Biomod2 Modeling for Predicting Suitable Distribution of Bamboo Bat (Tylonycteris pachypus) Under Climate Change
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
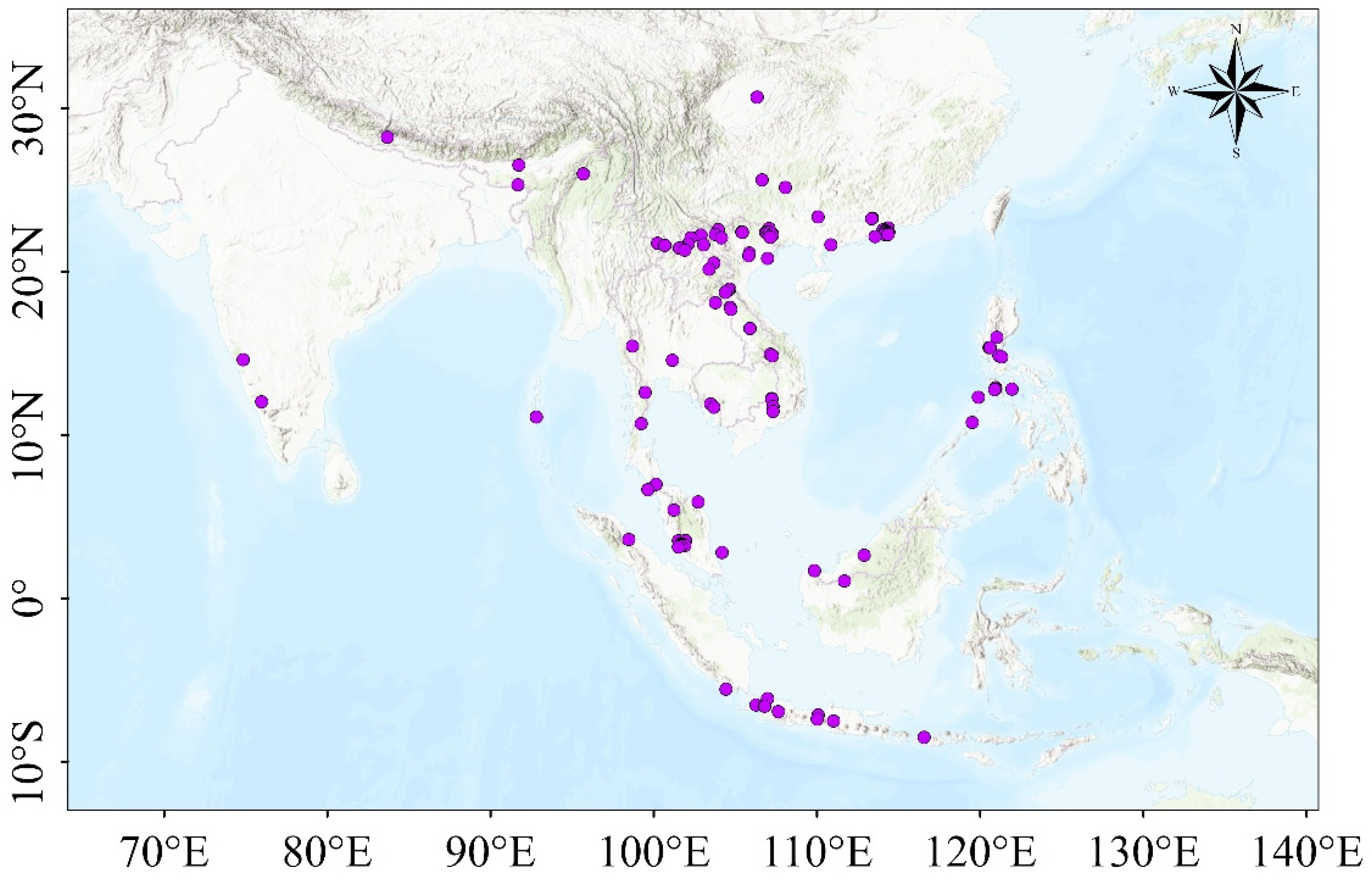
2.1. Data Collection
2.2. Environmental Variables Selection and Processing
2.3. Model Selection and Technical Framework
2.4. Model Construction and Evaluation
2.4.1. Model Construction
2.4.2. Model Training and Validation
2.4.3. Model Evaluation
2.4.4. Habitat Suitability Classification
3. Results
3.1. Model Performance Evaluation
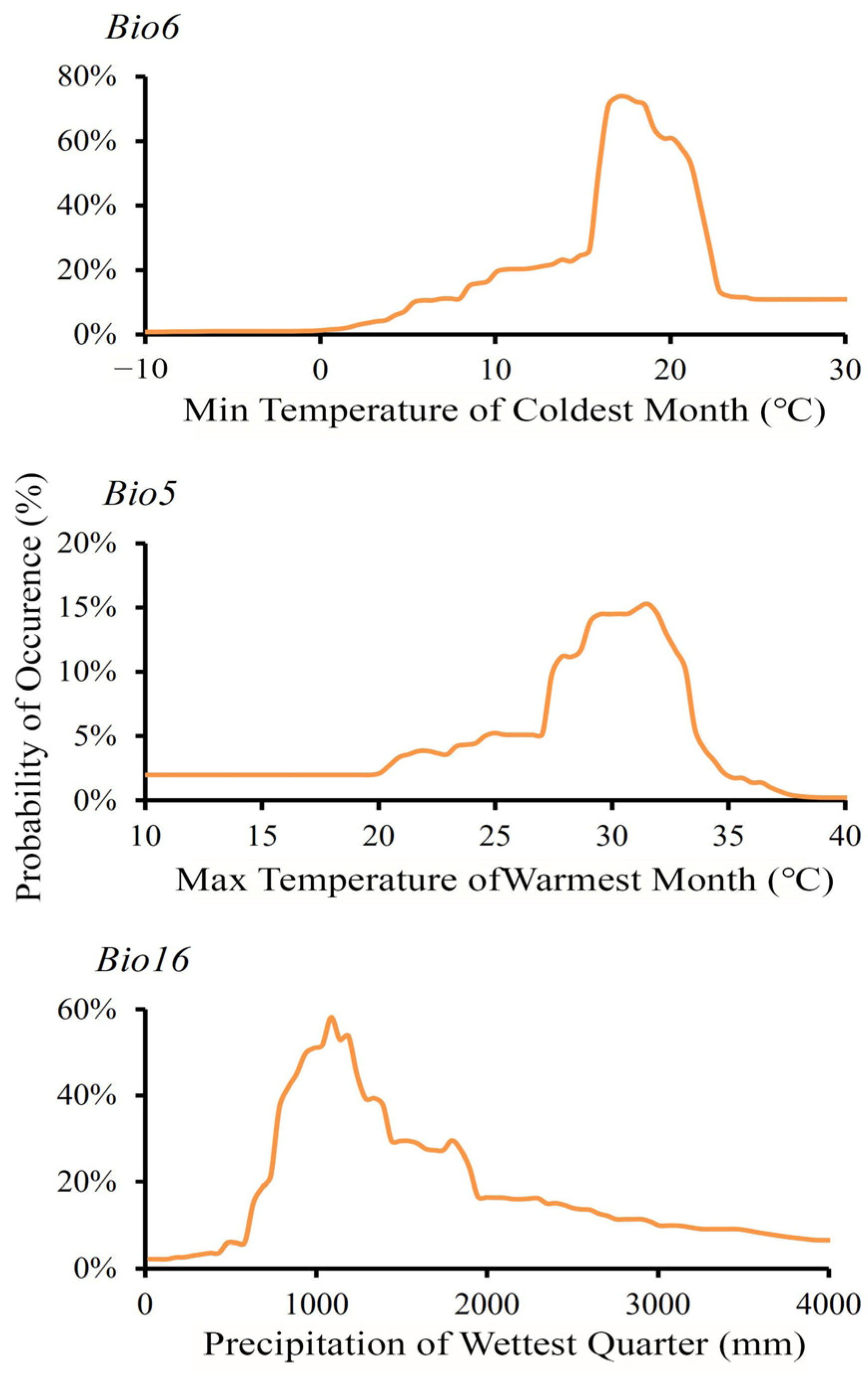
3.2. Key Factor Influencing the Spatial Distribution
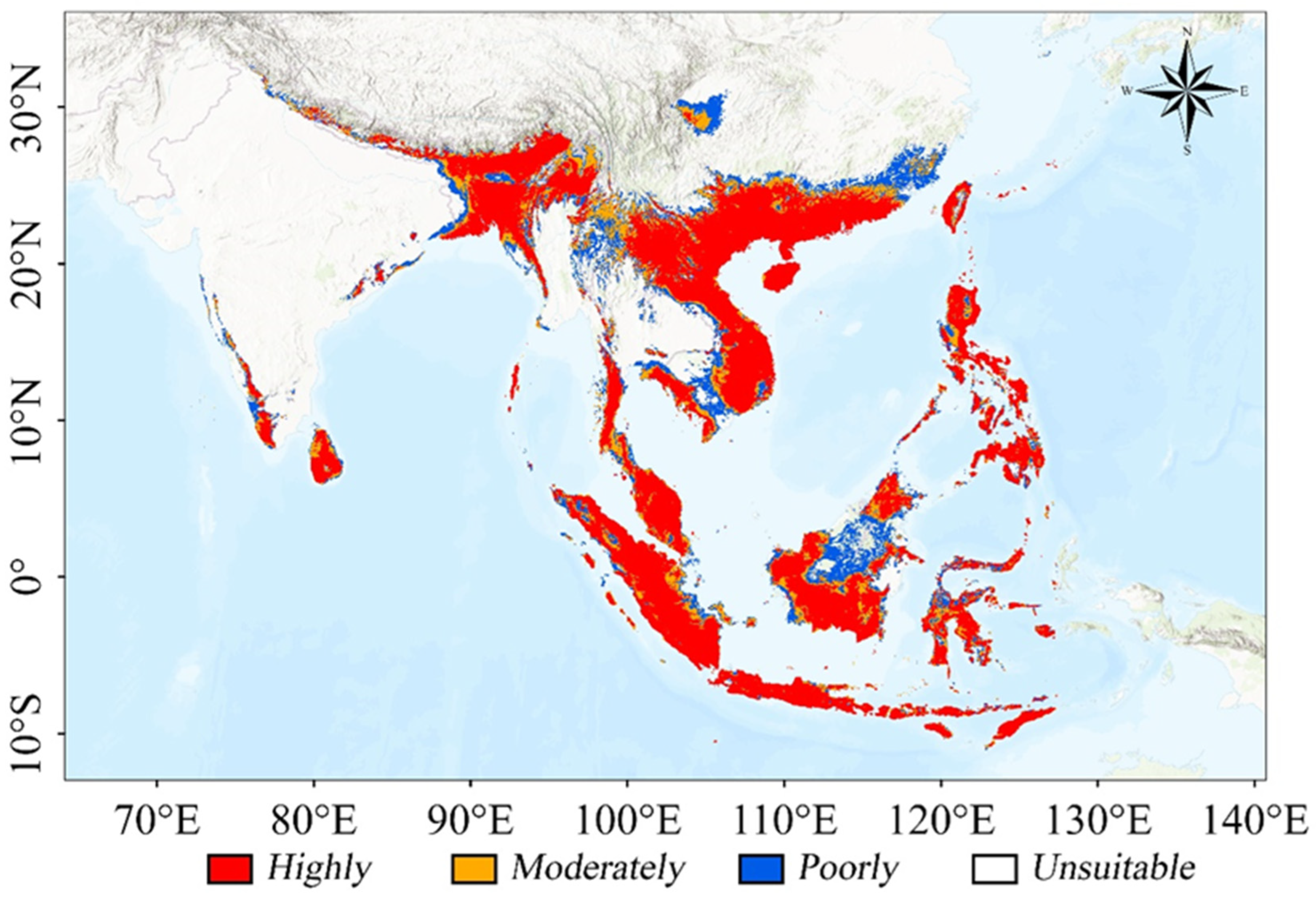
3.3. Potential Suitable Habitats for Current Climate
3.4. Potential Suitable Habitats for Future Scenarios
4. Discussion
4.1. Efficacy of Ensemble Modeling and Key Environmental Factors
4.2. Current Distribution and Ecological Preferences of T. pachypus
4.3. Multiple Constraints on Distribution and Dispersal
4.4. Future Distribution Under Climate Change and Conservation Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zandalinas, S.I.; Fritschi, F.B.; Mittler, R. Global warming, climate change, and environmental pollution: Recipe for a multifactorial stress combination disaster. Trends Plant Sci. 2021, 26, 588–599. [Google Scholar] [CrossRef]
- Yang, J.M.; Fu, Z.H.; Xiao, K.Y.; Dong, H.J.; Zhou, Y.D.; Zhan, Q.H. Climate change potentially leads to habitat expansion and increases the invasion risk of Hydrocharis (Hydrocharitaceae). Plants 2023, 12, 4124. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.R.; Cheng, C.Y.; Bai, D.F.; Zhang, Z.B. Ecological impacts of climate change and adaption strategies. Bull. Chin. Acad. Sci. 2023, 38, 518–527. [Google Scholar] [CrossRef]
- Legg, S. IPCC, 2021: Climate change 2021-the physical science basis. Interaction 2021, 49, 44–45. [Google Scholar]
- Hou, J.; Xiang, J.; Li, D.; Liu, X. Prediction of potential suitable distribution areas of Quasipaa spinosa in China based on MaxEnt optimization model. Biology 2023, 12, 366. [Google Scholar] [CrossRef] [PubMed]
