Clinical Utility of Patient-Derived Cell-Based In Vitro Drug Sensitivity Testing for Optimizing Adjuvant Therapy in Dogs with Solid Tumors: A Retrospective Study (2019–2023)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
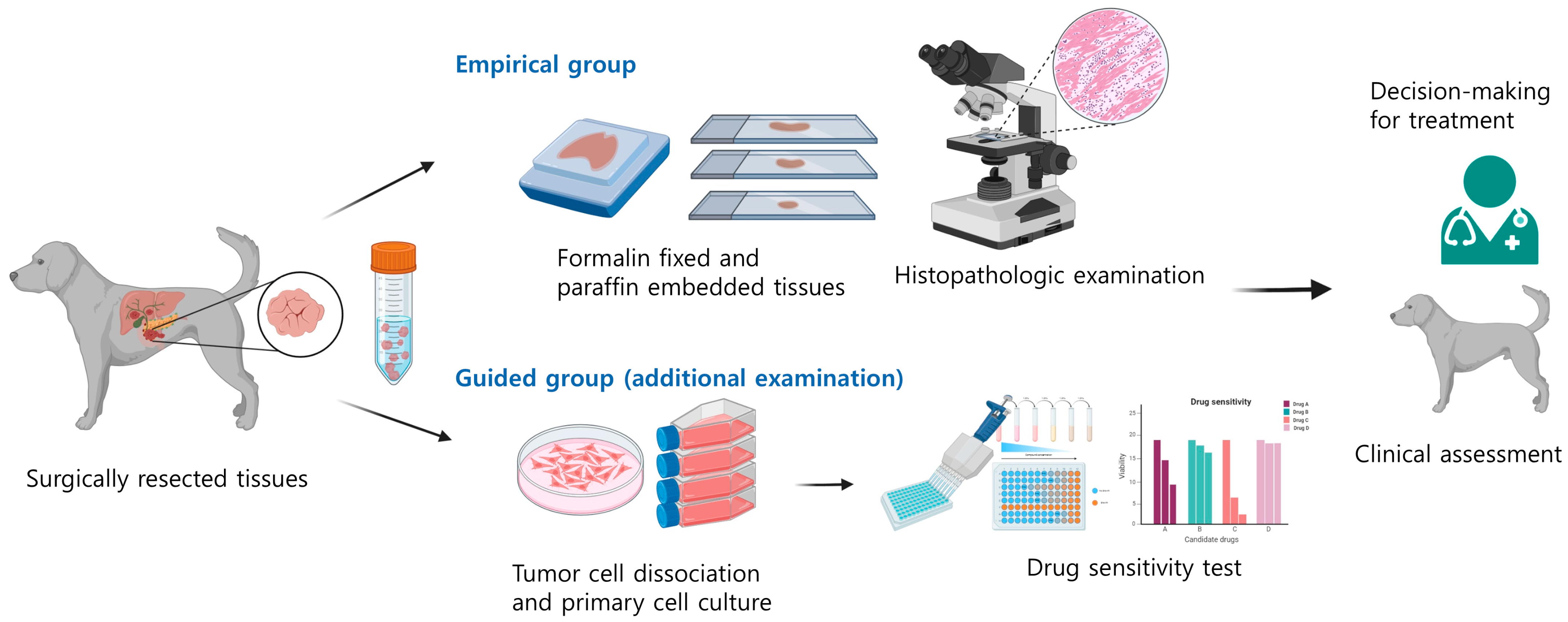
2.2. Study Design
2.3. In Vitro Drug Sensitivity Testing
2.4. Statistical Analysis
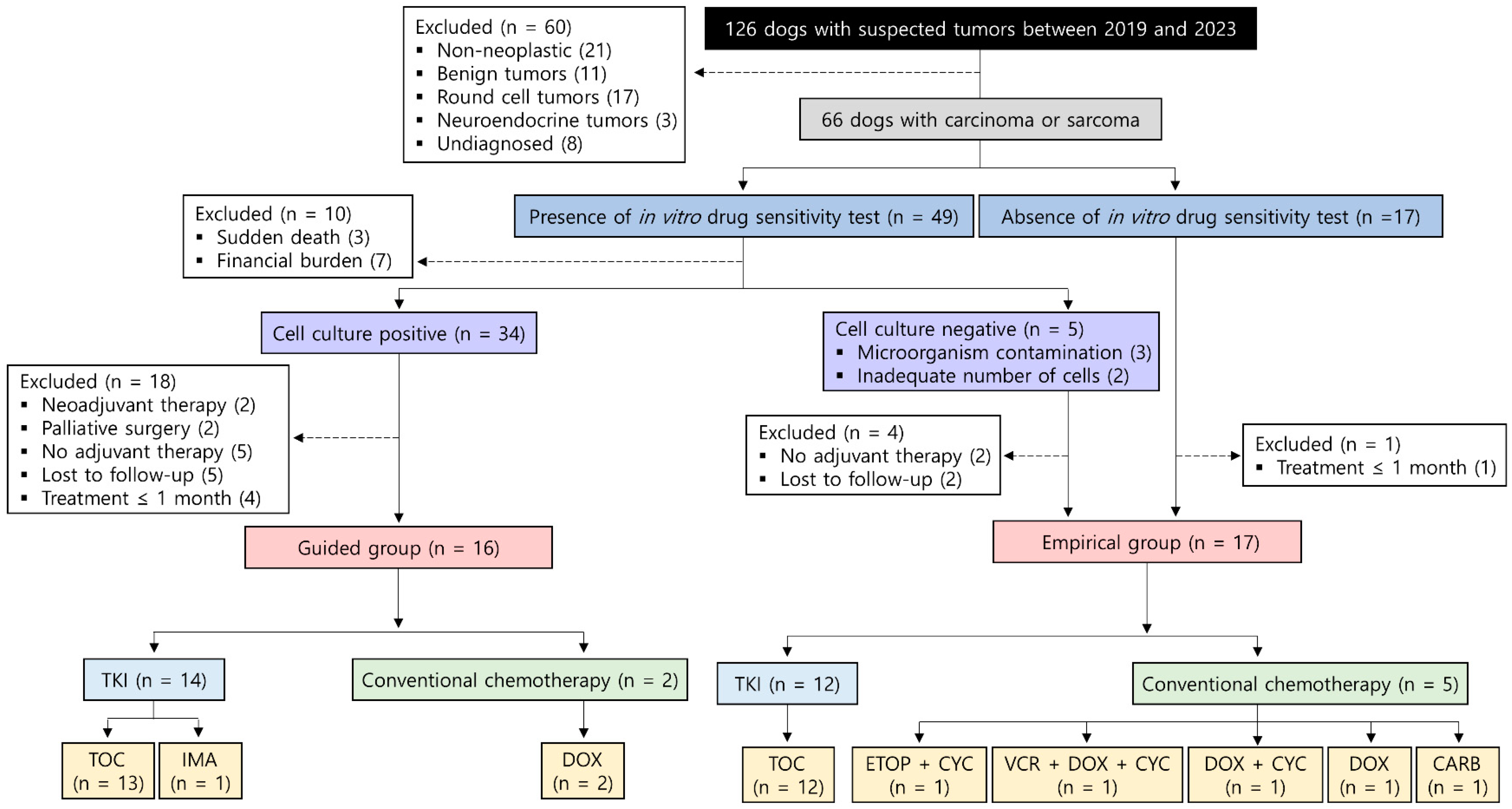
3. Results
3.1. Patient Characteristics
3.2. Treatments
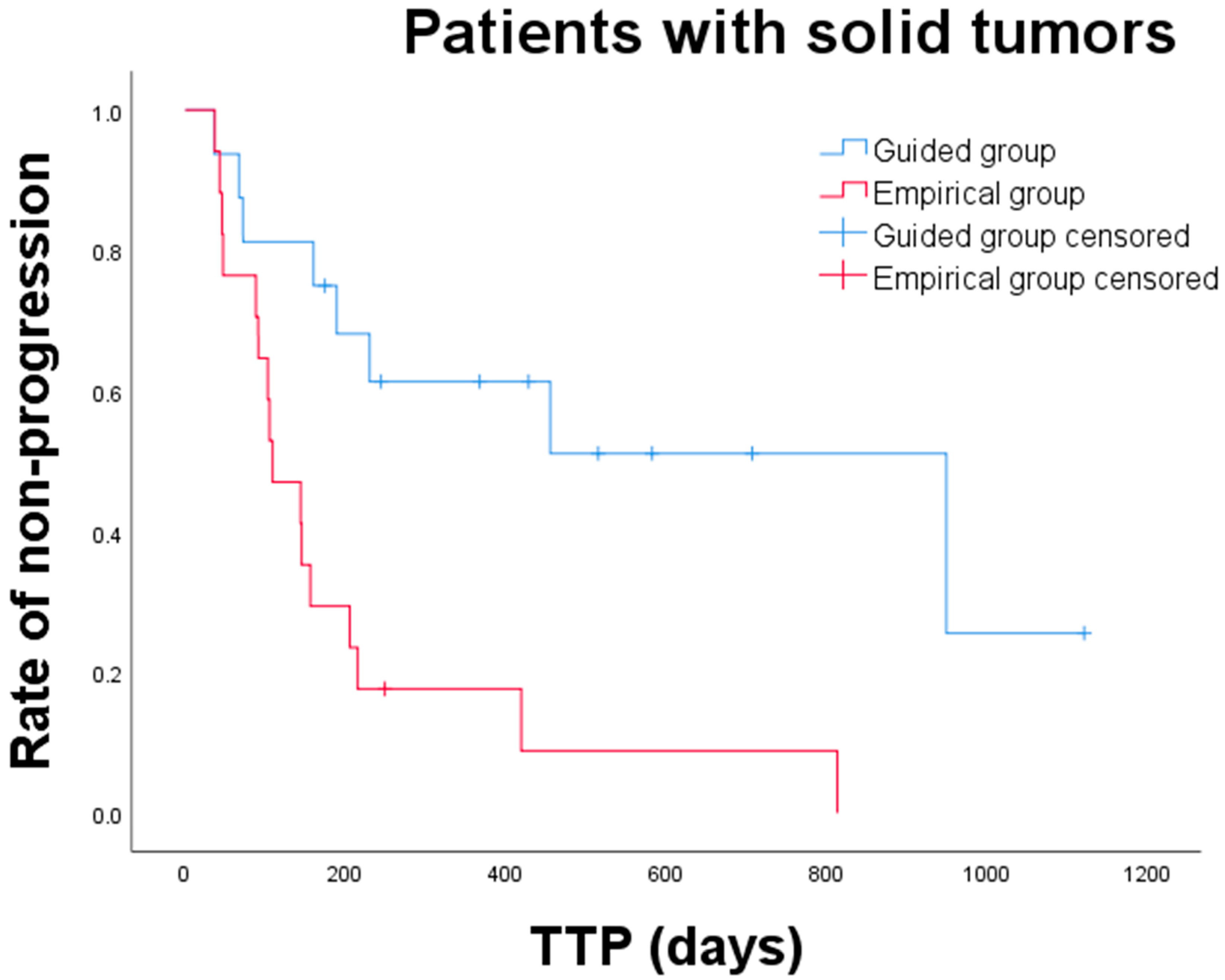
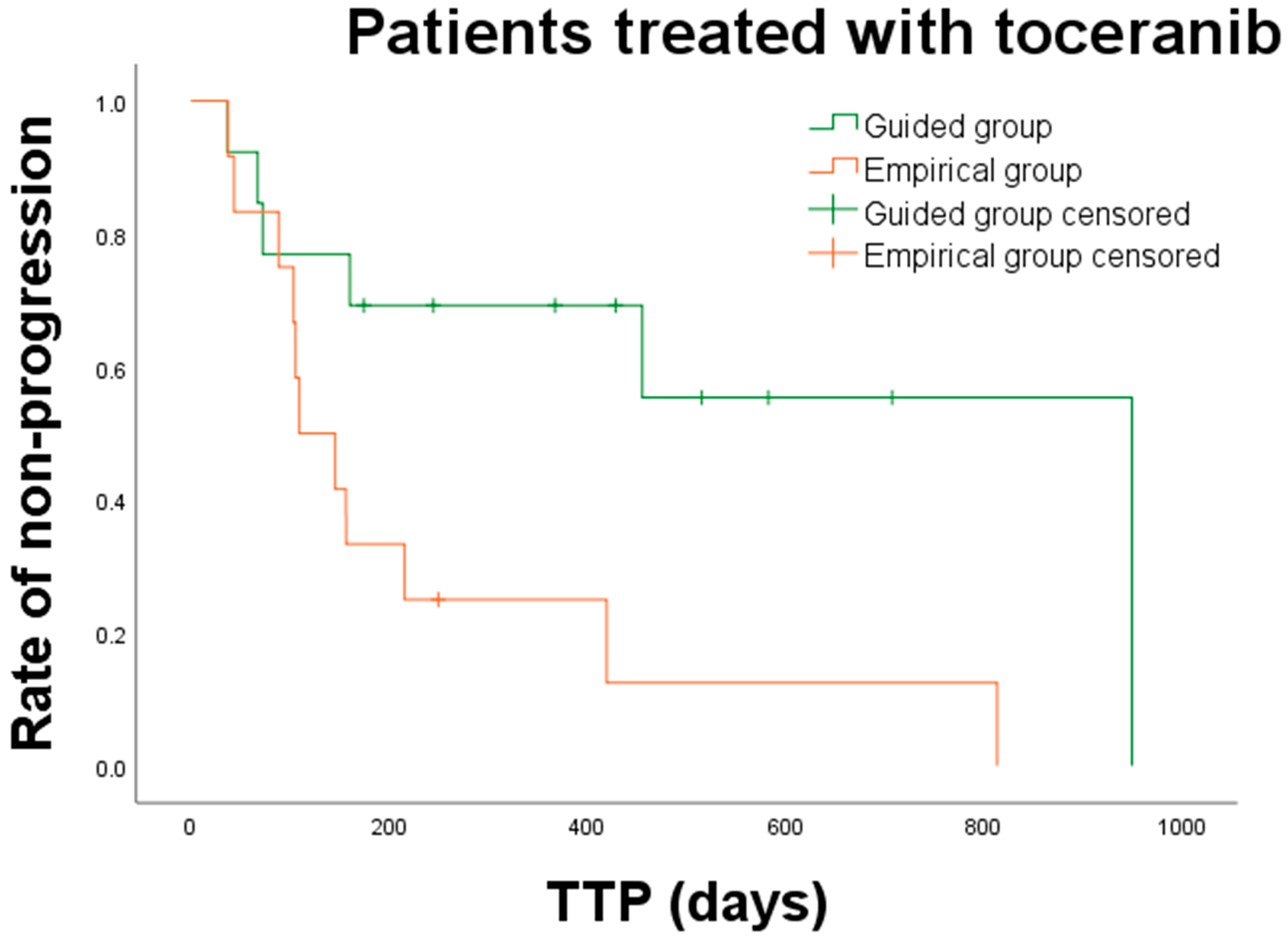
3.3. Clinical Outcomes and Prognostic Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Chibuk, J.; Flory, A.; Kruglyak, K.M.; Leibman, N.; Nahama, A.; Dharajiya, N.; van den Boom, D.; Jensen, T.J.; Friedman, J.S.; Shen, M.R.; et al. Horizons in veterinary precision oncology: Fundamentals of cancer genomics and applications of liquid biopsy for the detection, characterization, and management of cancer in dogs. Front. Vet. Sci. 2021, 8, 664718. [Google Scholar] [CrossRef] [PubMed]
- Biller, B.; Berg, J.; Garrett, L.; Ruslander, D.; Wearing, R.; Abbott, B.; Patel, M.; Smith, D.; Bryan, C. 2016 AAHA oncology guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 2016, 52, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Hammer, A.S.; Couto, C.G. Adjuvant chemotherapy for sarcomas and carcinomas. Vet. Clin. N. Am. Small Anim. Pract. 1990, 20, 1015–1036. [Google Scholar] [CrossRef]
- Arora, A.; Scholar, E.M. Role of tyrosine kinase inhibitors in cancer therapy. J. Pharmacol. Exp. Ther. 2005, 315, 971–979. [Google Scholar] [CrossRef]
- Klopfleisch, R.; Kohn, B.; Gruber, A.D. Mechanisms of tumour resistance against chemotherapeutic agents in veterinary oncology. Vet. J. 2016, 207, 63–72. [Google Scholar] [CrossRef]
- Londhe, P.; Gutwillig, M.; London, C. Targeted therapies in veterinary oncology. Vet. Clin. N. Am. Small Anim. Pract. 2019, 49, 917–931. [Google Scholar] [CrossRef] [PubMed]
- Min, H.Y.; Lee, H.Y. Molecular targeted therapy for anticancer treatment. Exp. Mol. Med. 2022, 54, 1670–1694. [Google Scholar] [CrossRef]
- Sposito, M.; Belluomini, L.; Pontolillo, L.; Tregnago, D.; Trestini, I.; Insolda, J.; Avancini, A.; Milella, M.; Bria, E.; Carbognin, L.; et al. Adjuvant targeted therapy in solid cancers: Pioneers and New Glories. J. Pers. Med. 2023, 13, 1427. [Google Scholar] [CrossRef]
- Sakthikumar, S.; Facista, S.; Whitley, D.; Byron, S.A.; Ahmed, Z.; Warrier, M.; Zhu, Z.; Chon, E.; Banovich, K.; Haworth, D.; et al. Standing in the canine precision medicine knowledge gap: Improving annotation of canine cancer genomic biomarkers through systematic comparative analysis of human cancer mutations in COSMIC. Vet. Comp. Oncol. 2023, 21, 482–491. [Google Scholar] [CrossRef]
- Korec, D.I.; Louke, D.S.; Breitbach, J.T.; Geisler, J.A.; Husbands, B.D.; Fenger, J.M. Characterization of receptor tyrosine kinase activation and biological activity of toceranib phosphate in canine urothelial carcinoma cell lines. BMC. Vet. Res. 2021, 17, 320. [Google Scholar] [CrossRef]
