Mixtures of Algal Oil and Terrestrial Oils in Diets of Tiger Puffer (Takifugu rubripes)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Diets
2.2. Feeding Procedure and Sampling
2.3. Proximate Composition Analysis for Fish and Diets
2.4. Biochemical Parameters of Serum
2.5. Fatty Acid Composition Histological Structure
2.6. Histological Structure
2.7. cDNA Preparation and Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.8. Calculation and Statistical Analysis
3. Results
3.1. Growth Performance, Somatic Indices, and Body Composition
3.2. Serum Biochemical Parameters
3.3. Fatty Acid Profiles in the Whole Fish, Muscle, and Liver Samples
3.4. Histological Structure of Tissues
3.5. Gene Expression
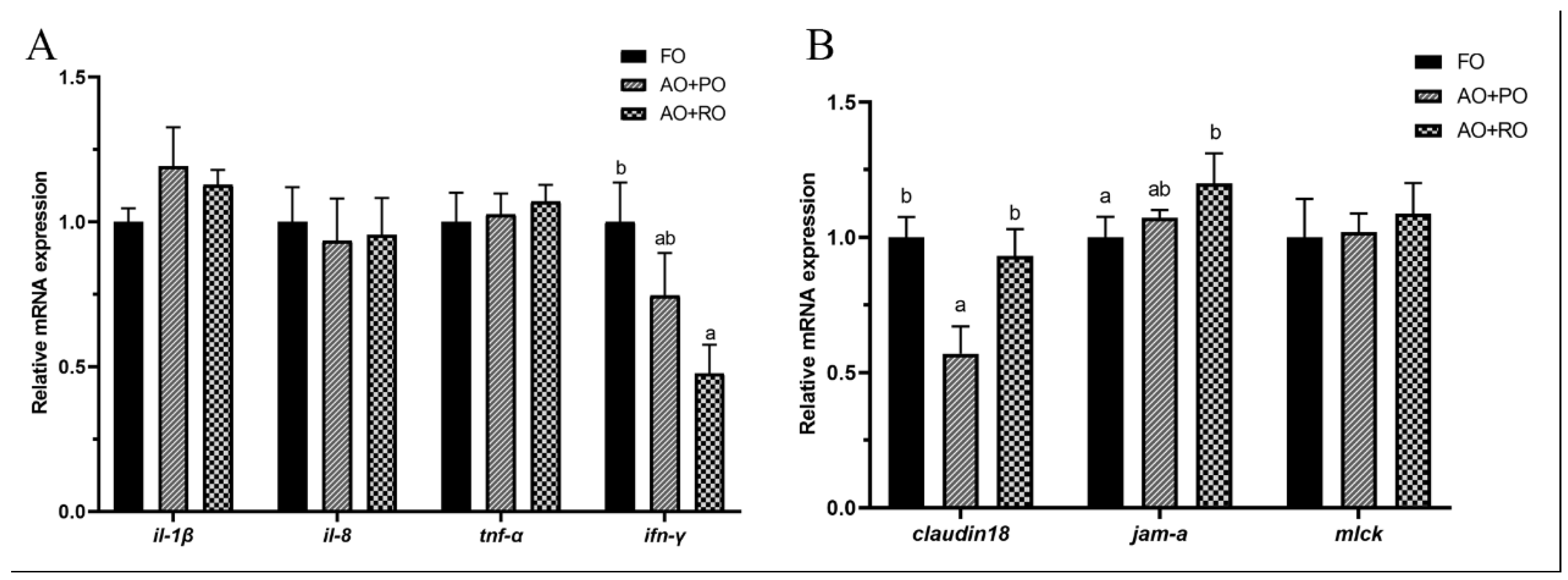
3.5.1. Liver Fibrosis and Inflammation Related Gene Expression
3.5.2. Intestinal Inflammation and Intestinal Barrier-Related Gene Expression
3.5.3. Muscle Differentiation Apoptosis Related Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Non-gene name | |
| LC-PUFA | long-chain polyunsaturated fatty acids |
| EPA | eicosapentaenoic acid |
| DHA | docosahexaenoic acid |
| ARA | arachidonic acid |
| FO | fish oil |
| AO | algal oil |
| SFA | saturated fatty acid |
| MUFA | monounsaturated fatty acid |
| PO | poultry oil |
| RO | rapeseed oil |
| RT-qPCR | real-time quantitative polymerase chain reaction |
| Gene name | |
| myod | myogenic differentiation antigen |
| myog | myogenin |
| myf6 | myogenic factor 6 |
| myf5 | myogenic factor 5 |
| bax | bcl-2-associated x |
| bcl-2 | b-cell lymphoma-2 |
| acta2 | actin alpha 2 |
| il-1β | interleukin—1β |
| il-8 | interleukin 8 |
| tnf-α | tumor necrosis factor alpha |
| ifn-γ | interferon gamma |
| jam-a | junctional adhesion molecule a |
| mlck | myosin light chain kinase |
| rpl19 | ribosomal protein l19 |
| rpl13 | ribosomal protein l13 |
| keap1 | Kelch-like ECH-associated protein 1 |
| nrf2 | nuclear factor erythroid 2 |
| col1a2 | collagen type I alpha 2 chain |
References
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, D.; Zhou, M.M.; Du, L.; Xu, J.; Xue, C.H.; Wang, Y.M. Comparative Study of EPA-enriched Phosphatidylcholine and EPA-enriched Phosphatidylserine on Lipid Metabolism in Mice. J. Oleo Sci. 2016, 65, 593–602. [Google Scholar] [CrossRef]
- Liu, J.H.; Wang, Q.; You, Q.L.; Li, Z.L.; Hu, N.Y.; Wang, Y.; Jin, Z.L.; Li, S.J.; Li, X.W.; Yang, J.M.; et al. Acute EPA-induced learning and memory impairment in mice is prevented by DHA. Nat. Commun. 2020, 11, 5465. [Google Scholar] [CrossRef]
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2021, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, F.R.; Meng, X.X.; Cui, X.S.; Ma, Q.; Wei, Y.L.; Liang, M.Q.; Xu, H.G. Fish Oil Replacement with Poultry Oil in the Diet of Tiger Puffer (Takifugu rubripes): Effects on Growth Performance, Body Composition, and Lipid Metabolism. Aquac. Nutr. 2022, 2022, 2337933. [Google Scholar] [CrossRef]
- Chen, Y.F.; Sun, Z.Z.; Liang, Z.M.; Xie, Y.D.; Su, J.L.; Luo, Q.L.; Zhu, J.Y.; Liu, Q.Y.; Han, T.; Wang, A.L. Effects of dietary fish oil replacement by soybean oil and l-carnitine supplementation on growth performance, fatty acid composition, lipid metabolism and liver health of juvenile largemouth bass, Micropterus salmoides. Aquaculture 2020, 516, 734596. [Google Scholar] [CrossRef]
- Zatti, K.M.; Ceballos, M.J.; Vega, V.V.; Denstadli, V. Full replacement of fish oil with algae oil in farmed Atlantic salmon (Salmo salar)—Debottlenecking omega 3. Aquaculture 2023, 574, 739653. [Google Scholar] [CrossRef]
- Monge-Ortiz, R.; Tomás-Vidal, A.; Rodriguez-Barreto, D.; Martínez-Llorens, S.; Pérez, J.A.; Jover-Cerdá, M.; Lorenzo, A. Replacement of fish oil with vegetable oil blends in feeds for greater amberjack (Seriola dumerili) juveniles: Effect on growth performance, feed efficiency, tissue fatty acid composition and flesh nutritional value. Aquac. Nutr. 2018, 24, 605–615. [Google Scholar] [CrossRef]
- Sperling, P.; Ternes, P.; Zank, T.K.; Heinz, E. The evolution of desaturases. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 73–95. [Google Scholar] [CrossRef]
- Sayanova, O.; Haslam, R.; Guschina, I.; Lloyd, D.; Christie, W.W.; Harwood, J.L.; Napier, J.A. A Bifunctional Δ12,Δ15-Desaturase from Acanthamoeba castellanii Directs the Synthesis of Highly Unusual n-1 Series Unsaturated Fatty Acids. J. Biol. Chem. 2006, 281, 36533–36541. [Google Scholar] [CrossRef]
- Alloatti, A.; Uttaro, A.D. Highly specific methyl-end fatty-acid desaturases of trypanosomatids. Mol. Biochem. Parasitol. 2011, 175, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.W.; Zhang, Y.T.; Jiang, J.Y.; Guo, D.S.; Gao, S.; Gao, Z. Efficient docosahexaenoic acid production by Schizochytrium sp. via a two-phase pH control strategy using ammonia and citric acid as pH regulators. Process Biochem. 2019, 77, 1–7. [Google Scholar] [CrossRef]
