Pleistocene layers of El Harhoura 2 Cave (Témara, Morocco)? Comptes Rendu Palevol. 2017, 16, 333–350. [Google Scholar] [CrossRef]
- Lofgren, D.L.; Shen, C.Y.; Buday, N.N.; Ylagan, C.A.C.; Lofgren, K.K.; Lai, R.; Santana-Grace, D.D.; Tabrum, A.R. Coprolites and mammalian carnivores from Pipestone Springs, Montana, and their paleoecological significance. Ann. Carnegie Mus. 2017, 84, 265–285. [Google Scholar] [CrossRef]
- Romaniuk, A.; Panciroli, E.; Buckley, M.; Chowdhury, M.P.; Willars, C.; Herman, J.S.; Troalen, L.G.; Shepherd, A.N.; Clarke, D.V.; Sheridan, A.; et al. Combined visual and biochemical analyses confirm depositor and diet for Neolithic coprolites from Skara Brae. Archaeol. Anthropol. Sci. 2020, 12, 274. [Google Scholar] [CrossRef]
- Drea, C.M.; Coscia, E.M.; Glickman, S.E. Hyenas. In Encyclopedia of Reproduction, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 637–645. [Google Scholar]
- Kruuk, H. The Spotted Hyena; University of Chicago Press: Chicago, IL, USA, 1972; 335p. [Google Scholar]
- East, M.L.; Hofer, H. Crocuta crocuta, spotted hyaena. In Mammals of Africa, Vol. 5: Carnivores, Pangolins, Equids and Rhinoceroses; Kingdon, J., Ed.; A&C Black: London, UK, 2014; pp. 273–281. [Google Scholar]
- Wagner, A.P. Hyaena hyaena, striped hyaena. In Mammals of Africa: Vol. 5: Carnivores, Pangolins, Equids and Rhinoceroses; Kingdon, J., Ed.; A&C Black: London, UK, 2014; pp. 267–272. [Google Scholar]
- Mwebi, O.; Ogara, W.; Adhola, T.; Fourvel, J.B.; Brugal, J.P. Striped hyaena Hyaena hyaena (Linnaeus 1758). Feeding ecology based on Den Prey remains in a pastoralist landscape, southern Kenya. Mammalia 2024, 88, 384–399. [Google Scholar] [CrossRef]
