Exploring Captive Giant Panda Reproduction: Maternal and Offspring Factor Correlations from 324 Breeding Events
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection and Selection Criteria
2.2. Female Reproductive History
2.3. Breeding Management Consistency
2.4. Data Analyzed
3. Results
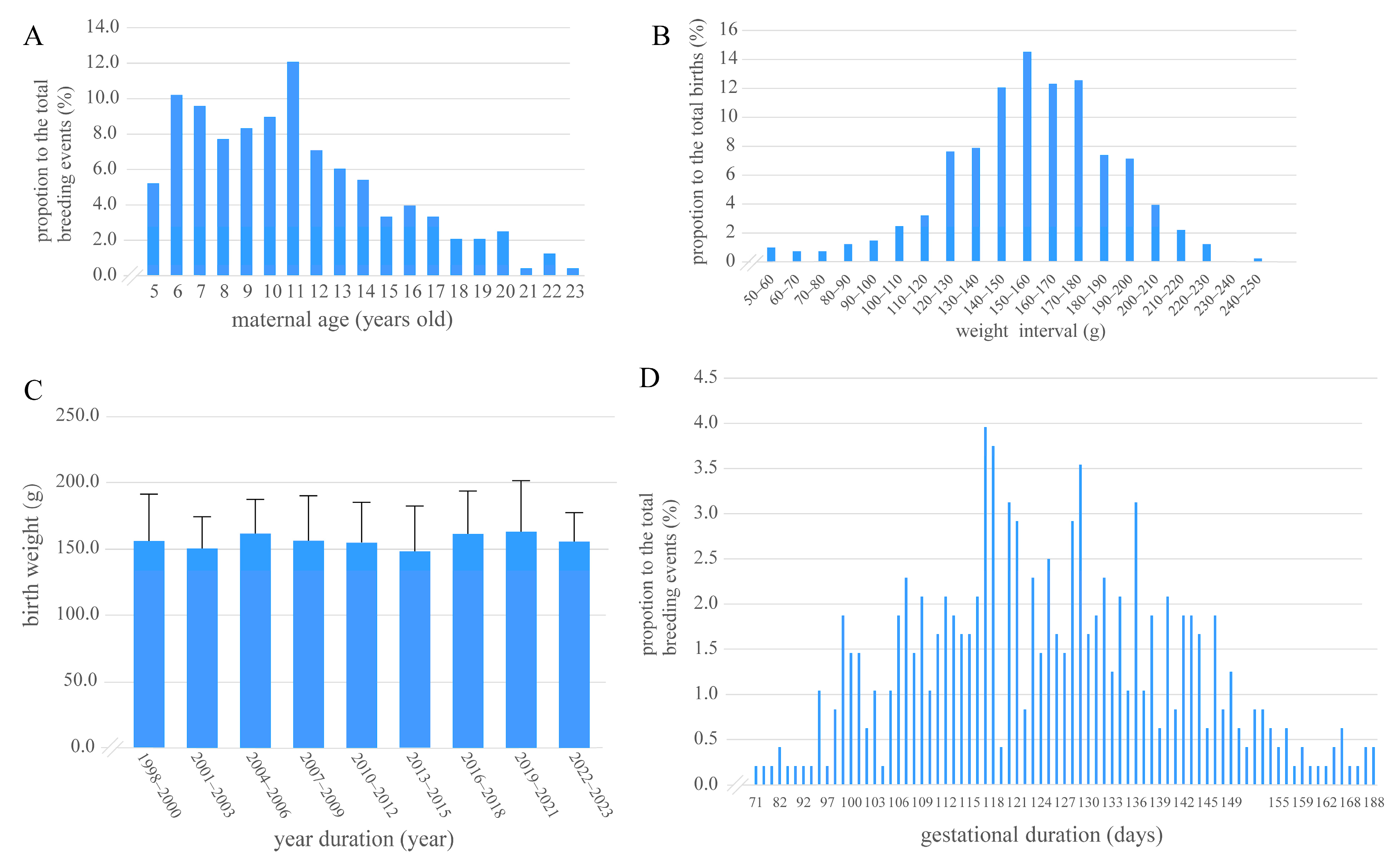
3.1. Distribution of Maternal Age, Cub Birth Weight, Gestational Duration, Number of Pregnancies, and Interbirth Intervals
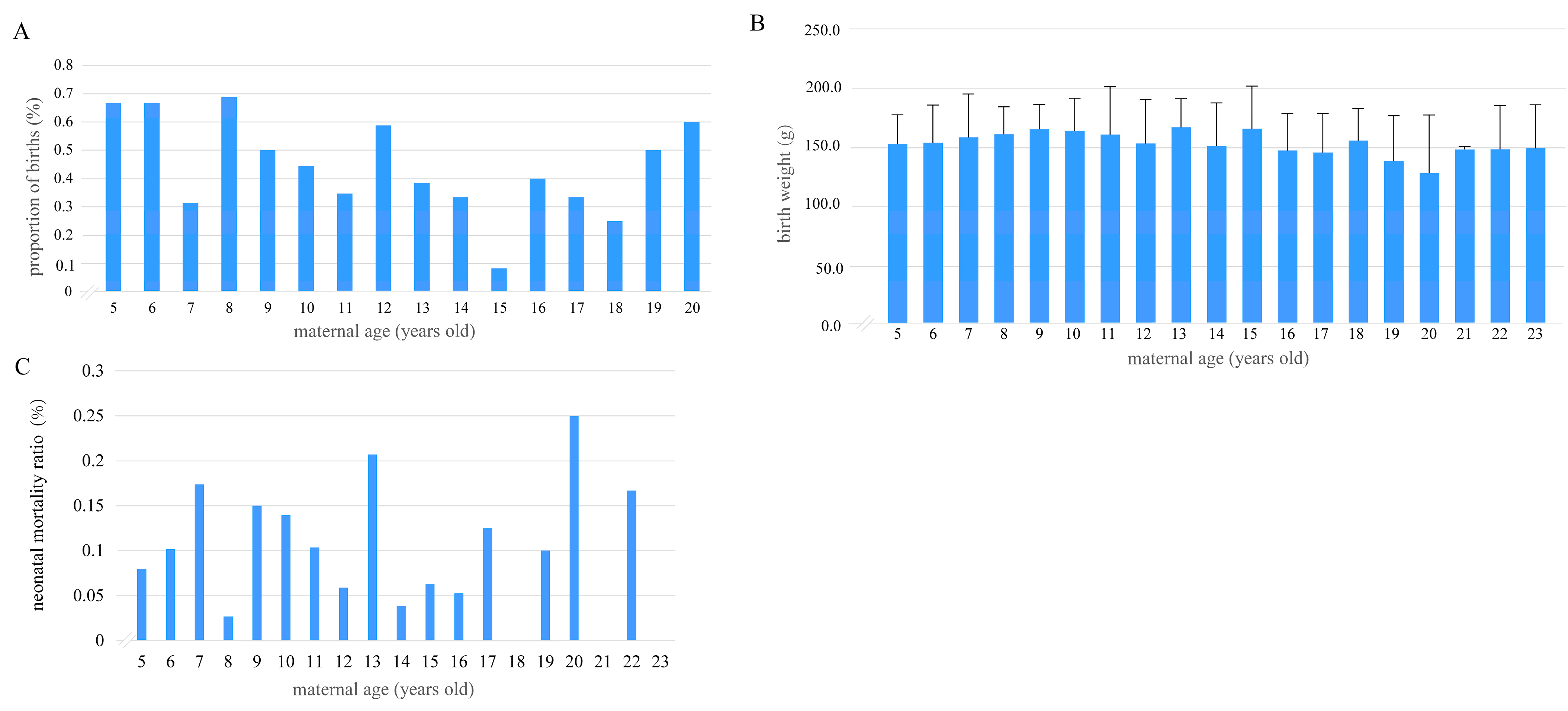
3.2. Relationships Between Maternal Age and the Number of Cubs per Breeding Event, Cub Birth Weight, and Neonatal Mortality Ratio
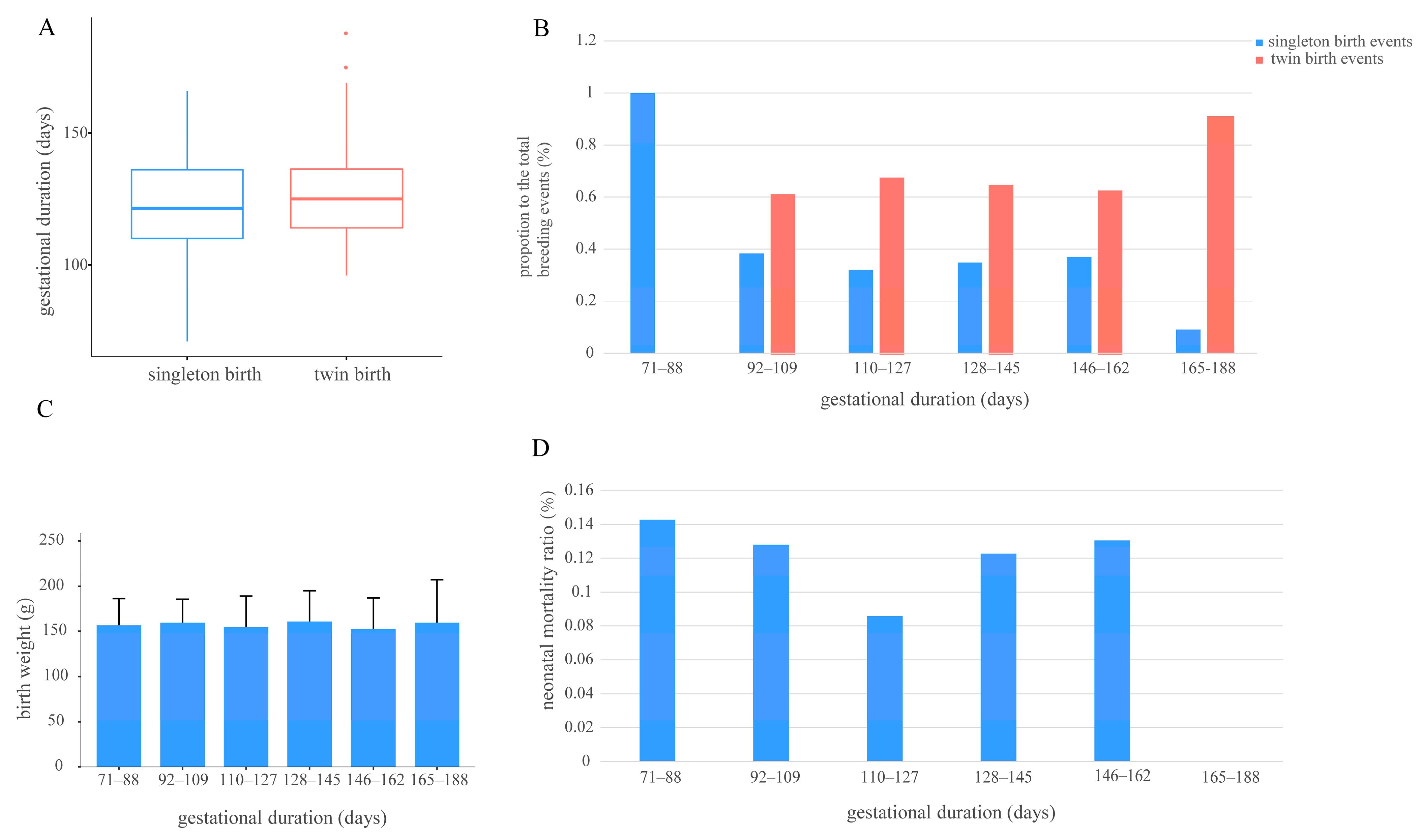
3.3. Relationships Between Gestational Duration and the Number of Cubs per Breeding Event, Cub Birth Weight, and Neonatal Mortality Ratio
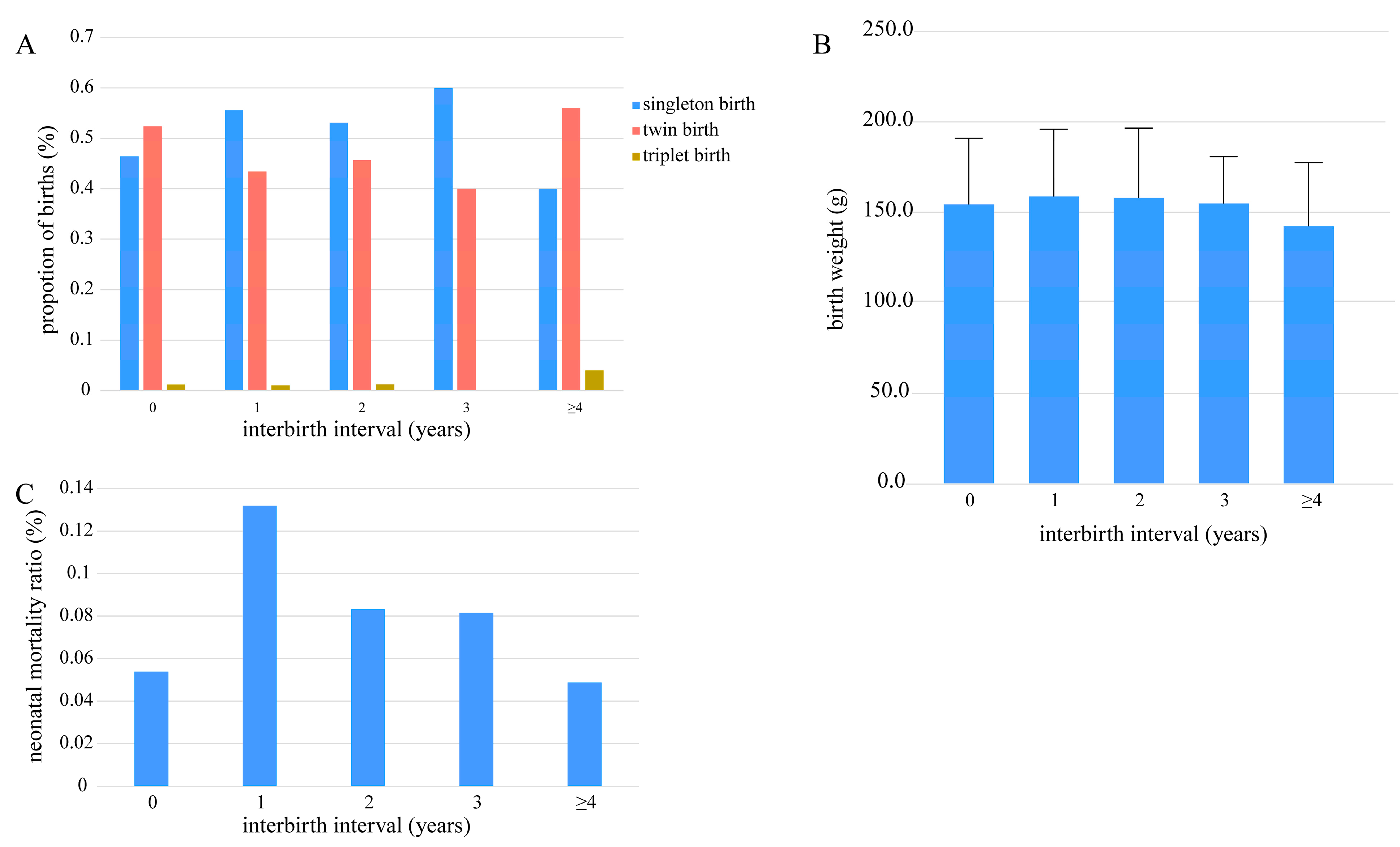
3.4. Relationships Between the Interbirth Interval and the Number of Cubs per Breeding Event, Cub Birth Weight, and Neonatal Mortality Ratio
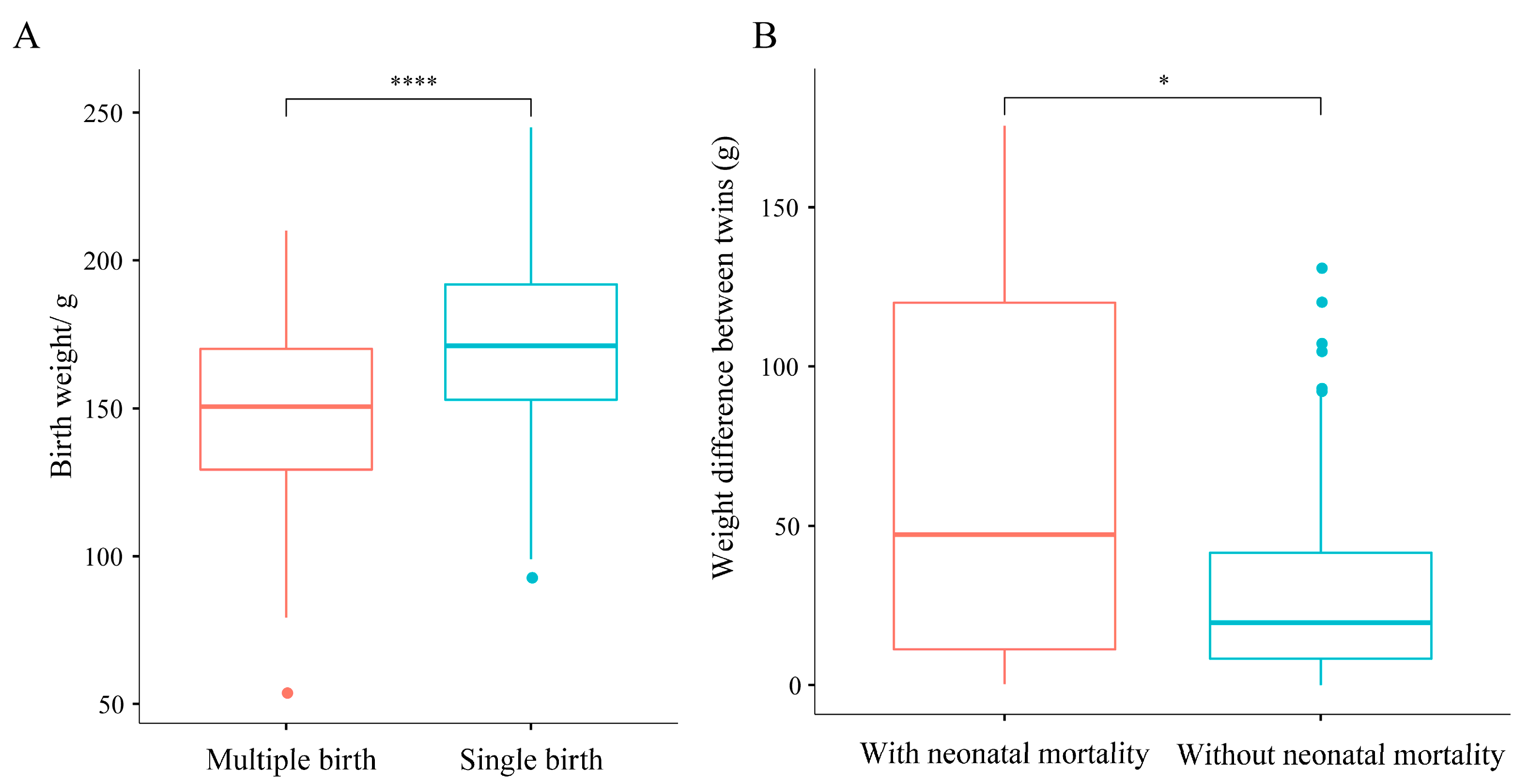