- Macedo, T.R.; de Queiroz, G.F.; Casagrande, T.A.C.; Alexandre, P.A.; Brandão, P.E.; Fukumasu, H.; Melo, S.R.; Dagli, M.L.Z.; Pinto, A.C.B.C.F.; Matera, J.M. Imatinib Mesylate for the Treatment of Canine Mast Cell Tumors: Assessment of the Response and Adverse Events in Comparison with the Conventional Therapy with Vinblastine and Prednisone. Cells 2022, 11, 571. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.P.; Johannes, C.M.; Jergens, A.E.; Allenspach, K.; Powers, B.E.; Du, Y.; Mochel, J.P.; Fox, L.E.; Musser, M.L. Retrospective evaluation of toceranib phosphate (Palladia®) use in the treatment of gastrointestinal stromal tumors of dogs. J. Vet. Intern. Med. 2018, 32, 2045–2053. [Google Scholar] [CrossRef]
- Maeda, S.; Sakai, K.; Kaji, K.; Iio, A.; Nakazawa, M.; Motegi, T.; Yonezawa, T.; Momoi, Y. Lapatinib as first-line treatment for muscle-invasive urothelial carcinoma in dogs. Sci. Rep. 2022, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Tanaka, T.; Mie, K.; Nishida, H.; Miura, N.; Akiyoshi, H. Assessment of postoperative adjuvant treatment using toceranib phosphate against adenocarcinoma in dogs. J. Vet. Intern. Med. 2020, 34, 1272–1281. [Google Scholar] [CrossRef]
- Janku, F. Tumor heterogeneity in the clinic: Is it a real problem? Ther. Adv. Med. Oncol. 2014, 6, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Zhong, W.Z.; Zhang, X.C.; Su, J.; Yang, X.N.; Chen, Z.H.; Yang, J.J.; Zhou, Q.; Yan, H.H.; An, S.J.; et al. EGFR mutation heterogeneity and the mixed response to EGFR tyrosine kinase inhibitors of lung adenocarcinomas. Oncologist 2012, 17, 978–985. [Google Scholar] [CrossRef]
- Hou, X.; Du, C.; Lu, L.; Yuan, S.; Zhan, M.; You, P.; Du, H. Opportunities and challenges of patient-derived models in cancer research: Patient-derived xenografts, patient-derived organoid and patient-derived cells. World J. Surg. Oncol. 2022, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell viability assays. In Assay Guidance Manual; Markossian, S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Brimacombe, K., Chung, T.D.Y., Coussens, N.P., et al., Eds.; Eli Lilly and Company: Bethesda, MD, USA; The National Center for Advancing Translational Sciences: Bethesda, MD, USA, 2013. [Google Scholar]
- Popova, A.A.; Levkin, P.A. Precision medicine in oncology: In vitro drug sensitivity and resistance test (DSRT) for selection of personalized anticancer therapy. Adv. Ther. 2020, 3, 1900100. [Google Scholar] [CrossRef]
- Dietrich, S.; Oleś, M.; Lu, J.; Sellner, L.; Anders, S.; Velten, B.; Wu, B.; Hüllein, J.; da Silva Liberio, M.; Walther, T.; et al. Drug-perturbation-based stratification of blood cancer. J. Clin. Investig. 2018, 128, 427–445. [Google Scholar] [CrossRef]
- Ge, W.Q.; Pu, J.X.; Zheng, S.Y. Clinical application of the adenosine triphosphate-based response assay in intravesical chemotherapy for superficial bladder cancer. Asian Pac. J. Cancer Prev. 2012, 13, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.U.; Bae, J.W.; Kim, H.G.; Choi, S.H.; Kang, D.H.; Lee, J.B.; Koo, B.W. Correlation between the in vitro ATP-based chemosensitivity assay and HER2/neu expression in women with breast cancer. J. Int. Med. Res. 2007, 35, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, K.W.; Kim, Y.H.; Lee, K.H.; Oh, D.Y.; Kim, J.; Yang, S.H.; Im, S.A.; Choi, S.H.; Bang, Y.J. Individualized tumor response testing for prediction of response to paclitaxel and cisplatin chemotherapy in patients with advanced gastric cancer. J. Korean Med. Sci. 2010, 25, 684–690. [Google Scholar] [CrossRef]