- Mills, M.G. The comparative behavioral ecology of hyenas: The importance of diet and food dispersion. In Carnivore Behavior, Ecology, and Evolution; Springer: Boston, MA, USA, 1989; pp. 125–142. [Google Scholar]
- Mills, G. Hyaena brunnea, brown hyaena. In Mammals of Africa: Volume V: Carnivores, Pangolins, Equids and Rhinoceroses; Kingdon, J., Ed.; A&C Black: London, UK, 2014; pp. 263–267. [Google Scholar]
- Gittleman, J.L. Carnivore Group Living: Comparative Trends. In Carnivore Behavior, Ecology, and Evolution; Gittleman, J.L., Ed.; Springer: Boston, MA, USA, 1989; pp. 183–207. [Google Scholar] [CrossRef]
- Fourvel, J.B.; Fosse, P.; Avery, G. Spotted, striped or brown? Taphonomical studies of eastern and southern African extant hyena dens. Quat. Int. 2015, 369, 38–50. [Google Scholar] [CrossRef]
- Brugal, J.-P.; Fourvel, J.B.; Fosse, P. A view from Europe: The case of digested teeth from Pleistocene hyaena dens. In Workshop Taphonomy Reference Collection; National Museum of Kenya (Nairobi), Smithsonian Nat. Mus. Nat. Hist., The Leakey Foundation: Nairobi, Kenya, 2024. [Google Scholar]
- Kruuk, H.; Sands, W.A. The aardwolf (Proteles cristatus Sparrman) 1783 as predator of termites. Afr. J. Ecol. 1972, 10, 211–227. [Google Scholar] [CrossRef]
- Anderson, M.D. Proteles cristatus, aardwolf. In Mammals of Africa: Volume V: Carnivores, Pangolins, Equids and Rhinoceroses; Kingdon, J., Ed.; A&C Black: London, UK, 2014; pp. 282–292. [Google Scholar]
- Adler, H.H.; Kerr, P.F. Infrared study of aragonite and calcite. Am. Mineral. J. Earth Planet. Mater. 1962, 47, 700–717. [Google Scholar]