3.5. Relationships Between the Number of Cubs per Breeding Event and Cub Birth Weight, and Neonatal Mortality Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Che, T.; Wang, C.; Jin, L.; Wei, M.; Wu, K.; Zhang, Y.; Zhang, H.; Li, D. Estimation of the growth curve and heritability of the growth rate for giant panda (Ailuropoda melanoleuca) cubs. Genet. Mol. Res. 2015, 14, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Lindburg, D.G.; Pan, W.; Forney, K.A.; Wang, D. The reproductive strategy of giant pandas (Ailuropoda melanoleuca): Infant growth and development and mother–infant relationships. J. Zool. 2001, 253, 141–155. [Google Scholar] [CrossRef]
- Czekala, N.; McGeehan, L.; Steinman, K.; Xuebing, L.; Gual-Sil, F. Endocrine monitoring and its application to the management of the giant panda. Zoo Biol. Publ. Affil. Am. Zoo Aquar. Assoc. 2003, 22, 389–400. [Google Scholar] [CrossRef]
- Liao, M.-J.; Zhu, M.-Y.; Zhang, Z.-H.; Zhang, A.-J.; Li, G.-H.; Sheng, F.-J. Cloning and sequence analysis of FSH and LH in the giant panda (Ailuropoda melanoleuca). Anim. Reprod. Sci. 2003, 77, 107–116. [Google Scholar] [CrossRef]