- Kim, C.D.; Kim, S.H.; Jung, S.H.; Kim, J.H. Clinical value of an adenosine triphosphate-based chemotherapy response assay in resectable stage III colorectal cancer. Ann. Surg. Treat. Res. 2019, 97, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Cortese, A.; Pantaleo, G.; Amato, M.; Lawrence, L.; Mayes, V.; Brown, L.; Sarno, M.R.; Valluri, J.; Claudio, P.P. A new complementary procedure for patients affected by head and neck cancer: Chemo-predictive assay. Int. J. Surg. Case Rep. 2016, 26, 42–46. [Google Scholar] [CrossRef]
- Kang, S.M.; Park, M.S.; Chang, J.; Kim, S.K.; Kim, H.; Shin, D.H.; Chung, K.Y.; Kim, D.J.; Sohn, J.H.; Choi, S.H.; et al. A feasibility study of adenosine triphosphate-based chemotherapy response assay (ATP-CRA) as a chemosensitivity test for lung cancer. Cancer Res. Treat. 2005, 37, 223–227. [Google Scholar] [CrossRef]
- Singh, T.; Neal, A.S.; Moatamed, N.A.; Memarzadeh, S. Exploring the potential of drug response assays for precision medicine in ovarian cancer. Int. J. Mol. Sci. 2020, 22, 305. [Google Scholar] [CrossRef]
- Ugurel, S.; Loquai, C.; Terheyden, P.; Schadendorf, D.; Richtig, E.; Utikal, J.; Gutzmer, R.; Rass, K.; Sunderkötter, C.; Stein, A.; et al. Chemosensitivity-directed therapy compared to dacarbazine in chemo-naive advanced metastatic melanoma: A multicenter randomized phase-3 DeCOG trial. Oncotarget 2017, 8, 76029–76043. [Google Scholar] [CrossRef]
- Michalski, C.W.; Erkan, M.; Sauliunaite, D.; Giese, T.; Stratmann, R.; Sartori, C.; Giese, N.A.; Friess, H.; Kleeff, J. Ex vivo chemosensitivity testing and gene expression profiling predict response towards adjuvant gemcitabine treatment in pancreatic cancer. Br. J. Cancer 2008, 99, 760–767. [Google Scholar] [CrossRef]
- Kwon, H.Y.; Kim, I.K.; Kang, J.; Sohn, S.K.; Lee, K.Y. In vitro adenosine triphosphate-based chemotherapy response assay as a predictor of clinical response to fluorouracil-based adjuvant chemotherapy in Stage II colorectal cancer. Cancer Res. Treat. 2016, 48, 970–977. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, M.C.; Oh, S.Y.; Kwon, H.C.; Kim, S.H.; Kwon, K.A.; Lee, S.; Jeong, J.S.; Choi, S.R.; Kim, H.J. Predictive value of in vitro adenosine triphosphate-based chemotherapy response assay in advanced gastric cancer patients who received oral 5-fluorouracil after curative resection. Cancer Res. Treat. 2011, 43, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Nakamori, M.; Iwahashi, M.; Nakamura, M.; Yamaue, H. Clinical benefit of chemosensitivity test for patients with regional lymph node-positive esophageal squamous cell carcinoma. J. Surg. Oncol. 2003, 84, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Obmińska-Mrukowicz, B.; Zbyryt, I.; Rapak, A. In vitro drug sensitivity in canine lymphoma. J. Vet. Res. 2016, 60, 55–61. [Google Scholar] [CrossRef]