- Jones, G.C.; Jackson, B. Infrared Transmission Spectra of Carbonate Mineral; Chapman & Hall: London, UK, 1993; 254p. [Google Scholar]
- Rey, C.; Shimuzu, M.; Collins, B.; Glimcher, M.J. Resolution enhanced Fourier transform infrared spectroscopy study of the environment of phosphate ion in the early deposits of a solid phase of calcium phosphate in bone and enamel and their evolution with age. 2. Investigations in the ν3 PO4 domain. Calcif. Tissue Int. 1991, 49, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Pleshko, N.; Boskey, A.; Mendelsohn, R. Novel infrared spectroscopic method for the determination of crystallinity of hydroxyapatite minerals. Biophys. J. 1991, 60, 786–793. [Google Scholar] [CrossRef]
- Cael, J.J.; Isaac, D.H.; Blackwell, J.; Koenig, J.L. Polarized infrared spectra of crystalline glycosaminoglycans. Carbohydr. Res. 1976, 50, 169–179. [Google Scholar] [CrossRef]
- Cabassi, F.; Casu, D.; Perlin, A.S. Infrared absorption and Raman scattering of sulfate groups of heparin and related glycosaminoglycans in aqueous solutions. Carbohydr. Res. 1978, 63, 1–11. [Google Scholar] [CrossRef]
- Machovic, V.; Lapcák, L.; Havelcová, M.; Borecká, L.; Novotná, M.; Javůrková, I.; Langrová, I.; Hájková, Š.; Brožová, A.; Titěra, D. Analysis of european honeybee (Apis mellifera) wings using ATR-FTIR and Raman spectroscopy: A pilot study. Sci. Agric. Bohem. 2017, 48, 22–29. [Google Scholar] [CrossRef]
- Paradkar, M.M.; Irudayaraj, J. Determination of cholesterol in dairy products using infrared techniques: 1. FTIR spectroscopy. Int. J. Dairy Technol. 2002, 55, 127–132. [Google Scholar] [CrossRef]