- Sutherland-Smith, M.; Morris, P.J.; Silverman, S. Pregnancy detection and fetal monitoring via ultrasound in a giant panda (Ailuropoda melanoleuca). Zoo Biol. 2004, 5, 449–461. [Google Scholar] [CrossRef]
- Zhang, H.; Li, D.; Wang, C.; Hull, V. Delayed implantation in giant pandas: The first comprehensive empirical evidence. Reproduction 2009, 138, 979–986. [Google Scholar] [CrossRef]
- National Forestry and Grassland Administration. National Forestry and Grassland Administration in the First Quarter of 2024 Routine Press Conference; National Forestry and Grassland Administration: Beijing, China, 2024. Available online: https://www.forestry.gov.cn/ (accessed on 14 April 2025).
- Zhou, S.; Luo, B.; Song, S.; Huang, J.; Li, W.; Zhou, J.; He, S.; Zhang, H. Analysis of Factors Influencing the Viability of Captive-Bred Pandas:Based on the Data of 2019 International Studbook for Giant Panda. Sichuan J. Zool. 2021, 40, 10. [Google Scholar]
- Wang, H.; Hou, R.; Liu, Y.; Cai, Z.; Luo, L.; Huang, H.; Zhang, Z. An Observational Analysis of the Relevant Factors on Twin Rate of Giant Panda. Sichuan J. Zool. 2015, 34, 650–655. [Google Scholar]
- China Conservation and Research Center for the Giant Panda. Available online: https://www.forestry.gov.cn/c/dxm/jgsz/311545.jhtml (accessed on 1 January 2022).
- Simopoulos, A.P.; Van Itallie, T.B. Body weight, health, and longevity. Ann. Intern. Med. 1984, 100, 285–295. [Google Scholar] [CrossRef]
- Lee, W.; Balasubramaniam, M.; Deter, R.; Hassan, S.; Gotsch, F.; Kusanovic, J.; Gonçalves, L.; Romero, R. Fetal growth parameters and birth weight: Their relationship to neonatal body composition. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2009, 33, 441–446. [Google Scholar] [CrossRef]
- Reynolds, L.P.; Borowicz, P.P.; Caton, J.S.; Crouse, M.S.; Dahlen, C.R.; Ward, A.K. Developmental programming of fetal growth and development. Vet. Clin. N. Am. Food Anim. Pract. 