- Taborda, T.; Moniz, P.; Reis, A.; da Silva, T.L. Evaluating low-cost substrates for Crypthecodinium cohnii lipids and DHA production, by flow cytometry. J. Appl. Phycol. 2021, 33, 263–274. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Scaife, M.A.; Armenta, R.E. Apparent digestibility of proximate nutrients, energy and fatty acids in nutritionally-balanced diets with partial or complete replacement of dietary fish oil with microbial oil from a novel Schizochytrium sp. (T18) by juvenile Atlantic salmon (Salmo salar L.). Aquaculture 2020, 520, 735003. [Google Scholar] [CrossRef]
- Osmond, A.T.Y.; Arts, M.T.; Hall, J.R.; Rise, M.L.; Bazinet, R.P.; Armenta, R.E.; Colombo, S.M. Schizochytrium sp. (T18) Oil as a Fish Oil Replacement in Diets for Juvenile Rainbow Trout (Oncorhynchus mykiss): Effects on Growth Performance, Tissue Fatty Acid Content, and Lipid-Related Transcript Expression. Animals 2021, 11, 1185. [Google Scholar] [CrossRef]
- Usman, M.; Zhao, S.; Jeon, B.H.; Salama, E.S.; Li, X.K. Microbial β-oxidation of synthetic long-chain fatty acids to improve lipid biomethanation. Water Res. 2022, 213, 118164. [Google Scholar] [CrossRef]
- Mu, H.; Wei, C.Q.; Xu, W.Q.; Gao, W.H.; Zhang, W.B.; Mai, K.S. Effects of replacement of dietary fish oil by rapeseed oil on growth performance, anti-oxidative capacity and inflammatory response in large yellow croaker Larimichthys crocea. Aquac. Rep. 2020, 16, 100251. [Google Scholar] [CrossRef]
- Turchini, G.M.; Mentasti, T.; Froyland, L.; Orban, E.; Caprino, F.; Moretti, V.M.; Valfré, F. Effects of alternative dietary lipid sources on performance, tissue chemical composition, mitochondrial fatty acid oxidation capabilities and sensory characteristics in brown trout (Salmo trutta L.). Aquaculture 2003, 225, 251–267. [Google Scholar] [CrossRef]
- Yun, B.A.; Xue, M.; Wang, J.; Fan, Z.Y.; Wu, X.F.; Zheng, Y.H.; Qin, Y.C. Effects of lipid sources and lipid peroxidation on feed intake, growth, and tissue fatty acid compositions of largemouth bass (Micropterus salmoides). Aquac. Int. 2013, 21, 97–110. [Google Scholar] [CrossRef]
- Bowyer, J.N.; Qin, J.G.; Smullen, R.P.; Stone, D.A.J. Replacement of fish oil by poultry oil and canola oil in yellowtail kingfish (Seriola lalandi) at optimal and suboptimal temperatures. Aquaculture 2012, 356, 211–222. [Google Scholar] [CrossRef]
- Seternes, T.; Johansson, G.S.; Evje, I.; Olsen, R.L. The level of eicosapentaenoic acid (EPA), but not docosahexaenoic acid (DHA), in blood of Atlantic salmon (Salmo salar L.) is related to formulation and concentration of EPA or DHA in feed. Aquaculture 2020, 526, 735407. [Google Scholar] [CrossRef]
- Stone, D.A.J.; Oliveira, A.C.M.; Plante, S.; Smiley, S.; Bechtel, P.; Hardy, R.W. Enhancing highly unsaturated omega-3 fatty acids in phase-fed rainbow trout (Oncorhynchus mykiss) using Alaskan fish oils. Aquac. Nutr. 2011, 17, E501–E510. [Google Scholar] [CrossRef]
- Xu, H.G.; Meng, X.X.; Jia, L.L.; Wei, Y.L.; Sun, B.; Liang, M.Q. Tissue distribution of transcription for 29 lipid metabolism-related genes in Takifugu rubripes, a marine teleost storing lipid predominantly in liver. Fish Physiol. Biochem. 2020, 46, 1603–1619. [Google Scholar] [CrossRef]
- Zhang, F.R.; Li, L.; Meng, X.X.; Liu, J.; Cui, X.S.; Ma, Q.; Wei, Y.L.; Liang, M.Q.; Xu, H.G.; Rombenso, A. Feeding Strategy to Use Beef Tallow and Modify Farmed Tiger Puffer Fatty Acid Composition. Animals 2023, 13, 3037. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.M.O.; Mackenzie, M.G.; Elgar, G.; Suzuki, Y.; Watabe, S.; Kinghorn, J.R.; Johnston, I.A. A genomic approach to reveal novel genes associated with myotube formation in the model teleost, Takifugu rubripes. Physiol Genom. 2005, 22, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, S.; Kaneko, T.; Suzuki, Y.; Hino, A. Individual variations in behavior and free cortisol responses to acute stress in tiger pufferfish Takifugu rubripes. Fish. Sci. 2008, 74, 755–763. [Google Scholar] [CrossRef]
- Gupta, S.C.; Siddique, H.R.; Mathur, N.; Mishra, R.K.; Mitra, K.; Saxena, D.K.; Chowdhuri, D.K. Adverse effect of organophosphate compounds, dichlorvos and chlorpyrifos in the reproductive tissues of transgenic Drosophila melanogaster: 70 kDa heat shock protein as a marker of cellular damage. Toxicology 2008, 243, 246. [Google Scholar] [CrossRef]
- Martins, D.A.; Valente, L.M.P.; Lall, S.P. Partial replacement of fish oil by flaxseed oil in Atlantic halibut (Hippoglossus hippoglossus L.) diets: Effects on growth, nutritional and sensory quality. Aquac. Nutr. 2011, 17, 671–684. [Google Scholar] [CrossRef]
- Wang, B.; Tontonoz, P. Phospholipid Remodeling in Physiology and Disease. Annu. Rev. Physiol. 2019, 81, 165–188. [Google Scholar] [CrossRef]
- Takii, K.; Ukawa, M.; Nakamura, M.; Kumai, H. Suitable Lipid Level in Brown Fish Meal Diet for Tiger Puffer. Fish. Sci. 1995, 61, 841–844. [Google Scholar] [CrossRef]