- Kleiner, O.; Ramesh, J.; Huleihel, M.; Cohen, B.; Kantarovich, K. A comparative study of gallstones from children and adults using FTIR spectroscopy and fluorescence microscopy. BMC Gastroenterol. 2002, 2, 14. [Google Scholar] [CrossRef]
- De Marinis, A.M.; Asprea, A. Hair identification key of wild and domestic ungulates from southern Europe. Wildl. Biol. 2006, 12, 305–320. [Google Scholar] [CrossRef]
- Souza, F.C.; Azevedo, F.C. Hair as a tool for identification of predators and prey: A study based on scats of jaguars (Panthera onca) and pumas (Panthera concolor). Biota Neotrop. 2021, 21, e20201044. [Google Scholar] [CrossRef]
- Pesquero, M.D.; Souza-Egipsy, V.; Alcalá, L.; Ascaso, C.; Fernández-Jalvo, Y. Calcium phosphate preservation of faecal bacterial negative moulds in hyaena coprolites. Acta Palaeont. Pol. 2014, 59, 997–1005. [Google Scholar] [CrossRef]
- Asscher, Y.; Regev, L.; Weiner, S.; Boaretto, E. Atomic Disorder in Fossil Tooth and Bone Mineral: An FTIR Study Using the Grinding Curve Method. ArcheoSciences 2011, 35, 135–141. [Google Scholar] [CrossRef]
- Shimoda, S.; Aoba, T.; Moreno, E.C.; Miake, Y. Effect of solution composition on morphological and structural features of carbonated calcium apatites. J. Dent. Res. 1990, 69, 1731–1740. [Google Scholar] [CrossRef]
- Legeros, R.Z.; Trautz, O.R.; Klein, E.; Legeros, J.P. Two types of carbonate substitution in the apatite structure. Experientia 1969, 25, 5–7. [Google Scholar] [CrossRef]
- Gadaleta, S.J.; Gericke, A.; Boskey, A.L.; Mendelsohn, R. Two-dimensional infrared correlation spectroscopy of synthetic and biological apatites. Biospectroscopy 1996, 2, 353–364. [Google Scholar] [CrossRef]