2019, 35, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, X.; Wu, H.; Qiu, J.; Zhang, Y.; Huang, S. Variation of body weight in all age groups of captive giant pandas (Ailuropoda melanoleuca). Acta Theriol. Sin. 2021, 41, 468–475. [Google Scholar]
- Wang, P.; Li, D.; Zhang, H.; Zhang, G.; Wei, R.; Zhou, X.; Huang, Y. Husbandary of Giant Panda; China Forestry Publishing House: Beijing, China, 2003. [Google Scholar]
- Zeng, W.; Zhou, Y.; Zhang, Y.; Wei, M.; Zhang, G.; Li, D.; Xie, Q.; Liu, H.; Dong, C.; Xiao, Y.; et al. Evaluation of the Artificial Rearing Level of Giant Panda Cubs (0~120 Days Old). Graziery Vet. Sci. (Electron. Version) 2020, 4, 5–7. [Google Scholar]
- Huang, X.; Feng, Y.; Lan, J.; Wu, K.; Yang, K.; Peng, W.; Yuan, B.; Xie, Y.; Wang, S.; Chen, M. Changes in body weight of captive giant panda cubs at early age. Acta Theriol. Sin. 2021, 2, 224–234. [Google Scholar]
- Chu, Q.; Yu, J.; Sun, Q.; Xu, C.; Xie, C.; Min, Y.; Jin, X.; Zeng, W.; Li, D.; Wu, F. Growth and Development Differences of Panda Cubs Raised by Different Methods in Shanghai and Ya’an. Sichuan J. Zool. 2019, 38, 77–86. [Google Scholar]