- Kim, S.S.; Lee, K.J. Dietary protein requirement of juvenile tiger puffer (Takifugu rubripes). Aquaculture 2009, 287, 219–222. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of Official Analytical Chemists International, 16th ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1995. [Google Scholar]
- Meng, X.X.; Bi, Q.Z.; Cao, L.; Ma, Q.; Wei, Y.L.; Duan, M.; Liang, M.Q.; Xu, H.G. Evaluation of Necessity of Cholesterol Supplementation in Diets of Two Marine Teleosts, Turbot (Scophthalmus maximus) and Tiger Puffer (Takifugu rubripes): Effects on Growth and Lipid Metabolism. Aquac. Nutr. 2022, 2022, 4160991. [Google Scholar] [CrossRef]
- Xu, H.G.; Liao, Z.B.; Zhang, Q.G.; Wei, Y.L.; Liang, M.Q. A moderately high level of dietary lipid inhibited the protein secretion function of liver in juvenile tiger puffer Takifugu rubripes. Aquaculture 2019, 498, 17–27. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034-1. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Morais, S.; Monroig, O.; Zheng, X.Z.; Leaver, M.J.; Tocher, D.R. Highly Unsaturated Fatty Acid Synthesis in Atlantic Salmon: Characterization of ELOVL5-and ELOVL2-like Elongases. Mar. Biotechnol. 2009, 11, 627–639. [Google Scholar] [CrossRef]
- Monroig, O.; Webb, K.; Ibarra-Castro, L.; Holt, G.J.; Tocher, D.R. Biosynthesis of long-chain polyunsaturated fatty acids in marine fish: Characterization of an Elovl4-like elongase from cobia Rachycentron canadum and activation of the pathway during early life stages. Aquaculture 2011, 312, 145–153. [Google Scholar] [CrossRef]
- Trushenski, J.T.; Rombenso, A.N. Trophic Levels Predict the Nutritional Essentiality of Polyunsaturated Fatty Acids in Fish—Introduction to a Special Section and a Brief Synthesis. N. Am. J. Aquac. 2020, 82, 241–250. [Google Scholar] [CrossRef]
- Santigosa, E.; Constant, D.; Prudence, D.; Wahli, T.; Verlhac-Trichet, V. A novel marine algal oil containing both EPA and DHA is an effective source of omega-3 fatty acids for rainbow trout (Oncorhynchus mykiss). J. World Aquac. Soc. 2020, 51, 649–665. [Google Scholar] [CrossRef]
- Sobczak, M.; Panicz, R.; Eljasik, P.; Sadowski, J.; Tórz, A.; Zochowska-Kujawska, J.; Barbosa, V.; Domingues, V.; Marques, A.; Dias, J. Quality improvement of common carp (Cyprinus carpio L.) meat fortified with n-3 PUFA. Food Chem. Toxicol. 2020, 139, 111261. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, S.K.A.; Schorer, M.; Moura, G.D.; Lanna, E.A.T.; Pedreira, M.M. Evaluation of growth and fatty acid profile of Nile tilapia (Oreochromis niloticus) fed with Schizochytrium sp. Aquac. Res. 2019, 50, 1068–1074. [Google Scholar] [CrossRef]
- Neylan, K.A.; Johnson, R.B.; Barrows, F.T.; Marancik, D.P.; Hamilton, S.L.; Gardner, L.D. Evaluating a microalga (Schizochytrium sp.) as an alternative to fish oil in fish-free feeds for sablefish (Anoplopoma fimbria). Aquaculture 2024, 578, 740000. [Google Scholar] [CrossRef]
- Lee, S.; Park, C.O.; Choi, W.; Bae, J.; Kim, J.; Choi, S.; Katya, K.; Kim, K.W.; Bai, S.C. Partial Substitution of Fish Oil with Microalgae (Schizochytrium sp.) Can Improve Growth Performance, Nonspecific Immunity and Disease Resistance in Rainbow Trout, Oncorhynchus mykiss. Animals 2022, 12, 1220. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Filer, K.; Xue, Y.; Ai, Q.; Mai, K. Replacement of fish oil with a DHA-rich Schizochytrium meal on growth performance, activities of digestive enzyme and fatty acid profile of Pacific white shrimp (Litopenaeus vannamei) larvae. Aquac. Nutr. 2017, 23, 1113–1120. [Google Scholar] [CrossRef]
- Ganuza, E.; Benítez-Santana, T.; Atalah, E.; Vega-Orellana, O.; Ganga, R.; Izquierdo, M.S. Crypthecodinium cohnii and Schizochytrium sp. as potential substitutes to fisheries-derived oils from seabream (Sparus aurata) microdiets. Aquaculture 2008, 277, 109–116. [Google Scholar] [CrossRef]
- Hu, S.X.; Wang, J.T.; Han, T.; Li, X.Y.; Jiang, Y.D.; Wang, C.L. Effects of dietary DHA/EPA ratios on growth performance, survival and fatty acid composition of juvenile swimming crab (Portunus trituberculatus). Aquac. Res. 2017, 48, 1291–1301. [Google Scholar] [CrossRef]
- Jin, M.; Monroig, O.; Lu, Y.; Yuan, Y.; Li, Y.; Ding, L.Y.; Tocher, D.R.; Zhou, Q.C. Dietary DHA/EPA ratio affected tissue fatty acid profiles, antioxidant capacity, hematological characteristics and expression of lipid-related genes but not growth in juvenile black seabream (Acanthopagrus schlegelii). PLoS ONE 2017, 12, e0176216. [Google Scholar] [CrossRef]
- Xu, H.G.; Cao, L.; Wei, Y.L.; Zhang, Y.Q.; Liang, M.Q. Lipid contents in farmed fish are influenced by dietary DHA/EPA ratio: A study with the marine flatfish, tongue sole (Cynoglossus semilaevis). Aquaculture 2018, 485, 183–190. [Google Scholar] [CrossRef]