- Paschalis, E.P.; Betts, F.; DiCarlo, E.; Mendelsohn, R.; Boskey, A.L. FTIR microspectroscopic analysis of normal human cortical and trabecular bone. Calcif. Tissue Int. 1997, 61, 480–486. [Google Scholar] [CrossRef]
- Boskey, A.L.; Mendelsohn, R. Infrared spectroscopic characterization of mineralized tissues. Vib. Spectrosc. 2005, 38, 107–114. [Google Scholar] [CrossRef]
- Boskey, A.L. Mineralization of bones and teeth. Elements 2007, 3, 385–391. [Google Scholar] [CrossRef]
- Sullasi, H.S.L.; dos Santos, A.L.C.; da Silva Simões, S.; Martin, G.; da Silva, S.F.S.M.; de Faria, A.A. A Note on diagenetic parameters for bone remains from Pedra do Alexandre Paleoamerican site without sample destruction. Fumdhamentos 2017, XIX, 74–85. [Google Scholar]
- Berzina-Cimdina, L.; Borodajenko, N. Research of calcium phosphates using Fourier transform infrared spectroscopy. In Materials Science, Engineering and Technology; Theophile, T., Ed.; Intech: Vienna, Austria, 2012; 510p. [Google Scholar]
- Álvarez-Lloret, P.; Rodriguez-Navarro, A.B.; Romanek, C.S. Quantitative analysis of bone mineral using FTIR. Rev. Soc. Española Mineral. 2006, 6, 45–47. [Google Scholar]
- Featherstone, J.D.B.; Pearson, S.; Legeros, R.Z. An infrared method for quantification of carbonate in carbonated apatites. Caries Res. 1984, 18, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Marxen, J.C.; Hammer, M.; Gehrke, T.; Becker, W. Carbohydrates of the organic shell matrix and the shell-forming tissue of the snail Biomphalaria glabrata (Say). Biol. Bull. 1998, 194, 231–240. [Google Scholar] [CrossRef]
- Espinoza, E.O.; Baker, B.W.; Moores, T.D.; Voin, D. Forensic identification of elephant and giraffe hair artifacts using HATR FTIR spectroscopy and discriminant analysis. Endanger. Species Res. 2008, 9, 239–246. [Google Scholar] [CrossRef]