- Remya, K.; Ramachandran, A.; Jayakumar, S. Predicting the current and future suitable habitat distribution of Myristica dactyloides Gaertn. using MaxEnt model in the Eastern Ghats, India. Ecol. Eng. 2015, 82, 184–188. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Wei, Y.N.; Yue, J.J.; Wang, Z.Y.; Zou, H.C.; Ji, X.D.; Zhang, S.J.; Liu, Z. Prediction of potential suitable areas and priority protection for Cupressus gigantea on the Tibetan Plateau. Plants 2024, 13, 896. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis: Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Cambridge, UK; New York, NY, USA, 2013.
- Dhami, B.; Bhusal, A.; Adhikari, B.; Miya, M.S.; Maharjan, S.K.; Neupane, D.; Adhikari, H. Habitat suitability and conflict zone mapping for the blue bull (Boselaphus tragocamelus) across Nepal. Animals 2023, 13, 937. [Google Scholar] [CrossRef]
- Warren, R.; VanDerWal, J.; Price, J.; Welbergen, J.A.; Atkinson, I.; Villegas, J.R.; Osborn, T.J.; Jarvis, A.; Shoo, L.P.; Williams, S.E. Quantifying the benefit of early climate change mitigation in avoiding biodiversity loss. Nat. Clim. Change 2014, 3, 678–682. [Google Scholar] [CrossRef]
- Anand, V.; Oinam, B.; Singh, I.H. Predicting the current and future potential spatial distribution of endangered Rucervus eldii eldii (Sangai) using MaxEnt model. Environ. Monit. Assess. 2021, 193, 147. [Google Scholar] [CrossRef]
- Wu, S.H.; Yan, J.Z.; Yang, L.; Cheng, X.; Wu, Y. Farmers and herders reclaim cropland to adapt to climate change in the eastern Tibetan Plateau: A case study in Zamtang County, China. Clim. Change 2021, 165, 69. [Google Scholar] [CrossRef]
- Momblanch, A.; Beevers, L.; Srinivasalu, P.; Kulkarni, A.; Holman, I.P. Enhancing production and flow of freshwater ecosystem services in a managed Himalayan river system under uncertain future climate. Clim. Change 2020, 162, 343–361. [Google Scholar] [CrossRef]
- Zhang, B.X.; Li, C.L.; Zuo, X.K.; Na, X.D. Impacts of climate change on potential habitat suitability of red-crowned cranes in the au-tumn migration. Acta Ecol. Sin. 2024, 44, 5194–5205. [Google Scholar] [CrossRef]