- Franco Molina, M.A.; Santamaría-Martínez, E.A.; Santana Krimskaya, S.E.; Zarate-Triviño, D.G.; Kawas, J.R.; Ramos Zayas, Y.; Palacios Estrada, N.; Prado García, H.; García Coronado, P.L.; Rodríguez Padilla, C. In vitro chemosensitivity of a canine tumor venereal transmissible cancer cell line. Front. Vet. Sci. 2022, 9, 972185. [Google Scholar] [CrossRef]
- Pinello, K.C.; Nagamine, M.; Silva, T.C.; Matsuzaki, P.; Caetano, H.V.; Torres, L.N.; Fukumasu, H.; Avanzo, J.L.; Matera, J.M.; Dagli, M.L. In vitro chemosensitivity of canine mast cell tumors grades II and III to all-trans-retinoic acid (ATRA). Vet. Res. Commun. 2009, 33, 581–588. [Google Scholar] [CrossRef]
- Takada, M.; Parys, M.; Gregory-Bryson, E.; Vilar Saavedra, P.; Kiupel, M.; Yuzbasiyan-Gurkan, V. A novel canine histiocytic sarcoma cell line: Initial characterization and utilization for drug screening studies. BMC Cancer 2018, 18, 237. [Google Scholar] [CrossRef]
- Jung, H.; Bae, K.; Lee, J.Y.; Kim, J.H.; Han, H.J.; Yoon, H.Y.; Yoon, K.A. Establishment of canine transitional cell carcinoma cell lines harboring BRAF V595E mutation as a therapeutic target. Int. J. Mol. Sci. 2021, 22, 9151. [Google Scholar] [CrossRef]
- Li, R.; Wu, H.; Sun, Y.; Zhu, J.; Tang, J.; Kuang, Y.; Li, G. A novel canine mammary cancer cell line: Preliminary identification and utilization for drug screening studies. Front. Vet. Sci. 2021, 8, 665906. [Google Scholar] [CrossRef]
- Henry, C.J.; McCaw, D.L.; Buss, M.S.; Pope, E.R.; Tyler, J.W.; Tate, D.; Russell, L. Clinical assessment of a chemosensitivity assay as a treatment planning tool for dogs with cancer. J. Am. Anim. Hosp. Assoc. 2001, 37, 165–171. [Google Scholar] [CrossRef]
- Owen, L.A. TNM Classification of Tumours in Domestic Animals; World Health Organization: Geneva, Switzerland, 1980. [Google Scholar]
- LeBlanc, A.K.; Atherton, M.; Bentley, R.T.; Boudreau, C.E.; Burton, J.H.; Curran, K.M.; Dow, S.; Giuffrida, M.A.; Kellihan, H.B.; Mason, N.J.; et al. Veterinary Cooperative Oncology Group-Common Terminology Criteria for Adverse Events (VCOG-CTCAE v2) following investigational therapy in dogs and cats. Vet. Comp. Oncol. 2021, 19, 311–352. [Google Scholar] [CrossRef]
- Laver, T.; London, C.A.; Vail, D.M.; Biller, B.J.; Coy, J.; Thamm, D.H. Prospective evaluation of toceranib phosphate in metastatic canine osteosarcoma. Vet. Comp. Oncol. 2018, 16, E23–E29. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Bae, K.; Kim, J.H.; Han, H.J.; Yoon, H.Y.; Yoon, K.A. Establishment and characterization of six canine hepatocellular carcinoma cell lines. Front. Vet. Sci. 2024, 11, 1392728. [Google Scholar] [CrossRef]
- Dennis, M.M.; McSporran, K.D.; Bacon, N.J.; Schulman, F.Y.; Foster, R.A.; Powers, B.E. Prognostic factors for cutaneous and subcutaneous soft tissue sarcomas in dogs. Vet. Pathol. 2011, 48, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Bernabe, L.F.; Portela, R.; Nguyen, S.; Kisseberth, W.C.; Pennell, M.; Yancey, M.F.; London, C.A. Evaluation of the adverse event profile and pharmacodynamics of toceranib phosphate administered to dogs with solid tumors at doses below the maximum tolerated dose. BMC Vet. Res. 2013, 9, 190. [Google Scholar] [CrossRef]