- Grunenwald, A.; Keyser, C.; Sautereau, A.M.; Crubézy, E.; Ludes, B.; Drouet, C. Revisiting carbonate quantification in apatite (bio)minerals: A validated FTIR methodology. J. Archaeol. Sci. 2014, 49, 134–141. [Google Scholar] [CrossRef]
- Hausman, L.A. Structural characteristics of the hair of mammals. Am. Nat. 1920, 54, 496–523. [Google Scholar] [CrossRef]
- Hausman, L.A. Recent studies of hair structure relationships. Sci. Mon. 1930, 30, 258–277. [Google Scholar]
- Taru, P.; Blackwell, L. Identification of fossil hairs in Parahyaena brunnea coprolites from Middle Pleistocene deposits at Gladysvale cave, South Africa. J. Archaeol. Sci. 2013, 40, 3674–3685. [Google Scholar] [CrossRef]


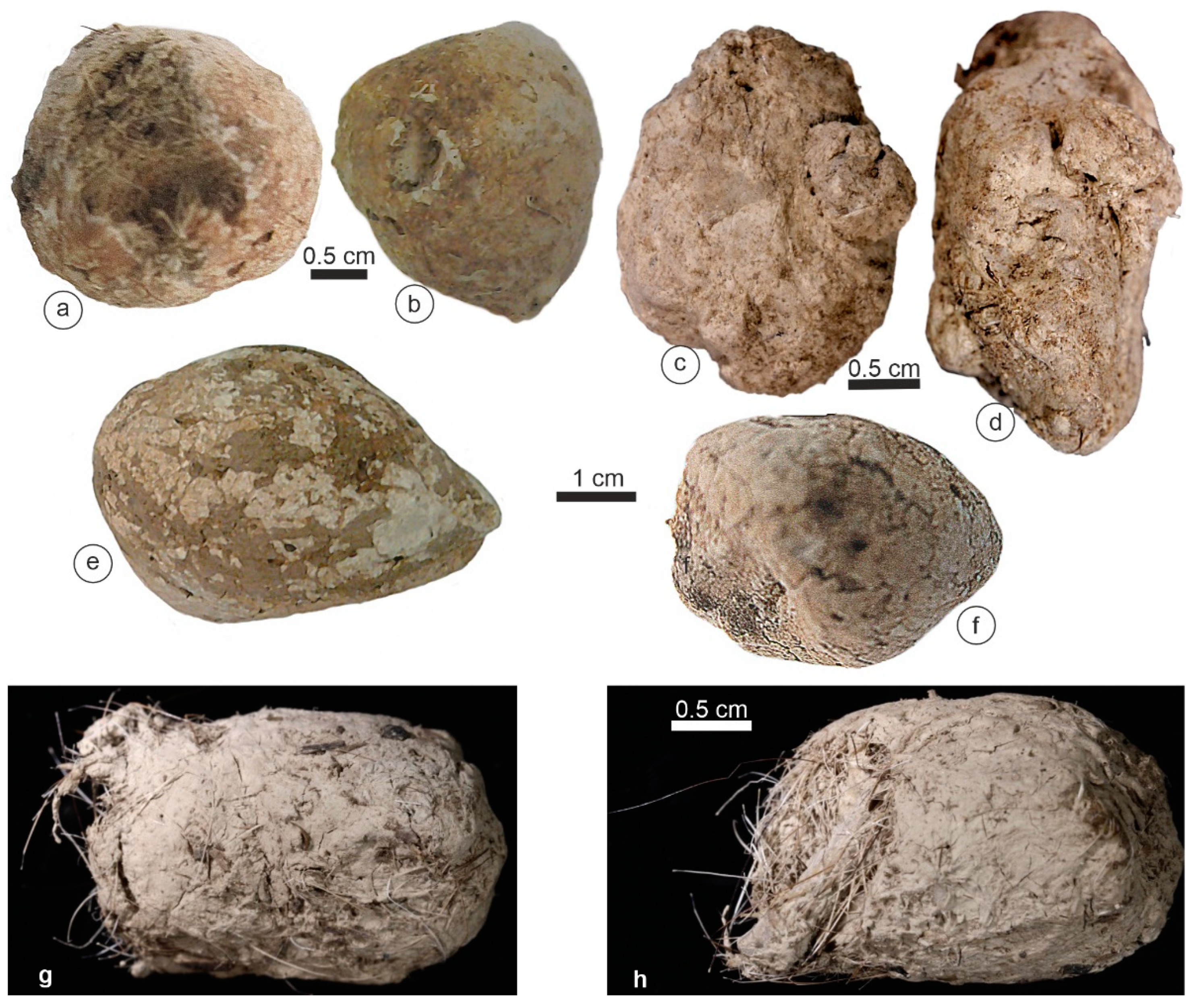


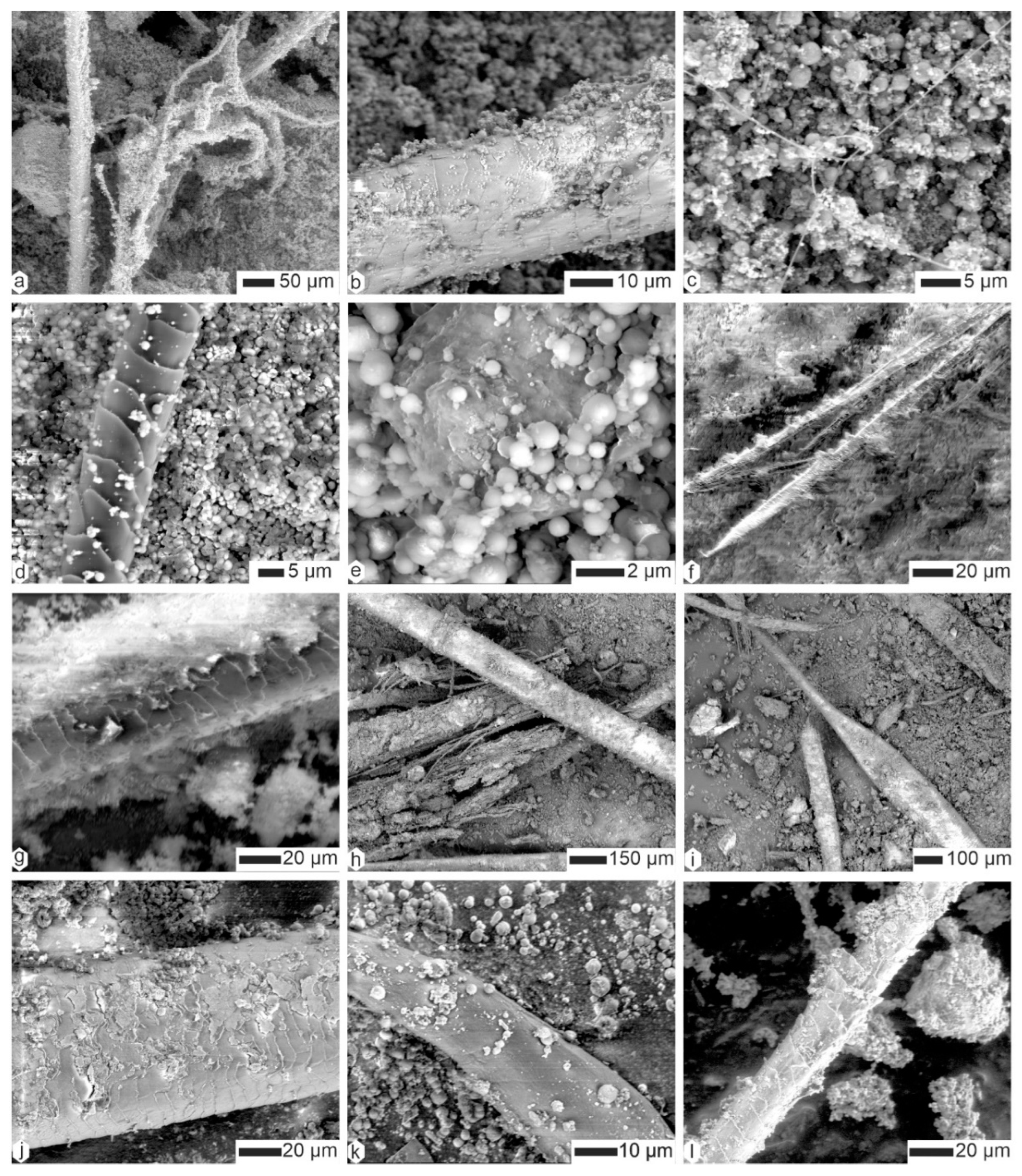
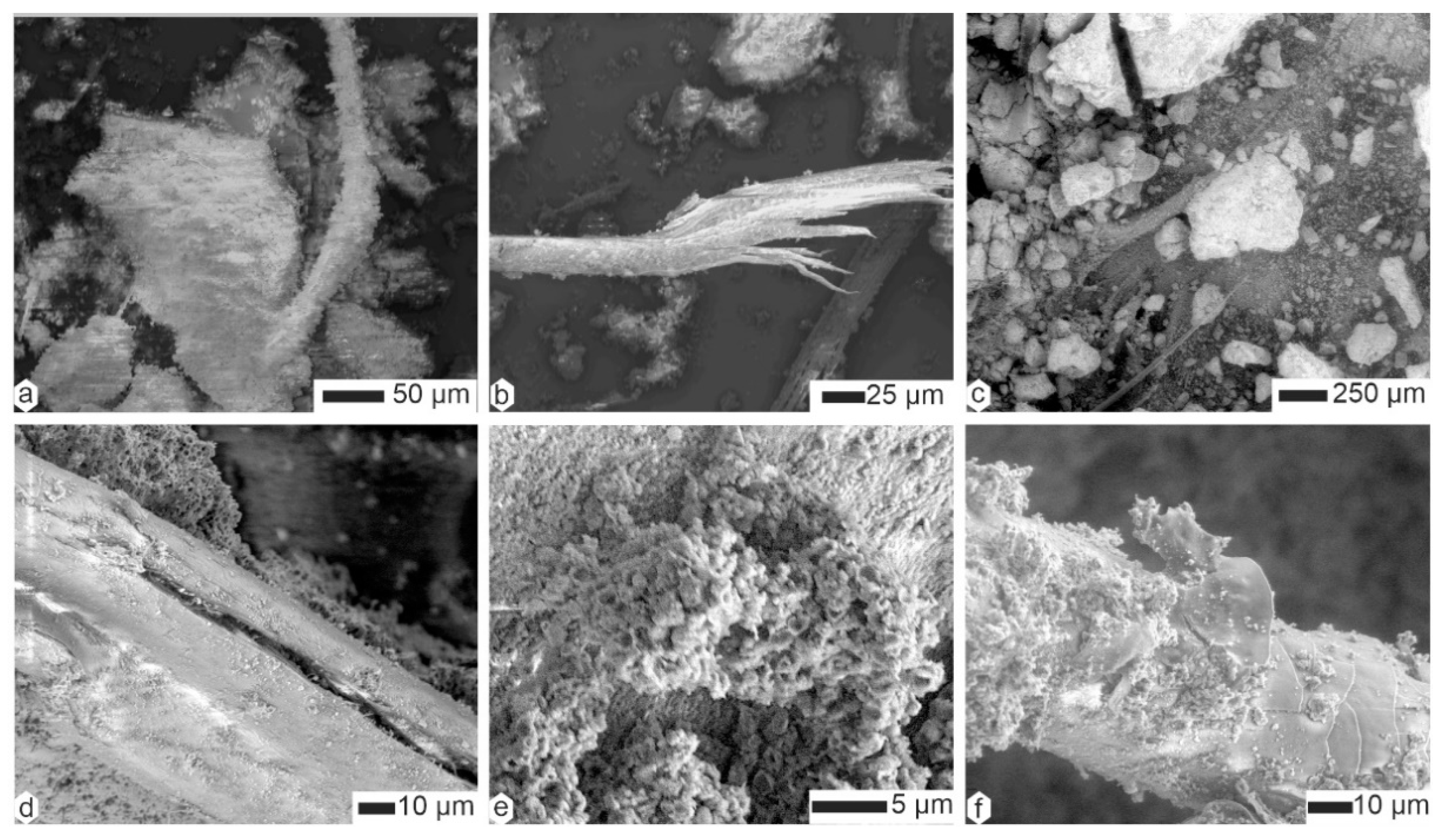
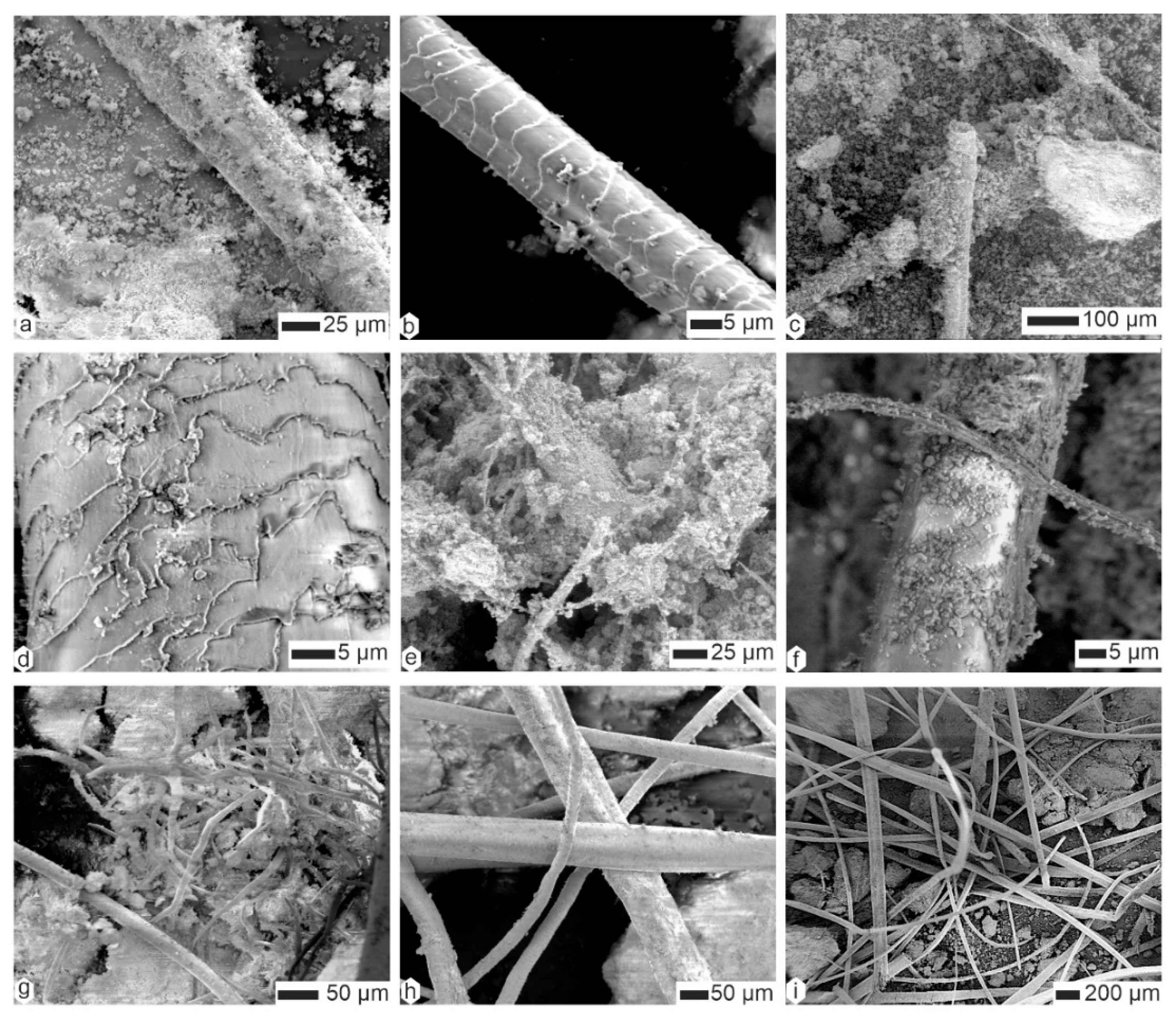




| Species | Country | Region | Collector/Date | # This Study | L x ep (mm) | Note |
|---|---|---|---|---|---|---|
| Crocuta crocuta | Kenya | Kipeto, Kajiado | J.P. Brugal, 2019 | CC-kip4 | 28x27.5 | isolated |
| Kenya | Kipeto, Kajiado | J.P. Brugal, 2019 | CC-kip5 | 28x38.5 | isolated | |
| Kenya | Kipeto, Kajiado | J.P. Brugal, 2019 | CC-kip | - | isolated | |
| Kenya | Samburu/Seva Cons. | T. Adhola, 2017 | CC-Sam | 38x27 | isolated | |
| Kenya | Soysambu | J.P. Brugal, 2017 | CC-soy | 34x28 | isolated | |
| Djibouti | Barogali | J.B. Fourvel, 2019 | CC-djib1 | 24x24 | isolated | |
| Djibouti | Barogali | J.B. Fourvel, 2019 | CC-djib2 | -x19.5 | isolated | |