- Gao, M.; Zhao, G.; Zhang, S.; Wang, Z.; Wen, X.; Liu, L.; Zhang, C.; Tie, N.; Sa, R. Priority conservation area of Larix gmelinii under climate change: Application of an ensemble modeling. Front. Plant Sci. 2023, 14, 1177307. [Google Scholar] [CrossRef] [PubMed]
- Guisan, A.; Thuiller, W. Predicting species distribution: Offering more than simple habitat models. Ecol. Lett. 2005, 8, 993–1009. [Google Scholar] [CrossRef]
- Thuiller, W.; Lavorel, S.; Araújo, M.B.; Sykes, M.T.; Prentice, I.C. Climate change threats to plant diversity in Europe. Proc. Natl. Acad. Sci. USA 2005, 102, 8245–8250. [Google Scholar] [CrossRef]
- Gao, C.; Fan, Z.F.; Ma, C.L.; Yang, J.X.; Guo, S.L. Modelling the suitable area of Camellia reticulata under climate change based on the Biomod2. Chin J. Ecol. 2024, 43, 13. [Google Scholar]
- Buisson, L.; Thuiller, W.; Casajus, N.; Lek, S.; Grenouillet, G. Uncertainty in ensemble forecasting of species distribution. Glob. Change Biol. 2010, 16, 1145–1157. [Google Scholar] [CrossRef]
- Gao, M.L.; Tie, N.; Zhang, C.; Li, F.Z.; Wu, Y.H.; Luo, Q.H.; Wang, Z.R.; Liu, L.; Sa, R.L. Modlleing the potential distribution area of Populus davidiana in China based on the Biomod2. J. Nanjing For. Univ. (Nat. Sci. Edit.) 2024, 48, 247–255. [Google Scholar]
- Zhao, J.Y.; Jiang, C.L.; Ding, Y.Y.; Li, G.R.; Li, Q.L. Analyzing potential distribution and disturbance intensity of plateau pika in the source region of Yellow River via Biomod2 integrated model. Chin. J. Ecol. 2024, 43, 1192–1201. [Google Scholar] [CrossRef]
- Zhang, L.B.; Liang, B.; Parsons, S.; Wei, L.; Zhang, S.Y. Morphology, echolocation and foraging behaviour in two sympatric sibling species of bat (Tylonycteris pachypus and Tylonycteris robustula) (Chiroptera: Vespertilionidae). J. Zool. 2007, 271, 344–351. [Google Scholar] [CrossRef]
- Eguren, R.E.; McBee, K. Tylonycteris pachypus (Chiroptera: Vespertilionidae). Mamm. Species 2014, 46, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.B.; Lu, L.R.; Zhou, S.Y.; Dai, Q.; Zhao, H.H.; Luo, G.H.; Zhang, S.Y. Comparison of the echolocation signals in two species of flat-headed bats at flying. Zool. Res. 2002, 23, 296–300. [Google Scholar]