- Delgado, A.; Guddati, A.K. Clinical endpoints in oncology—A primer. Am. J. Cancer Res. 2021, 11, 1121–1131. [Google Scholar] [PubMed]
- Tran, C.M.; Moore, A.S.; Frimberger, A.E. Surgical treatment of mammary carcinomas in dogs with or without postoperative chemotherapy. Vet. Comp. Oncol. 2016, 14, 252–262. [Google Scholar] [CrossRef]
- Boston, S.E.; Lu, X.; Culp, W.T.N.; Montinaro, V.; Romanelli, G.; Dudley, R.M.; Liptak, J.M.; Mestrinho, L.A.; Buracco, P. Efficacy of systemic adjuvant therapies administered to dogs after excision of oral malignant melanomas: 151 cases (2001–2012). J. Am. Vet. Med. Assoc. 2014, 245, 401–407. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Mazcko, C.N.; Cherukuri, A.; Berger, E.P.; Kisseberth, W.C.; Brown, M.E.; Lana, S.E.; Weishaar, K.; Flesner, B.K.; Bryan, J.N.; et al. Adjuvant sirolimus does not improve outcome in pet dogs receiving standard-of-care therapy for appendicular osteosarcoma: A prospective, randomized trial of 324 dogs. Clin. Cancer Res. 2021, 27, 3005–3016. [Google Scholar] [CrossRef]
- Marconato, L.; Murgia, D.; Finotello, R.; Meier, V.; Morello, E.M.; Pisoni, L.; Foglia, A.; Guerra, D.; Chalfon, C.; Aralla, M.; et al. Clinical features and outcome of 79 dogs with digital squamous cell carcinoma undergoing treatment: A SIONCOV observational study. Front. Vet. Sci. 2021, 8, 645982. [Google Scholar] [CrossRef]
- Faroni, E.; Sabattini, S.; Guerra, D.; Iannuzzi, C.; Chalfon, C.; Agnoli, C.; Stefanello, D.; Polton, G.; Ramos, S.; Aralla, M.; et al. Timely adjuvant chemotherapy improves outcome in dogs with non-metastatic splenic hemangiosarcoma undergoing splenectomy. Vet. Comp. Oncol. 2023, 21, 123–130. [Google Scholar] [CrossRef]
- Matsuyama, A.; Takagi, S.; Hosoya, K.; Kagawa, Y.; Nakamura, K.; Deguchi, T.; Takiguchi, M. Impact of surgical margins on survival of 37 dogs with massive hepatocellular carcinoma. N. Z. Vet. J. 2017, 65, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Elpiner, A.K.; Brodsky, E.M.; Hazzah, T.N.; Post, G.S. Single-agent gemcitabine chemotherapy in dogs with hepatocellular carcinomas. Vet. Comp. Oncol. 2011, 9, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.A.; Rodrigues, P.; Wesolowski, L.; Vanharanta, S. Genomic control of metastasis. Br. J. Cancer 2021, 124, 3–12. [Google Scholar] [CrossRef]
- Coelho, Y.N.B.; Soldi, L.R.; da Silva, P.H.R.; Mesquita, C.M.; Paranhos, L.R.; Dos Santos, T.R.; Silva, M.J.B. Tyrosine kinase inhibitors as an alternative treatment in canine mast cell tumor. Front. Vet. Sci. 2023, 10, 1188795. [Google Scholar] [CrossRef]
- Marconato, L.; Sabattini, S.; Marisi, G.; Rossi, F.; Leone, V.F.; Casadei-Gardini, A. Sorafenib for the treatment of unresectable hepatocellular carcinoma: Preliminary toxicity and activity data in dogs. Cancers 2020, 12, 1272. [Google Scholar] [CrossRef]