| Tchad | Zakuma, Bar Salamat | C. Denys, 2000 | CC-Tchad | 27x25 | isolated | |
| Tanzanie | Kingu Pira | C. Denys, 2003 | CC-Tanz | 32x21 | isolated | |
| Hyaena hyaena | Kenya | Shompole | O. Mwebi, 2009 | FF-F105 | 19x9.5 | isolated |
| Kenya | Shompole | O. Mwebi, 2008 | HH-M113 | 24x16 | isolated | |
| Kenya | West Turkana | J.P. Brugal, 2012 | HM-LA2c | 28.5x22 | isolated | |
| Kenya | West Turkana | J.P. Brugal, 2013 | HH-Lom | 23x18.5 | isolated | |
| Parahyaena brunnea | South Africa | Limpopo, BRS | J.P. Brugal, 2016 | HB-lim | 20x15.7 | isolated |
| Botswana | Gewihaba Hills | J.B. Fourvel, 2021 | HBp | small specimen | isolated | |
| Botswana | Gewihaba Hills | J.B. Fourvel, 2021 | HBg | larger specimen | isolated | |
| Proteles cristatus | Kenya | Samburu | J.P. Brugal, O. Mwebi, 2017 | Pro | 90x85 | latrine |
| Kenya | Samburu | J.P. Brugal, O. Mwebi, 2017 | Pro2023 | 30x18 | latrine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dauphin, Y.; Brugal, J.-P. Coprolite Multimodal Analysis: A Tool for Hyaenid Predator Identification. Animals 2025, 15, 1145. https://doi.org/10.3390/ani15081145
Dauphin Y, Brugal J-P. Coprolite Multimodal Analysis: A Tool for Hyaenid Predator Identification. Animals. 2025; 15(8):1145. https://doi.org/10.3390/ani15081145
Chicago/Turabian StyleDauphin, Yannicke, and Jean-Philip Brugal. 2025. "Coprolite Multimodal Analysis: A Tool for Hyaenid Predator Identification" Animals 15, no. 8: 1145. https://doi.org/10.3390/ani15081145
APA StyleDauphin, Y., & Brugal, J.-P. (2025). Coprolite Multimodal Analysis: A Tool for Hyaenid Predator Identification. Animals, 15(8), 1145. https://doi.org/10.3390/ani15081145