- Xu, H.G.; Turchini, G.M.; Francis, D.S.; Liang, M.Q.; Mock, T.S.; Rombenso, A.; Ai, Q.H. Are fish what they eat? A fatty acid’s perspective. Prog. Lipid Res. 2020, 80, 101064. [Google Scholar] [CrossRef]
- Carvalho, M.; Izquierdo, M.; Valdes, M.; Montero, D.; Farias, A. Oils Combination with Microalgal Products as a Strategy for Increasing the N-3 Long-Chain Polyunsaturated Fatty Acid Content in Fish Oil-Free Diets for Meagre (Argyrosomus regius). Aquac. Nutr. 2022, 2022, 5275570. [Google Scholar] [CrossRef]
- Sprague, M.; Walton, J.; Campbell, P.J.; Strachan, F.; Dick, J.R.; Bell, J.G. Replacement of fish oil with a DHA-rich algal meal derived from Schizochytrium sp. on the fatty acid and persistent organic pollutant levels in diets and flesh of Atlantic salmon (Salmo salar, L.) post-smolts. Food Chem. 2015, 185, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Guerra, N.; Parrish, C.C.; Wei, M.M.; Perry, J.; Armenta, R.E.; Colombo, S.M. Effects of Replacement of Fish Oil with Microbial Oil (Schizochytrium sp. T18) on Membrane Lipid Composition of Atlantic Salmon Parr Muscle and Liver Tissues. Sustainability 2023, 15, 4594. [Google Scholar] [CrossRef]
- Qiao, H.; Wang, H.; Song, Z.; Ma, J.; Li, B.; Liu, X.; Zhang, S.; Wang, J.; Zhang, L. Effects of dietary fish oil replacement by microalgae raw materials on growth performance, body composition and fatty acid profile of juvenile olive flounder, Paralichthys olivaceus. Aquac. Nutr. 2014, 20, 646–653. [Google Scholar] [CrossRef]
- Seong, T.; Kitagima, R.; Haga, Y.; Satoh, S. Non-fish meal, non-fish oil diet development for red sea bream, Pagrus major, with plant protein and graded levels of Schizochytrium sp.: Effect on growth and fatty acid composition. Aquac. Nutr. 2020, 26, 1173–1185. [Google Scholar] [CrossRef]
- Ma, W.; Liu, M.Z.; Zhang, Z.X.; Xu, Y.S.; Huang, P.W.; Guo, D.S.; Sun, X.M.; Huang, H. Efficient co-production of EPA and DHA by Schizochytrium sp. via regulation of the polyketide synthase pathway. Commun. Biol. 2022, 5, 1356. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, F. Effects of temperature and temperature shift on docosahexaenoic acid production by the marine microalga Crypthecodinium cohnii. J. Am. Oil Chem. Soc. 2000, 77, 613–617. [Google Scholar] [CrossRef]
- Vásquez-Sandoval, C.; Herrera-Herrera, P.; Navarrete, J.; Contreras, P.; Dantagnan, P.; Oviedo, C. Arachidonic acid and antioxidant behavior in Ulkenia visurgensis strain Lng2 under adaptive laboratory evolution. Algal Res.-Biomass Biofuels Bioprod. 2025, 85, 103859. [Google Scholar] [CrossRef]
- Adarme-Vega, T.C.; Lim, D.K.Y.; Timmins, M.; Vernen, F.; Li, Y.; Schenk, P.M. Microalgal biofactories: A promising approach towards sustainable omega-3 fatty acid production. Microb. Cell Factories 2012, 11, 96. [Google Scholar] [CrossRef]
- Ward, O.P.; Singh, A. Omega-3/6 fatty acids: Alternative sources of production. Process Biochem. 2005, 40, 3627–3652. [Google Scholar] [CrossRef]
- Lutfi, E.; Berge, G.M.; Bæverfjord, G.; Sigholt, T.; Bou, M.; Larsson, T.; Morkore, T.; Evensen, O.; Sissener, N.H.; Rosenlund, G.; et al. Increasing dietary levels of the n-3 long-chain PUFA, EPA and DHA, improves the growth, welfare, robustness and fillet quality of Atlantic salmon in sea cages. Br. J. Nutr. 2023, 129, 10–28. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.J.; Hu, Y.X.; Xu, W.; Luo, H.R.; Chen, J.; Tao, B.B.; Luo, D.J.; Han, D.; Zhu, X.M.; Xie, S.Q.; et al. Effects of dietary arachidonic acid on growth, gonadal development, and tissue fatty acid composition in the hermaphroditic swamp eel (Monopterus albus). Aquac. Rep. 2023, 33, 101791. [Google Scholar] [CrossRef]
- Wang, X.X.; Jin, M.; Cheng, X.; Hu, X.Y.; Zhao, M.M.; Yuan, Y.; Sun, P.; Jiao, L.F.; Betancor, M.B.; Tocher, D.R.; et al. Dietary DHA/EPA ratio affects growth, tissue fatty acid profiles and expression of genes involved in lipid metabolism in mud crab Scylla paramamosain supplied with appropriate n-3 LC-PUFA at two lipid levels. Aquaculture 2021, 532, 736028. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.; Rosenberg, J.N.; Betenbaugh, M.J.; Wang, F. Optimization of One-Step In Situ Transesterification Method for Accurate Quantification of EPA in Nannochloropsis gaditana. Appl. Sci. 2016, 6, 343. [Google Scholar] [CrossRef]
- Ahern, T.J.; Katoh, S.; Sada, E. Arachidonic acid production by the red alga Porphyridium cruentum. Biotechnol. Bioeng. 1983, 25, 1057–1070. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Venegas-Venegas, E.; Rincón-Cervera, M.A.; Suárez, M.D. Fatty acid profiles of livers from selected marine fish species. J. Food Compos. Anal. 2011, 24, 217–222. [Google Scholar] [CrossRef]
- Kaneko, G.; Yamada, T.; Han, Y.N.; Hirano, Y.; Khieokhajonkhet, A.; Shirakami, H.; Nagasaka, R.; Kondo, H.; Hirono, I.; Ushio, H.; et al. Differences in lipid distribution and expression of peroxisome proliferator-activated receptor gamma and lipoprotein lipase genes in torafugu and red seabream. Gen. Comp. Endocrinol. 2013, 184, 51–60. [Google Scholar] [CrossRef]