- Liang, J.; Yang, J.; Xie, H.W.; Peng, X.W.; He, X.Y.; Sun, Y.X.; Zhang, L.B. Impact of external odor on self-grooming of lesser flat-headed bats, Tylonycteris pachypus. Ecol. Evol. 2019, 9, 8113–8118. [Google Scholar] [CrossRef]
- Zhang, L.B.; Liang, B.; Zhou, S.Y.; Lu, L.R.; Zhang, S.Y. Group structure of lesser flat -headed bat Tylonycteris pachypus and greater flat -headed bat T. robustula. Acta Zool. Sinica 2004, 50, 326–333. [Google Scholar]
- Zhang, L.B.; Liang, B.; Zhou, S.Y.; Lu, L.R.; Zhang, S.Y. Prey Selection of Tylonycteris pachypus and T. robustula(Chiroptera: Vesper-tilioninae) in Guangxi, China. Zool. Res. 2004, 25, 105–110. [Google Scholar]
- Hua, P.Y.; Zhang, L.B.; Zhu, G.J.; Jones, G.; Zhang, S.Y.; Rossiter, S.J. Hierarchical polygyny in multiparous lesser flat-headed bats. Mol. Ecol. 2011, 20, 3669–3680. [Google Scholar] [CrossRef]
- Guo, W.J.; Liang, X.L.; Wu, Y.; Yu, W. The complete mitochondrial genome of Tylonycteris fulvida (Chiroptera: Vespertilionidae) in South China. Mitochondrial DNA B 2021, 6, 3274–3275. [Google Scholar] [CrossRef]
- Chen, M.; Huang, C.J.; Qiu, Y.X.; Qiu, B.; Lin, S.W.; Yu, W.H.; Xu, Z.X.; Pan, W.Q.; Wu, Y. The protection of habitat of the en-dangered bats Tylonycteris pachypus in Yuexiu Park, Guangzhou. Sichuan J. Zool. 2013, 32, 205–211. [Google Scholar]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1 km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Zhao, J.; Wang, M.M.; Li, Z.P.; Lin, S.; Chen, H. Potential distribution prediction of Amaranthus palmeri S. Watson in China under current and future climate scenarios. Ecol. Evol. 2022, 12, e9505. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Han, S.Y.; Li, Y.; Wang, J.M.; He, H.X. Impacts and assessment of climate change on the global distribution of potentially suitable habitats for Panthera uncia. Acta Ecol. Sin. 2023, 43, 1412–1425. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Lai, Y.; Shao, H. Comparative study of potential habitats for two endemic grassland caterpillars on the Qinghai-Tibet plateau based on BIOMOD2 and land use data. Insects 2024, 15, 781. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, C.; Xie, G.; Wang, P.; Wang, W. Assessment of the potential suitable habitat of Apriona rugicollis Chevrolat, 1852 (Coleoptera: Cerambycidae) under climate change and human activities based on the Biomod2 ensemble model. Insects 2024, 15, 930. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhang, X.; Zhou, Y.; Zong, S. Prediction of the global distribution of Arhopalus rusticus under future climate change scenarios of the CMIP6. Forests 2024, 15, 955. [Google Scholar] [CrossRef]
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2010, 43, 1223–1232. [Google Scholar] [CrossRef]
- Fawcett, T. An introduction to ROC analysis. Pattern Recogn. Lett. 2005, 27, 861–874. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, H.P.; Chen, C.; Fan, S.Y.; Wei, J.F.; Cai, B.; Zhang, H.F. Potential global distribution of Paracoccus marginatus, under climate change conditions, using MaxEnt. Insects 2024, 15, 98. [Google Scholar] [CrossRef]
- de Oliveira, L.R.D.; Brito, G.; Gama, M.; Ovando, X.; Anastácio, P.; Cardoso, S. Non-native decapods in south America: Risk assessment and potential impacts. Diversity 2023, 15, 841. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Hou, X.Y.; Wang, X.L.; Li, X.W.; Zhang, Y.X. Habitat suitability assessment for Saunders’s Gull (Saundersilarus saundersi) in the Yellow River Delta, China. Ecol. Inform. 2024, 79, 102393. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing whether ensemble modelling is advantageous for maximising predictive performance of species distribution models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Chen, L.L.; Li, Z.F.; Liu, T.M. Changes in potentially suitable habitats for Epilobium hirsutum based on Biomod2 models. Horticult Seed 2023, 43, 9–15. [Google Scholar] [CrossRef]
- Medway, L.; Marshall, A.G. Roosting associations of flat-headed bats, Tylonycteris species (Chiroptera: Vespertilionidae) in Malaysia. J. Zool. 1972, 168, 463–482. [Google Scholar] [CrossRef]