- Isotani, M.; Ishida, N.; Tominaga, M.; Tamura, K.; Yagihara, H.; Ochi, S.; Kato, R.; Kobayashi, T.; Fujita, M.; Fujino, Y.; et al. Effect of tyrosine kinase inhibition by imatinib mesylate on mast cell tumors in dogs. J. Vet. Intern. Med. 2008, 22, 985–988. [Google Scholar] [CrossRef]
- Weishaar, K.M.; Ehrhart, E.J.; Avery, A.C.; Charles, J.B.; Elmslie, R.E.; Vail, D.M.; London, C.A.; Clifford, C.A.; Eickhoff, J.C.; Thamm, D.H. c-Kit Mutation and Localization Status as Response Predictors in mast cell Tumors in Dogs Treated with prednisone and toceranib or vinblastine. J. Vet. Intern. Med. 2018, 32, 394–405. [Google Scholar] [CrossRef]
- Kamstock, D.A.; Russell, D.S.; Powers, B.E. The pathology of neoplasia. In Withrow and MacEwen’s Small Animal Clinical Oncology, 6th ed.; MacEwen, E.G., Withrow, S.J., Eds.; Elsevier: St. Louis, MO, USA, 2020; pp. 61–80. [Google Scholar]
| Case | Breed | Age (Years) | Sex | Tumor Location | Diagnosis | TNM | Anticancer Drugs | Treatment Duration (Days) | TTP (Days) a |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Pomeranian | 10 | SF | Liver | HCC-CC | T1N0M0 | Toceranib | 734 | 949 |
| 2 | Mixed | 11 | IF | Liver | HCC-CC | T3N0M0 | Toceranib | 685 | 455 |
| 3 | Cocker spaniel | 8 | CM | Liver | HCC | T1N0M0 | Toceranib | 93 | 174 b |
| 4 | Mixed | 9 | CM | Liver | HCC | T1NXM0 | Toceranib | 172 | 582 c |
| 5 | Bichon frise | 10 | SF | Liver | HCC | T1N0M0 | Toceranib | 376 | 428 c |
| 6 | Scottish terrier | 8 | CM | Liver | HCC | T1N0M0 | Toceranib | 323 | 367 b |
| 7 | Maltese | 5 | CM | Kidney | RCC | T3NXM1 | Toceranib | 41 | 36 |
| 8 | Chihuahua | 6 | SF | Kidney | RCC | T3NXM1 | Toceranib | 142 | 72 |
| 9 | Pomeranian | 5 | SF | Oral cavity | SCC | T1N1M0 | Toceranib | 143 | 160 |
| 10 | French bulldog | 10 | SF | Lung | PC | T1NXM0 | Doxorubicin | 183 | 189 |
| 11 | Great Pyrenees | 12 | CM | Muscle | HSA | T3N0M0 | Toceranib | 79 | 67 |
| 12 | Yorkshire terrier | 13 | SF | Spleen | HSA | T1N0M0 | Doxorubicin | 264 | 230 |
| 13 | Shih tzu | 15 | SF | Skin | STS | T1N0M0 | Toceranib | 651 | 707 b |
| 14 | Maltese | 11 | SF | Intestine | GIST | T1N0M0 | Imatinib | 1093 | 1121 c |
| 15 | Coton de Tulear | 7 | CM | Lung | HS | T1N0M0 | Toceranib | 197 | 244 c |
| 16 | Golden retriever | 6 | CM | Bone | OSA | T2N0M0 | Toceranib | 471 | 515 b |
| Case | Breed | Age (Years) | Sex | Tumor Location | Diagnosis | TNM | Anticancer Drugs | Treatment Duration (Days) | TTP (Days) a |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Shih tzu | 12 | CM | Liver | HCC | T2N0M0 | Toceranib | 78 | 103 |
| 2 | Poodle | 7 | IF | Liver | HCC | T1N0M0 | Toceranib | 593 | 813 |
| 3 | Poodle | 11 | CM | Liver | HCC | T1N0M0 | Toceranib | 227 | 249 b |
| 4 | Japanese spitz | 9 | CM | Lung | PC | T1NXM1 | Toceranib | 130 | 109 |
| 5 | Pekingese | 14 | SF | Lung | PC | T1N0M0 | Toceranib | 29 | 43 |
| 6 | Maltese | 6 | SF | Anal sac | AGASACA | T3N0M0 | Toceranib | 264 | 419 |
| 7 | Silky terrier | 10 | CM | Intestine | SBA | T2NXM1 | Toceranib | 145 | 145 |
| 8 | Maltese | 10 | SF | Pancreas | PAC | T1N1M0 | Toceranib | 133 | 105 |
| 9 | Poodle | 8 | CM | Tonsil | SCC | T2N1M0 | Toceranib | 123 | 88 |