- de Souza, F.P.; de Lima, E.C.S.; Urrea-Rojas, A.M.; Suphoronski, S.A.; Facimoto, C.T.; Bezerra, J., Jr.; de Oliveira, T.E.S.; Pereira, U.D.; Di Santis, G.W.; de Oliveira, C.A.L.; et al. Effects of dietary supplementation with a microalga (Schizochytrium sp.) on the hemato-immunological, and intestinal histological parameters and gut microbiota of Nile tilapia in net cages. PLoS ONE 2020, 15, e0226977. [Google Scholar] [CrossRef]
- Twibell, R.G.; Watkins, B.A.; Brown, P.B. Dietary Conjugated Linoleic Acids and Lipid Source Alter Fatty Acid Composition of Juvenile Yellow Perch, Perca flavescens. J. Nutr. 2001, 131, 2322–2328. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, W.; Zhi, S.; Zhao, M.; Liu, M.; Qin, C.; Feng, J.; Yan, X.; Nie, G. Evaluation of dietary genistein on the antioxidant capacity, non-specific immune status, and fatty acid composition of common carp (Cyprinus carpio L). Aquaculture 2022, 550, 737822. [Google Scholar] [CrossRef]
- Sun, S.X.; Cao, X.J.; Gao, J. C24:0 avoids cold exposure-induced oxidative stress and fatty acid β-oxidation damage. Iscience 2021, 24, 103409. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Karahashi, M.; Sakamoto, T.; Tsuji, Y.; Yamazaki, T.; Okazaki, M.; Mitsumoto, A.; Kudo, N.; Kawashima, Y. Fatty Acid β-Oxidation Plays a Key Role in Regulating cis-Palmitoleic Acid Levels in the Liver. Biol. Pharm. Bull. 2016, 39, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Colombo, S.M.; Budge, S.M.; Hall, J.R.; Kornicer, J.; White, N. Atlantic salmon adapt to low dietary n-3 PUFA and warmer water temperatures by increasing feed intake and expression of n-3 biosynthesis-related transcripts. Fish Physiol. Biochem. 2023, 49, 39–60. [Google Scholar] [CrossRef]
- Nayak, S.; Khozin-Goldberg, I.; Cohen, G.; Zilberg, D. Dietary Supplementation with ω6 LC-PUFA-Rich Algae Modulates Zebrafish Immune Function and Improves Resistance to Streptococcal Infection. Front. Immunol. 2018, 9, 1960. [Google Scholar] [CrossRef] [PubMed]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- He, J.L.; Liu, T.; Weng, X.Z.; Zheng, P.Q.; Xu, H.Y.; Wang, J.T.; Han, T. Effects of different vegetable oil sources on growth performance, body composition and tissue fatty acid composition of spotted knifejaw, Oplegnathus punctatus. Aquac. Rep. 2022, 27, 101401. [Google Scholar] [CrossRef]
- Marques, A.; Canada, P.; Costa, C.; Basto, A.; Piloto, F.; Salgado, M.A.; Abreu, H.; Dias, J.; Valente, L.M.P. Replacement of fish oil by alternative n-3 LC-PUFA rich lipid sources in diets for European sea bass (Dicentrarchus labrax). Front. Mar. Sci. 2023, 10, 1189319. [Google Scholar] [CrossRef]
- Liang, C.; Zhao, X.Y.; Jiao, L.F.; Shen, Y.D.; Luo, J.X.; Zhu, T.T.; Zhao, W.L.; Gen, Z.; Zhou, Q.C.; Jin, M. Effects of different lipid sources on growth performance, fatty acids composition in tissue and expression of genes related to lipid metabolism in largemouth bass (Micropterus salmoides). Aquac. Rep. 2022, 23, 101013. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; López, G.; Sáez, M.I.; Jiménez, J.A.; Barros, A.; Hidalgo, L.; Camacho-Rodríguez, J.; Martínez, T.F.; Cerón-García, M.C.; Alarcón, F.J. Effects of the microalga Scenedesmus almeriensis as fishmeal alternative in diets for gilthead sea bream, Sparus aurata, juveniles. Aquaculture 2014, 431, 34–43. [Google Scholar] [CrossRef]
- Yao, X.Z.; Lin, Y.Y.; Shi, M.L.; Chen, L.T.; Qu, K.Y.; Liu, Y.C.; Tan, B.P.; Xie, S.W. Effect of Schizochytrium limacinum supplementation to a low fish-meal diet on growth performance, lipid metabolism, apoptosis, autophagy and intestinal histology of Litopenaeus vannamei. Front. Mar. Sci. 2022, 9, 1090235. [Google Scholar] [CrossRef]
- Xie, J.J.; Fang, H.H.; Liao, S.Y.; Guo, T.Y.; Yin, P.; Liu, Y.J.; Tian, L.X.; Niu, J. Study on Schizochytrium sp. improving the growth performance and non-specific immunity of golden pompano (Trachinotus ovatus) while not affecting the antioxidant capacity. Fish Shellfish. Immunol. 2019, 95, 617–623. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, X.Y.; Zhu, F.Y.; Wen, H.; Dong, L.X.; Tian, J.; Zhang, J.M.; Yang, C.G.; Xiao, J.R.; Duan, X.B.; et al. Schizochytrium sp. can improve feed utilization, fillet DHA content, and non-specific immunity of juvenile Nile tilapia (Oreochromis niloticus) fed fish oil free diet. J. Appl. Phycol. 2024, 36, 3341–3352. [Google Scholar] [CrossRef]
- Yang, B.; Li, R.T.; Greenlief, C.M.; Fritsche, K.L.; Gu, Z.Z.; Cui, J.K.; Lee, J.C.; Beversdorf, D.Q.; Sun, G.Y. Unveiling anti-oxidative and anti-inflammatory effects of docosahexaenoic acid and its lipid peroxidation product on lipopolysaccharide-stimulated BV-2 microglial cells. J. Neuroinflamm. 2018, 15, 202. [Google Scholar] [CrossRef]