- Liu, B.L.; Miao, R.Z.; Chen, X.S.; Ma, S.; Yang, Y.J.; Liu, G.; Zhu, H.Q. Ensemble model-based prediction of suitable distribution of Moschus moschiferus in the eastern mountainous area of Jilin Province. Sichuan J. Zool. 2023, 42, 121–130. [Google Scholar] [CrossRef]
- Hua, P.Y.; Zhang, L.B.; Guo, T.T.; Flanders, J.; Zhang, S.Y. Dispersal, mating events and fine-scale genetic structure in the lesser flat-headed bats. PLoS ONE 2013, 8, e54428. [Google Scholar] [CrossRef]
- Noga, K.S.; Tamar, D. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 153–181. [Google Scholar] [CrossRef]
- Zeng, X. The Research on Bambusa chungii’s Characterstics of Biology and Physiology in Different Climate Regions. Master’s Thesis, Central South University of Forestry and Technology, Changsha, China, 2011. [Google Scholar]
- Zhang, L.B.; Hong, T.Y.; Wei, L.; Zhu, G.J.; Zhang, G.L.; Gong, Y.Y.; Yang, J.; Hu, H.J. Dispersal behaviour of the lesser flat-headed bat, Tylonycteris pachypus (Chiroptera: Vespertilionidae). Acta Theriol. Sinica 2011, 31, 244–250. [Google Scholar]
- Che, Y.J.; Zhao, J.; Zhang, M.J.; Wang, S.J.; Qi, Y. Potential vegetation and its sensitivity under different climate change scenarios from 2070 to 2099 in China. Acta Ecol. Sinica 2016, 36, 2885–2895. [Google Scholar] [CrossRef]
- Korine, C.; Adams, R.; Russo, D.; Fisher-Phelps, M.; Jacobs, D. Bats and water: Anthropogenic alterations threaten global bat populations. In Bats in the Anthropocene: Conservation of Bats in a Changing World; Voigt, C.C., Kingston, T., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 215–241. [Google Scholar]
- Sherwin, H.A.; Montgomery, W.I.; Lundy, M.G. The impact and implications of climate change for bats. Mammal. Rev. 2013, 43, 171–182. [Google Scholar] [CrossRef]
- Williams, P.; Hannah, L.; Andelman, S.; Midgley, G.; Araújo, M.; Hughes, G.; Manne, L.; Martinez-meye, E.; Pearson, R. Planning for climate change: Identifying minimum-dispersal corridors for the cape Proteaceae. Conserv. Biol. 2005, 19, 1063–1074. [Google Scholar] [CrossRef]
- Zhang, T.Z.; Jiang, F.; Xu, B.; Li, B.; Liang, C.B.; Gu, H.F. Research advances in conservation and management of endangered mammals on the Qinghai−Tibet Plateau. Acta Theriol. Sinica 2022, 42, 490–507. [Google Scholar]