| 10 | Italian greyhound | 3 | IF | Ovary | OC | T3N0M0 | Carboplatin | 176 | 205 |
| 11 | Bichon frise | 11 | CM | Nasal cavity | NC | T2N0M0 | Toceranib | 235 | 156 |
| 12 | French bulldog | 10 | CM | Spleen | HSA | T2N0M0 | ETOP + CYC | 150 | 144 |
| 13 | Mixed | 11 | CM | Spleen | HSA | T3NXM1 | Doxorubicin | 29 | 46 |
| 14 | Maltese | 9 | SF | Spleen | HSA | T2NXM0 | VCR + DOX + CYC | 28 | 91 |
| 15 | Maltese | 10 | CM | Spleen | HSA | T2N0M1 | DOX + CYC | 70 | 47 |
| 16 | Welsh corgi | 10 | CM | Oral cavity | STS | T1NXM0 | Toceranib | 40 | 37 |
| 17 | Beagle | 8 | SF | Intestine | GIST | T2NXM1 | Toceranib | 196 | 215 |
| Tumor Types (n) | Guided Group (n = 16) | Empirical Group (n = 17) |
|---|---|---|
| Carcinoma (n = 21) | ||
| Hepatocellular carcinoma | 4 (25.0%) | 3 (17.6%) |
| Pulmonary carcinoma | 1 (6.25%) | 2 (11.8%) |
| Squamous cell carcinoma | 1 (6.25%) | 1 (5.9%) |
| Combined hepatocellular cholangiocarcinoma | 2 (12.5%) | - |
| Renal cell carcinoma | 2 (12.5%) | - |
| Small bowel adenocarcinoma | - | 1 (5.9%) |
| Apocrine gland anal sac adenocarcinoma | - | 1 (5.9%) |
| Pancreatic adenocarcinoma | - | 1 (5.9%) |
| Ovarian carcinoma | - | 1 (5.9%) |
| Nasal carcinoma | - | 1 (5.9%) |
| Total | 10 | 11 |
| Sarcoma (n = 13) | ||
| Hemangiosarcoma | 2 (12.5%) | 4 (23.5%) |
| Gastrointestinal stromal tumor | 1 (6.25%) | 1 (5.9%) |
| Soft tissue sarcoma | 1 (6.25%) | 1 (5.9%) |
| Osteosarcoma | 1 (6.25%) | - |
| Histiocytic sarcoma | 1 (6.25%) | - |
| Total | 6 | 6 |
| Variables | Guided Group (n = 16) | Empirical Group (n = 17) | p |
|---|---|---|---|
| Median age (years) (range) | 9.5 (5–15) | 10 (3–14) | 0.811 |
| Median body weight (kg) (range) | 7.1 (2.0–54.0) | 5.8 (3.3–16.5) | 0.260 |
| Sex (n) Male Female | 7 (43.8%) 9 (56.3%) | 10 (58.8%) 7 (41.2%) | 0.387 |
| Neuter status (n) Neutered Intact | 15 (93.8%) 1 (6.3%) | 15 (88.2%) 2 (11.8%) | 1.000 |
| Tumor types (n) Carcinoma Sarcoma | 10 (62.5%) 6 (37.5%) | 11 (64.7%) 6 (35.3%) | 0.895 |
| Surgical margin (n) Complete Incomplete | 5 (31.3%) 11 (68.8%) | 6 (35.3%) 11 (64.7%) | 0.805 |
| Mitotic count (per 10 HPF) (n) <20 ≥20 | 11 (68.8%) 5 (31.3%) | 14 (82.4%) 3 (17.6%) | 0.438 |
| Median tumor size (mm) a (range) | 50.4 (11.2–125.0) | 55.0 (26.0–139.0) | 0.443 |
| Distant metastasis (n) Present Absent | 2 (12.5%) 14 (87.5%) | 5 (29.4%) 12 (70.6%) | 0.398 |
| Time interval from surgery to chemotherapy (n) ≤28 days >28 days | 9 (56.3%) 7 (43.8%) | 13 (76.5%) 4 (23.5%) | 0.218 |
| Median treatment duration (days) (range) | 231 (41–1093) | 133 (28–593) | 0.037 * |
| Types of cancer treatment (n) Targeted therapy (i.e., TKIs) Conventional chemotherapy | 14 (87.5%) 2 (12.5%) | 12 (70.6%) 5 (29.4%) | 0.398 |
| Variables | Median TTP (Range) (Days) | ||||
|---|---|---|---|---|---|
| Guided Group | N | Empirical Group | N | p | |
| Total (n = 33) | 949 (36–949) | 16 | 109 (37–813) | 17 | 0.002 * |
| Tumor types Carcinoma (n = 21) Sarcoma (n = 12) | 455 (36–949) NR (67–230) | 10 6 | 145 (43–813) 144 (37–215) | 11 6 | 0.084 0.005 * |