- Tong, Y.L.; Liu, Y.T.; Zheng, H.M.; Zheng, L.; Liu, W.Q.; Wu, J.J.; Ou, R.L.; Zhang, G.Y.; Li, F.Y.; Hu, M.; et al. Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/β-catenin signaling. Oncotarget 2016, 7, 31413–31428. [Google Scholar] [CrossRef] [PubMed]
- Habte-Tsion, H.M.; Kolimadu, G.D.; Rossi, W.; Filer, K.; Kumar, V. Effects of Schizochytrium and micro-minerals on immune, antioxidant, inflammatory and lipid-metabolism status of Micropterus salmoides fed high- and low-fishmeal diets. Sci. Rep. 2020, 10, 7457. [Google Scholar] [CrossRef]
- Cai, X.X.; Yan, A.; Fu, N.Y.; Wang, S.Y. In Vitro Antioxidant Activities of Enzymatic Hydrolysate from Schizochytrium sp. and Its Hepatoprotective Effects on Acute Alcohol-Induced Liver Injury In Vivo. Mar. Drugs 2017, 15, 115. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Ku, C.S.; Pham, T.X.; Park, Y.; Kim, B.; Shin, M.S.; Kang, I.; Lee, J. Edible blue-green algae reduce the production of pro-inflammatory cytokines by inhibiting NF-κB pathway in macrophages and splenocytes. Biochim. Biophys. Acta-Gen. Subj. 2013, 1830, 2981–2988. [Google Scholar] [CrossRef]
- Hassan, S.; Zil-E-Rubab; Shah, H.; Shawana, S. Dysregulated epidermal growth factor and tumor growth factor-beta receptor signaling through GFAP-ACTA2 protein interaction in liver fibrosis. Pak. J. Med. Sci. 2020, 36, 782–787. [Google Scholar] [CrossRef]
- Yang, J.C.Z.; Fernández-Galilea, M.; Martínez-Fernández, L.; González-Muniesa, P.; Pérez-Chávez, A.; Martínez, J.A.; Moreno-Aliaga, M.J. Oxidative Stress and Non-Alcoholic Fatty Liver Disease: Effects of Omega-3 Fatty Acid Supplementation. Nutrients 2019, 11, 872. [Google Scholar] [CrossRef]
- Vernon, G.; Baranova, A.; Younossi, Z.M. Systematic review: The epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment. Pharmacol. Ther. 2011, 34, 274–285. [Google Scholar] [CrossRef] [PubMed]
- Mrsny, R.J.; Brown, G.T.; Gerner-Smidt, K.; Buret, A.G.; Meddings, J.B.; Quan, C.; Koval, M.; Nusrat, A. A key claudin extracellular loop domain is critical for epithelial barrier integrity. Am. J. Pathol. 2008, 172, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Wardill, H.R.; Gibson, R.J.; Logan, R.M.; Bowen, J.M. TLR4/PKC-mediated tight junction modulation: A clinical marker of chemotherapy-induced gut toxicity? Int. J. Cancer 2014, 135, 2483–2492. [Google Scholar] [CrossRef]
- Zheng, Z.N.; Lin, K.; Hu, Y.B.; Zhou, Y.; Ding, X.Y.; Wang, Y.L.; Wu, W. Sulforaphane metabolites inhibit migration and invasion via microtubule-mediated Claudins dysfunction or inhibition of autolysosome formation in human non-small cell lung cancer cells. Cell Death Dis. 2019, 10, 259. [Google Scholar] [CrossRef]
- Ji, S.H.; Li, H.D.; Huang, X.C.; Sun, J.; Kaneko, G.; Ji, H. Docosahexaenoic acid (DHA) promotes grass carp (Ctenopharyngodon idella) muscle fiber development by activating MEK/ERK pathway in vitro and in vivo. Aquaculture 2024, 579, 740148. [Google Scholar] [CrossRef]
- Ji, S.H.; Bian, C.C.; Sun, J.; Li, H.D.; Kaneko, G.; Ji, H. Docosahexaenoic acid (DHA) regulates mitochondrial quality control via MEK/ERK activation to promote myoblast-to-myotube formation in grass carp (Ctenopharyngodon idellus). Aquaculture 2024, 591, 741123. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, C.D.; Wang, X.; Zhou, H.H.; Mai, K.S.; He, G. EPA and DHA promote cell proliferation and enhance activity of the Akt-TOR-S6K anabolic signaling pathway in primary muscle cells of turbot (Scophthalmus maximus L.). Fish Physiol. Biochem. 2024, 50, 1483–1494. [Google Scholar] [CrossRef]




| Ingredients | FO-C | AO+PO | AO+RO |
|---|---|---|---|
| Fish meal | 40.00 | 40.00 | 40.00 |
| Poultry by-product meal | 8.00 | 8.00 | 8.00 |
| Soybean meal | 11.00 | 11.00 | 11.00 |
| Corn gluten meal | 8.00 | 8.00 | 8.00 |
| Wheat meal | 17.68 | 17.68 | 17.68 |
| Brewer’s yeast | 5.00 | 5.00 | 5.00 |
| Mineral premix a | 0.50 | 0.50 | 0.50 |
| Vitamin premix a | 1.00 | 1.00 | 1.00 |
| Monocalcium phosphate | 1.00 | 1.00 | 1.00 |
| L-ascorbyl-2-polyphosphate | 0.20 | 0.20 | 0.20 |
| Choline chloride | 0.20 | 0.20 | 0.20 |
| Betaine | 0.30 | 0.30 | 0.30 |
| Ethoxyquin | 0.02 | 0.02 | 0.02 |
| Calcium propionic acid | 0.10 | 0.10 | 0.10 |
| Soya lecithin | 1.00 | 1.00 | 1.00 |
| Fish oil | 6.00 | ||
| Schizochytrium sp. oil b | 3.00 | 3.00 | |
| Poultry oil c | 3.00 | ||
| Rapeseed oil d | 3.00 | ||
| Proximate composition | |||
| Crude protein | 49.98 | 49.98 | 49.98 |
| Crude lipid | 11.24 | 11.24 | 11.24 |
| Ash | 89.08 | 88.85 | 88.90 |
| Energy kJ/g | 20.96 | 20.91 | 20.89 |
| Fatty Acid | Diet | Oil | |||||
|---|---|---|---|---|---|---|---|
| FO-C | AO+PO | AO+RO | FO | AO | PO | RO | |
| 14:0 | 5.42 | 2.07 | 1.97 | 7.02 | 0.93 | 0.67 | 0.05 |
| 16:0 | 23.44 | 21.43 | 18.78 | 22.04 | 21.37 | 24.28 | 4.17 |
| 18:0 | 5.12 | 3.96 | 3.68 | 0.36 | 0.03 | 5.25 | 1.63 |