- Hou, R.; Huang, X.; Li, G.; Yu, J.; Ye, Z.; Yang, Z.; Zhang, Z.; Zuo, H. Hand-rearing on newborn infant of low body weight of giant panda (Ailuropoda Melanleuca). Acta Theriol. Sin. 2000, 20, 146–150. [Google Scholar]
- Nie, Y.; Swaisgood, R.R.; Zhang, Z.; Liu, X.; Wei, F. Reproductive competition and fecal testosterone in wild male giant pandas (Ailuropoda melanoleuca). Behav. Ecol. Sociobiol. 2012, 66, 721–730. [Google Scholar] [CrossRef]
- Kersey, D.C.; Wildt, D.E.; Brown, J.L.; Snyder, R.J.; Huang, Y.; Monfort, S.L. Unique biphasic progestagen profile in parturient and non-parturient giant pandas (Ailuropoda melanoleuca) as determined by faecal hormone monitoring. Reproduction 2010, 140, 183–193. [Google Scholar] [CrossRef]
- Shenfei, W.; Mengshi, Z.; Feiping, L.; Xianbiao, H.; Yan, L.; Juan, W.; Xiangyu, L.; Rong, H.; Yuliang, L.; Kailai, C. Preliminary urinary profiles of prolactin during gestation and the synergistic effects of embryonic diapause hormones in the giant panda (Ailuropoda melanoleuca). Biol. Reprod. 2025, 112, 319–330. [Google Scholar] [CrossRef]
- Martorell, R.; González-Cossío, T. Maternal nutrition and birth weight. Am. J. Phys. Anthropol. 1987, 30, 195–220. [Google Scholar] [CrossRef]
- Wu, G.; Imhoff-Kunsch, B.; Girard, A.W. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr. Perinat. Epidemiol. 2012, 26, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Ptak, G.E.; Tacconi, E.; Czernik, M.; Toschi, P.; Modlinski, J.A.; Loi, P. Embryonic diapause is conserved across mammals. PLoS ONE 2012, 7, e33027. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wang, P.; Zhang, G.; Zhang, H.; Li, D.; Du, J.; Wei, R.; Tang, C.; Spindler, R.E.; Wildt, D.E. Use of artificial insemination to enhance propagation of giant pandas at the Wolong Breeding Center. In Proceedings of the International Symposium on Assisted Reproductive Technologies (ART) for Conservation and Genetic Management of Wildlife; 2002. Available online: https://repository.si.edu/bitstream/handle/10088/11601/Huang2002.pdf (accessed on 14 April 2025).