| Surgical margin Complete Incomplete | 230 (36–230) 949 (67–949) | 5 11 | 91 (46–419) 109 (37–813) | 6 11 | 0.232 0.005 * |
| Mitotic count (per 10 HPF) <20 ≥20 | 949 (67–949) 160 (36–160) | 11 5 | 105 (37–813) 109 (91–144) | 14 3 | 0.002 * 0.331 |
| Distant metastasis Present (n = 7) Absent (n = 26) | 67 (67–72) 455 (36–949) | 2 14 | 109 (46–215) 196 (37–813) | 5 12 | 0.429 0.002 * |
| Types of cancer treatment TKIs (n = 26) Conventional chemotherapy (n = 7) | 949 (36–949) 189 (189–230) | 14 2 | 109 (37–813) 91 (46–205) | 12 5 | 0.008 * 0.153 |
| Variables | Tumor Progression (Total) (n = 33) | |||
|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis | |||
| Hazard Ratio (95% CI) | p | Hazard Ratio (95% CI) | p | |
| Age ≥ 10 years | 1.191 (0.520–2.724) | 0.679 | - | - |
| Weight ≥ 6 kg | 0.941 (0.406–2.180) | 0.887 | - | - |
| Female sex | 0.694 (0.298–1.620) | 0.399 | - | - |
| Intact status | 0.839 (0.246–2.869) | 0.780 | - | - |
| Incomplete margin | 0.588 (0.250–1.383) | 0.224 | - | - |
| Mitotic count ≥ 20/10 HPF | 1.569 (0.609–4.042) | 0.351 | - | - |
| Tumor size ≥ 53 mm | 1.664 (0.726–3.815) | 0.229 | - | - |
| Distant metastasis | 4.013 (1.531–10.517) | 0.005 * | 1.565 (0.517–4.739) | 0.428 |
| Time interval from surgery to chemotherapy > 28 days | 0.435 (0.160–1.180) | 0.102 | 0.779 (0.258–2.350) | 0.657 |
| Treatment duration > 150 days | 0.022 (0.003–0.177) | <0.001 | 0.021 (0.002–0.194) | 0.001 * |
| Conventional chemotherapy | 2.571 (1.015–6.510) | 0.046 * | 1.458 (0.509–4.178) | 0.482 |
| Empirical treatment | 3.861 (1.549–9.628) | 0.004 * | 4.120 (1.347–12.607) | 0.013 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-R.; Bae, K.; Lee, J.-Y.; Jeong, S.-W.; Yoon, H.-Y.; Han, H.-J.; Hyun, J.-E.; Nam, A.; Park, J.-H.; Yoon, K.-A.; et al. Clinical Utility of Patient-Derived Cell-Based In Vitro Drug Sensitivity Testing for Optimizing Adjuvant Therapy in Dogs with Solid Tumors: A Retrospective Study (2019–2023). Animals 2025, 15, 1146. https://doi.org/10.3390/ani15081146
Kim Y-R, Bae K, Lee J-Y, Jeong S-W, Yoon H-Y, Han H-J, Hyun J-E, Nam A, Park J-H, Yoon K-A, et al. Clinical Utility of Patient-Derived Cell-Based In Vitro Drug Sensitivity Testing for Optimizing Adjuvant Therapy in Dogs with Solid Tumors: A Retrospective Study (2019–2023). Animals. 2025; 15(8):1146. https://doi.org/10.3390/ani15081146
Chicago/Turabian StyleKim, Young-Rok, Kieun Bae, Ja-Young Lee, Soon-Wuk Jeong, Hun-Young Yoon, Hyun-Jung Han, Jae-Eun Hyun, Aryung Nam, Ji-Hwan Park, Kyong-Ah Yoon, and et al. 2025. "Clinical Utility of Patient-Derived Cell-Based In Vitro Drug Sensitivity Testing for Optimizing Adjuvant Therapy in Dogs with Solid Tumors: A Retrospective Study (2019–2023)" Animals 15, no. 8: 1146. https://doi.org/10.3390/ani15081146
APA StyleKim, Y.-R., Bae, K., Lee, J.-Y., Jeong, S.-W., Yoon, H.-Y., Han, H.-J., Hyun, J.-E., Nam, A., Park, J.-H., Yoon, K.-A., & Kim, J.-H. (2025). Clinical Utility of Patient-Derived Cell-Based In Vitro Drug Sensitivity Testing for Optimizing Adjuvant Therapy in Dogs with Solid Tumors: A Retrospective Study (2019–2023). Animals, 15(8), 1146. https://doi.org/10.3390/ani15081146