| 20:0 | 0.48 | 0.20 | 0.24 | 0.12 | 0.39 | 0.06 | 0.39 |
| SFA | 34.46 | 27.66 | 24.68 | 29.54 | 22.41 | 30.26 | 6.25 |
| 16:1n-7 | 0.62 | 0.16 | 0.17 | 0.45 | 0.04 | 0.13 | 0.06 |
| 18:1n-9 | 14.48 | 20.37 | 21.96 | 13.79 | 0.38 | 40.97 | 58.97 |
| 20:1n-9 | 1.47 | 0.48 | 0.53 | 0.06 | ND | 0.37 | 0.99 |
| 22:1n-9 | 0.28 | 0.22 | 0.20 | 0.70 | 0.10 | ND | 0.69 |
| MUFA | 16.86 | 21.23 | 22.85 | 15.0 | 1.75 | 41.47 | 61.05 |
| 18:2n-6 | 10.17 | 14.36 | 14.54 | 0.16 | 0.15 | 19.64 | 19.71 |
| 20:2n-6 | 0.21 | 0.14 | 0.13 | 0.03 | 0.27 | 0.15 | 0.06 |
| 20:4n-6 | 0.99 | 0.68 | 0.60 | 0.02 | 0.06 | 0.281 | ND |
| n-6 PUFA | 11.36 | 15.19 | 15.27 | 0.21 | 1.35 | 20.35 | 19.77 |
| 18:3n-3 | 1.43 | 1.62 | 2.21 | 0.50 | 0.13 | 0.71 | 0.12 |
| 20:5n-3 | 6.99 | 2.99 | 3.02 | 8.94 | 0.07 | 0.08 | 0.04 |
| 22:5n-3 | 1.05 | 0.55 | 0.53 | 0.98 | 0.22 | 0.05 | ND |
| 22:6n-3 | 11.47 | 19.86 | 20.02 | 13.83 | 59.44 | 0.20 | 0.06 |
| n-3 PUFA | 20.94 | 25.03 | 25.78 | 24.25 | 59.86 | 1.04 | 0.22 |
| DHA/EPA | 1.64 | 6.67 | 6.65 | 1.55 | 849.14 | 1.63 | 1.50 |
| Primer | Sequence (5′−3′) | GenBank Reference | PL (bp) |
|---|---|---|---|
| myod-F | TTCATCATCACACCGAGGCG | NM_001032769.1 | 126 |
| myod-R | GTCGGTCCACGTTTGTAGTCT | ||
| myog-F | ACGCTAATCAGTGGGTCTGC | XM_003973605.3 | 89 |
| myog-R | TAACTCGTGGCTTCGACAGG | ||
| myf6-F | GATCTGCAAGCGCAAATCGG | NM_001032771.1 | 116 |
| myf6-R | CCACGGTCTTCCTCTTGAGC | ||
| myf5-F | GGAGTCCTCTGTCCAACTGC | NM_001032770.1 | 84 |
| myf5-R | CGCTGCTGTAAACTGCGTTC | ||
| bax-F | ACCGTTCCCAGTGCAAATCT | XM_003964782.3 | 108 |
| bax-R | TGGGAACACTTGAGCCCATC | ||
| bcl-2-F | GGGCCGGATTATCGCTTTCT | XM_029830403.1 | 111 |
| bcl-2-R | TATTCCGTCATCCACTCCGC | ||
| acta2-6F | ATTCCTCGTCCCTGTGTGGTC | XM_029845356.1 | 125 |
| acta2-6R | GGCATCATCTCCAGCGAAGC | ||
| il-1β-F | CATCACCCGCTGACCATGAA | NM_001280090.1 | 103 |
| il-1β-R | CATCCCTGAACTCGGTGCTC | ||
| il-8-F | CCTGCGGAGCCTCGGAGTG | AB125645.1 | 145 |
| il-8-R | TGACATCTTCAGAGTGGCAATGATCTC | ||
| tnf-α-F | CTACTGGAACGGAAGGCAAGAGATG | AB183465.1 | 100 |
| tnf-α-R | GATGCGGCTCAGCGTGTAGTG | ||
| ifn-γ-F | CTGTGATGACTCTTGGGGCT | XM_029825554.1 | 147 |
| ifn-γ-R | TGTACCGCTGACAGGAGTTG | ||
| claudin18-F | GACACAAGGGTCTGTGGCAG | AY554344.1 | 112 |
| claudin18-R | ATGATCATCAGGGCTCGCAC | ||
| jam-a-F | CAAAAACGGCGTGCCTCTAC | XM_003971244.3 | 122 |
| jam-a-R | CCGAGTCCGACCTTGATGTT | ||
| mlck-F | GACACGACTGGCACGCAGATC | XM_029830403.1 | 170 |
| mlck-R | CAGATGACTCCGATGCTCCACATG | ||
| rpl19-F | GTCTCATCATCCGCAAACC | XM_003964816 | 132 |
| rpl19-R | TCTCAGGCATACGAGCATT | ||
| rpl13-F | GTAACAGGTCCACAGAATCCC | XM_003969972 | 117 |
| rpl13-R | CCTCAGTGCTGTCTCCCTTC | ||
| keap1-F | ACCGTGATGGAGGAATCGAGC | XM_029851225.1 | 123 |
| keap1-R | TCAGCTTACCAGAACCGAGGG | ||
| nrf2-F | ACGCATTCGACAAACACGAC | XM_003961827.3 | 106 |
| nrf2-R | CCGTACACAGACTTCCCAGG | ||
| col1a2-F | TGTTGGAGAGGGTGGAAAGC | XM_011609483.2 | 136 |
| col1a2-R | GACTCCCATTGGACCCTGAG |
| Parameters | FO-C | AO+PO | AO+RO |
|---|---|---|---|
| Initial body weight (g) | 23.85 ± 0.02 | 23.79 ± 0.01 | 23.81 ± 0.01 |
| Final body weight (g) | 67.87 ± 2.49 | 69.13 ± 2.95 | 66.20 ± 2.78 |
| Weight gain (g) | 44.02 ± 2.50 | 45.36 ± 2.95 | 42.40 ± 2.78 |
| Weight gain ratio (%) | 184.60 ± 10.53 | 190.79 ± 12.42 | 178.09 ± 11.69 |
| Specific growth rate (%/d) | 1.87 ± 0.07 | 1.90 ± 0.08 | 1.82 ± 0.07 |
| Feed conversion ratio | 1.30 ± 0.02 | 1.31 ± 0.05 | 1.37 ± 0.04 |
| Survival (%) | 97.33 ± 1.33 | 98.67 ± 1.33 | 94.67 ± 1.33 |
| Hepatosomatic index (%) | 9.66 ± 0.60 | 9.94 ± 0.62 | 9.09 ± 0.37 |
| Viscerosomatic index (%) | 14.22 ± 0.56 | 14.78 ± 0.58 | 13.58 ± 0.56 |
| Condition factor (g/cm3) | 3.15 ± 0.09 | 3.31 ± 0.08 | 2.97 ± 0.09 |
| Parameters | FO-C | AO+PO | AO+RO |
|---|---|---|---|
| Whole fish | |||
| Crude protein (% w.w.) | 18.84 ± 0.51 | 19.20 ± 0.54 | 19.62 ± 0.67 |
| Crude lipid (% w.w.) | 8.54 ± 1.07 | 9.41 ± 1.12 | 8.27 ± 0.65 |
| Moisture (%) | 70.32 ± 1.22 | 70.05 ± 1.18 | 69.40 ± 1.03 |
| Ash (% w.w.) | 3.11 ± 0.30 | 3.08 ± 0.29 | 3.27 ± 0.19 |
| Muscle | |||
| Crude protein (% w.w.) | 18.96 ± 0.07 | 19.18 ± 0.23 | 18.86 ± 0.05 |
| Crude lipid (% w.w.) | 0.78 ± 0.01 b | 0.65 ± 0.01 a | 0.79 ± 0.03 b |
| Moisture (%) | 78.74 ± 0.27 | 78.80 ± 0.19 | 78.63 ± 0.26 |
| Liver | |||
| Crude protein (% w.w.) | 3.84 ± 0.13 | 3.74 ± 0.43 | 4.11 ± 0.55 |
| Crude lipid (% w.w.) | 44.76 ± 2.75 | 46.35 ± 1.95 | 45.30 ± 2.00 |
| Moisture (%) | 30.93 ± 0.84 | 30.28 ± 0.34 | 30.10 ± 0.78 |
| Parameters | FO-C | AO+PO | AO+RO |
|---|---|---|---|
| Triacylglycerol (mmol/L) | 1.76 ± 0.19 | 1.53 ± 0.38 | 1.71 ± 0.33 |
| Total cholesterol (mmol/L) | 3.82 ± 0.61 | 3.32 ± 0.31 | 3.02 ± 0.36 |
| High-density lipoprotein cholesterol (mmol/L) | 4.97 ± 1.00 | 4.38 ± 0.47 | 4.23 ± 0.82 |
| Low-density lipoprotein cholesterol (mmol/L) | 0.93 ± 0.22 a | 1.13 ± 0.13 b | 0.88 ± 0.10 a |