- Smucny, D.A.; Abbott, D.H.; Mansfield, K.G.; Schultz-Darken, N.J.; Yamamoto, M.E.; Alencar, A.I.; Tardif, S.D. Reproductive output, maternal age, and survivorship in captive common marmoset females (Callithrix jacchus). Am. J. Primatol. Off. J. Am. Soc. Primatol. 2004, 64, 107–121. [Google Scholar] [CrossRef]
- Tardif, S.D.; Araujo, A.; Arruda, M.F.; French, J.A.; Sousa, M.B.C.; Yamamoto, M.E. Reproduction and aging in marmosets and tamarins. Interdiscip. Top. Gerontol. 2008, 36, 29–48. [Google Scholar]
- Lunn, N.; Boyd, I.; Croxall, J. Reproductive performance of female Antarctic fur seals: The influence of age, breeding experience, environmental variation and individual quality. J. Anim. Ecol. 1994, 63, 827–840. [Google Scholar] [CrossRef]
- Robertson, R.J.; Rendell, W.B. A long-term study of reproductive performance in tree swallows: The influence of age and senescence on output. J. Anim. Ecol. 2001, 70, 1014–1031. [Google Scholar] [CrossRef]
- DeFranco, E.A.; Stamilio, D.M.; Boslaugh, S.E.; Gross, G.A.; Muglia, L.J. A short interpregnancy interval is a risk factor for preterm birth and its recurrence. Am. J. Obstet. Gynecol. 2007, 197, e261–e264. [Google Scholar] [CrossRef]
- Stieglitz, J.; Beheim, B.A.; Trumble, B.C.; Madimenos, F.C.; Kaplan, H.; Gurven, M. Low mineral density of a weight-bearing bone among adult women in a high fertility population. Am. J. Phys. Anthropol. 2015, 156, 637–648. [Google Scholar] [CrossRef]
- Glick, I.; Kadish, E.; Rottenstreich, M. Management of pregnancy in women of advanced maternal age: Improving outcomes for mother and baby. Int. J. Women’s Health 2021, 13, 751–759. [Google Scholar] [CrossRef]
- Fraser, A.M.; Brockert, J.E.; Ward, R.H. Association of young maternal age with adverse reproductive outcomes. New Engl. J. Med. 1995, 332, 1113–1118. [Google Scholar] [CrossRef]
- Shirasuna, K.; Iwata, H. Effect of aging on the female reproductive function. Contracept. Reprod. Med. 2017, 2, 23. [Google Scholar] [CrossRef] [PubMed]
- Talbert, G.B. Effect of maternal age on reproductive capacity. Am. J. Obstet. Gynecol. 1968, 102, 451–477. [Google Scholar] [CrossRef]
- Brunner Huber, L.R.; Smith, K.; Sha, W.; Vick, T. Interbirth interval and pregnancy complications and outcomes: Findings from the pregnancy risk assessment monitoring system. J. Midwifery Women’s Health 2018, 63, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Shults, R.A.; Arndt, V.; Olshan, A.F.; Martin, C.F.; Royce, R.A. Effects of short interpregnancy intervals on small-for-gestational age and preterm births. Epidemiology 1999, 10, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.A.; Sebire, N.J. Genetics and developmental pathology of twinning. Semin. Fetal Neonatal Med. 2010, 15, 313–318. [Google Scholar] [CrossRef]
- Leroy, F. Major fetal hazards in multiple pregnancy. Acta Genet. Medicae Gemellol. Twin Res. 1976, 25, 299–306. [Google Scholar] [CrossRef]
- Willis, E.L.; Kersey, D.C.; Durrant, B.S.; Kouba, A.J. The acute phase protein ceruloplasmin as a non-invasive marker of pseudopregnancy, pregnancy, and pregnancy loss in the giant panda. PLoS ONE 2011, 6, e21159. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, B.; Yang, B.; Zhou, Q.; Li, G.; Lai, Y.; Zeng, W.; Zhang, G.; Li, D.; Yang, L. Exploring Captive Giant Panda Reproduction: Maternal and Offspring Factor Correlations from 324 Breeding Events. Animals 2025, 15, 1182. https://doi.org/10.3390/ani15081182
Luo B, Yang B, Zhou Q, Li G, Lai Y, Zeng W, Zhang G, Li D, Yang L. Exploring Captive Giant Panda Reproduction: Maternal and Offspring Factor Correlations from 324 Breeding Events. Animals. 2025; 15(8):1182. https://doi.org/10.3390/ani15081182
Chicago/Turabian StyleLuo, Bo, Bo Yang, Qiang Zhou, Guo Li, Yanwu Lai, Wen Zeng, Guiquan Zhang, Desheng Li, and Liu Yang. 2025. "Exploring Captive Giant Panda Reproduction: Maternal and Offspring Factor Correlations from 324 Breeding Events" Animals 15, no. 8: 1182. https://doi.org/10.3390/ani15081182
APA StyleLuo, B., Yang, B., Zhou, Q., Li, G., Lai, Y., Zeng, W., Zhang, G., Li, D., & Yang, L. (2025). Exploring Captive Giant Panda Reproduction: Maternal and Offspring Factor Correlations from 324 Breeding Events. Animals, 15(8), 1182. https://doi.org/10.3390/ani15081182