- Vonhof, M.J.; Whitehead, H.; Fenton, M.B. Analysis of Spix’s discwinged bat association patterns and roosting home ranges reveal a novel social structure among bats. Anim. Behav. 2004, 68, 507–521. [Google Scholar] [CrossRef]

| Category | Environmental Variable | Unit | Participating in Modeling | % Contribution |
|---|---|---|---|---|
| Bioclimatic | Annual mean air temperature(Bio1) | °C | ||
| Mean diurnal range (Mean of monthly (max temp–min temp)) (Bio2) | °C | √ | 1.18 | |
| Isothermality (Bio2/Bio7) (×100) (Bio3) | / | |||
| Variation in temperature seasonality (Bio4) | % | |||
| Max temperature of warmest month (Bio5) | °C | √ | 38.38 | |
| Min temperature of coldest month (Bio6) | °C | √ | 40.90 | |
| Temperature annual range (BIO5–BIO6) (Bio7) | °C | |||
| Mean temperature of wettest quarter (Bio8) | °C | |||
| Mean temperature of driest quarter (Bio9) | °C | |||
| Mean temperature of warmest quarter (Bio10) | °C | |||
| Mean temperature of coldest quarter (Bio11) | °C | |||
| Annual precipitation (Bio12) | mm | |||
| Precipitation of wettest month (Bio13) | mm | |||
| Precipitation of driest month (Bio14) | mm | |||
| Precipitation seasonality (Coefficient of variation) (Bio15) | / | |||
| Precipitation of wettest quarter (Bio16) | mm | √ | 11.09 | |
| Precipitation of driest quarter (Bio17) | mm | √ | 6.32 | |
| Precipitation of warmest quarter (Bio18) | mm | √ | 1.86 | |
| Precipitation of coldest quarter (Bio19) | mm | |||
| Topographic | Elevation (Alt) | M | ||
| Anthropogenic | The land use and cover change (Lucc) | / | √ | 0.27 |
| Model | AUC | TSS | ||||
|---|---|---|---|---|---|---|
| Mean | SD | CV | Mean | SD | CV | |
| GBM | 0.963 | 0.015 | 0.016 | 0.766 | 0.083 | 0.108 |
| MARS | 0.947 | 0.022 | 0.023 | 0.773 | 0.083 | 0.108 |
| XGBOOST | 0.948 | 0.022 | 0.023 | 0.683 | 0.060 | 0.088 |
| GLM | 0.931 | 0.023 | 0.024 | 0.715 | 0.063 | 0.087 |
| FDA | 0.901 | 0.030 | 0.033 | 0.643 | 0.094 | 0.146 |
| MAXENT | 0.887 | 0.033 | 0.037 | 0.715 | 0.064 | 0.089 |
| CTA | 0.876 | 0.048 | 0.055 | 0.719 | 0.079 | 0.110 |