| Total bile acid (μmol/L) | 1.96 ± 0.47 | 1.61 ± 0.39 | 1.62 ± 0.41 |
| Malondialdehyde (nmol/mL) | 10.17 ± 1.39 | 9.56 ± 1.51 | 9.29 ± 1.44 |
| Fatty Acid | FO-C | AO+PO | AO+RO |
|---|---|---|---|
| 14:0 | 2.89 ± 0.15 b | 1.32 ± 0.09 a | 1.49 ± 0.17 a |
| 16:0 | 22.65 ± 0.47 b | 20.81 ± 0.64 a | 19.82 ± 1.36 a |
| 18:0 | 6.65 ± 0.51 ab | 7.29 ± 0.71 b | 6.06 ± 0.80 a |
| 20:0 | 0.29 ± 0.02 b | 0.19 ± 0.03 a | 0.21 ± 0.03 a |
| SFA | 32.47 ± 0.82 b | 29.60 ± 0.58 a | 27.58 ± 2.13 a |
| 16:1n-7 | 0.30 ± 0.02 b | 0.20 ± 0.05 a | 0.16 ± 0.01 a |
| 18:1n-9 | 17.83 ± 1.27 a | 21.46 ± 1.81 b | 22.97 ± 1.39 b |
| 20:1n-9 | 1.56 ± 0.08 b | 1.05 ± 0.07 a | 1.23 ± 0.21 a |
| 22:1n-9 | 0.27 ± 0.03 b | 0.15 ± 0.02 a | 0.18 ± 0.07 a |
| MUFA | 19.95 ± 1.16 a | 22.85 ± 1.79 b | 24.54 ± 1.43 b |
| 18:2n-6 | 7.38 ± 0.20 a | 10.26 ± 0.60 b | 10.01 ± 0.59 b |
| 20:2n-6 | 0.44 ± 0.04 | 0.48 ± 0.03 | 0.53 ± 0.08 |
| 20:4n-6 | 0.71 ± 0.06 | 0.68 ± 0.17 | 0.54 ± 0.03 |
| n-6 PUFA | 8.52 ± 0.25 a | 11.41 ± 0.56 b | 11.07 ± 0.63 b |
| 18:3n-3 | 1.07 ± 0.06 a | 0.91 ± 0.07 a | 1.76 ± 0.44 b |
| 20:5n-3 | 4.46 ± 0.18 b | 2.02 ± 0.25 a | 1.98 ± 0.28 a |
| 22:5n-3 | 2.63 ± 0.10 b | 1.87 ± 0.08 a | 1.98 ± 0.11 a |
| C22:6n-3 | 10.79 ± 0.46 a | 16.06 ± 0.87 b | 15.34 ± 0.71 b |
| n-3 PUFA | 18.94 ± 0.25 a | 20.83 ± 0.39 b | 21.11 ± 0.39 b |
| DHA/EPA | 2.42 ± 0.10 a | 8.06 ± 1.04 b | 7.87 ± 1.14 b |
| Fatty Acid | FO-C | AO+PO | AO+RO |
|---|---|---|---|
| 14:0 | 3.06 ± 0.17 b | 1.46 ± 0.16 a | 1.38 ± 0.23 a |
| 16:0 | 22.08 ± 0.86 c | 20.86 ± 0.90 b | 17.71 ± 0.43 a |
| 18:0 | 6.45 ± 0.66 b | 6.11 ± 0.61 ab | 5.31 ± 0.70 a |
| 20:0 | 0.32 ± 0.02 c | 0.17 ± 0.01 a | 0.21 ± 0.01 b |
| SFA | 31.91 ± 1.20 c | 28.59 ± 0.96 b | 24.61 ± 0.41 a |
| 16:1n-7 | 0.29 ± 0.01 b | 0.15 ± 0.01 a | 0.14 ± 0.01 a |
| 18:1n-9 | 17.98 ± 1.21 a | 20.86 ± 0.84 b | 23.97 ± 1.17 c |
| 20:1n-9 | 1.64 ± 0.11 c | 1.01 ± 0.08 a | 1.26 ± 0.16 b |
| 22:1n-9 | 0.30 ± 0.04 c | 0.13 ± 0.02 a | 0.19 ± 0.04 b |
| MUFA | 20.21 ± 1.07 a | 22.15 ± 0.85 b | 25.57 ± 1.04 c |
| 18:2n-6 | 7.50 ± 0.28 a | 10.55 ± 0.18 b | 10.69 ± 0.38 b |
| 20:2n-6 | 0.44 ± 0.06 | 0.45 ± 0.04 | 0.50 ± 0.05 |
| 20:4n-6 | 0.57 ± 0.04 | 0.56 ± 0.13 | 0.44 ± 0.06 |
| n-6 PUFA | 8.51 ± 0.33 a | 11.56 ± 0.27 b | 11.63 ± 0.36 b |
| 18:3n-3 | 1.18 ± 0.04 b | 0.96 ± 0.07 a | 2.08 ± 0.06 c |
| 20:5n-3 | 4.32 ± 0.15 b | 2.22 ± 0.15 a | 2.63 ± 1.12 a |
| 22:5n-3 | 2.86 ± 0.08 b | 1.96 ± 0.04 a | 2.05 ± 0.07 a |
| 22:6n-3 | 10.72 ± 0.53 a | 17.23 ± 0.61 b | 16.62 ± 0.72 b |
| n-3 PUFA | 19.08 ± 0.28 a | 22.37 ± 0.30 b | 23.38 ± 0.32 b |
| DHA/EPA | 2.48 ± 0.18 a | 7.78 ± 0.36 b | 7.04 ± 2.04 b |
| Fatty Acid | FO-C | AO+PO | AO+RO |
|---|---|---|---|
| 14:0 | 0.59 ± 0.06 b | 0.34 ± 0.05 a | 0.36 ± 0.00 a |
| 16:0 | 24.23 ± 0.58 | 25.36 ± 1.73 | 23.01 ± 0.92 |
| 18:0 | 7.88 ± 0.29 ab | 8.40 ± 0.28 b | 7.32 ± 0.21 a |
| 20:0 | 0.22 ± 0.02 b | 0.15 ± 0.04 a | 0.18 ± 0.01 ab |
| SFA | 32.92 ± 0.88 | 34.19 ± 1.97 | 30.86 ± 1.00 |
| 16:1n-7 | 1.03 ± 0.07 b | 0.76 ± 0.06 a | 0.73 ± 0.08 a |
| 18:1n-9 | 13.41 ± 0.86 | 13.56 ± 1.33 | 14.59 ± 0.68 |
| 20:1n-9 | 0.77 ± 0.05 | 0.67 ± 0.46 | 0.54 ± 0.03 |
| MUFA | 15.44 ± 0.80 | 15.00 ± 1.48 | 15.86 ± 0.67 |
| 18:2n-6 | 7.72 ± 0.02 | 8.64 ± 0.36 | 8.59 ± 0.66 |
| 20:2n-6 | 0.39 ± 0.05 | 0.45 ± 0.06 | 0.47 ± 0.04 |
| 20:4n-6 | 2.50 ± 0.05 b | 1.75 ± 0.21 a | 1.65 ± 0.02 a |
| n-6 PUFA | 10.61 ± 0.12 | 10.84 ± 0.11 | 10.71 ± 0.69 |
| 18:3n-3 | 0.41 ± 0.02 b | 0.20 ± 0.03 a | 0.55 ± 0.13 b |
| 20:5n-3 | 5.91 ± 0.44 b | 2.36 ± 0.75 a | 2.07 ± 0.41 a |
| 22:5n-3 | 2.34 ± 0.05 b | 1.12 ± 0.06 a | 1.17 ± 0.02 a |
| 22:6n-3 | 21.91 ± 0.71 a | 23.42 ± 0.45 a | 26.18 ± 0.30 b |
| n-3 PUFA | 30.57 ± 0.89 | 27.12 ± 0.92 | 29.97 ± 0.57 |
| DHA/EPA | 3.71 ± 0.12 a | 10.53 ± 2.63 b | 13.01 ± 2.70 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Li, H.; Song, Z.; Gao, Q.; Bian, C.; Ma, Q.; Wei, Y.; Liang, M.; Xu, H. Mixtures of Algal Oil and Terrestrial Oils in Diets of Tiger Puffer (Takifugu rubripes). Animals 2025, 15, 1187. https://doi.org/10.3390/ani15091187
Zhang L, Li H, Song Z, Gao Q, Bian C, Ma Q, Wei Y, Liang M, Xu H. Mixtures of Algal Oil and Terrestrial Oils in Diets of Tiger Puffer (Takifugu rubripes). Animals. 2025; 15(9):1187. https://doi.org/10.3390/ani15091187
Chicago/Turabian StyleZhang, Lu, Haoxuan Li, Ziling Song, Qingyan Gao, Chenchen Bian, Qiang Ma, Yuliang Wei, Mengqing Liang, and Houguo Xu. 2025. "Mixtures of Algal Oil and Terrestrial Oils in Diets of Tiger Puffer (Takifugu rubripes)" Animals 15, no. 9: 1187. https://doi.org/10.3390/ani15091187
APA StyleZhang, L., Li, H., Song, Z., Gao, Q., Bian, C., Ma, Q., Wei, Y., Liang, M., & Xu, H. (2025). Mixtures of Algal Oil and Terrestrial Oils in Diets of Tiger Puffer (Takifugu rubripes). Animals, 15(9), 1187. https://doi.org/10.3390/ani15091187