| SRE | 0.851 | 0.064 | 0.075 | 0.701 | 0.128 | 0.182 |
| ANN | 0.839 | 0.044 | 0.052 | 0.613 | 0.097 | 0.159 |
| RF | 0.956 | 0.013 | 0.013 | 0.646 | 0.064 | 0.100 |
| Ensemble models | 0.981 | 0.001 | 0.001 | 0.877 | 0.022 | 0.026 |
| Climate Scenarios | Poorly Suitable Habitat | Moderately Suitable Habitat | Highly Suitable Habitat | Total Area | Total Change | Area Percentage (%) |
|---|---|---|---|---|---|---|
| Current | 83.244 | 71.368 | 291.893 | 446.505 | / | 37.10 |
| SSP126_2050 | 75.209 | 53.864 | 187.914 | 316.988 | −129.518 | 26.34 |
| SSP126_2090 | 89.067 | 59.679 | 222.293 | 371.038 | −75.467 | 30.83 |
| SSP245_2050 | 75.837 | 55.819 | 192.804 | 324.460 | −122.045 | 26.96 |
| SSP245_2090 | 76.469 | 51.947 | 146.565 | 274.980 | −171.525 | 22.85 |
| SSP370_2050 | 67.626 | 52.974 | 171.727 | 292.327 | −154.179 | 24.29 |
| SSP370_2090 | 52.754 | 29.580 | 90.942 | 173.277 | −273.228 | 14.40 |
| SSP585_2050 | 75.859 | 53.244 | 156.306 | 285.409 | −161.096 | 23.71 |
| SSP585_2090 | 46.386 | 27.115 | 85.595 | 159.095 | −287.410 | 13.22 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, K.; Shao, W.; Li, Y.; Wang, L.; Lin, Z.; Guo, L.; Wei, L. Biomod2 Modeling for Predicting Suitable Distribution of Bamboo Bat (Tylonycteris pachypus) Under Climate Change. Animals 2025, 15, 1164. https://doi.org/10.3390/ani15081164
Chen K, Shao W, Li Y, Wang L, Lin Z, Guo L, Wei L. Biomod2 Modeling for Predicting Suitable Distribution of Bamboo Bat (Tylonycteris pachypus) Under Climate Change. Animals. 2025; 15(8):1164. https://doi.org/10.3390/ani15081164
Chicago/Turabian StyleChen, Kai, Weiwei Shao, Yalei Li, Lijin Wang, Zhihua Lin, Ling Guo, and Li Wei. 2025. "Biomod2 Modeling for Predicting Suitable Distribution of Bamboo Bat (Tylonycteris pachypus) Under Climate Change" Animals 15, no. 8: 1164. https://doi.org/10.3390/ani15081164
APA StyleChen, K., Shao, W., Li, Y., Wang, L., Lin, Z., Guo, L., & Wei, L. (2025). Biomod2 Modeling for Predicting Suitable Distribution of Bamboo Bat (Tylonycteris pachypus) Under Climate Change. Animals, 15(8), 1164. https://doi.org/10.3390/ani15081164






