Pilot Study: Simultaneous Daily Recording of Total Locomotor Activity and Heart Rate in Horses for Application in Precision Livestock Farming
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
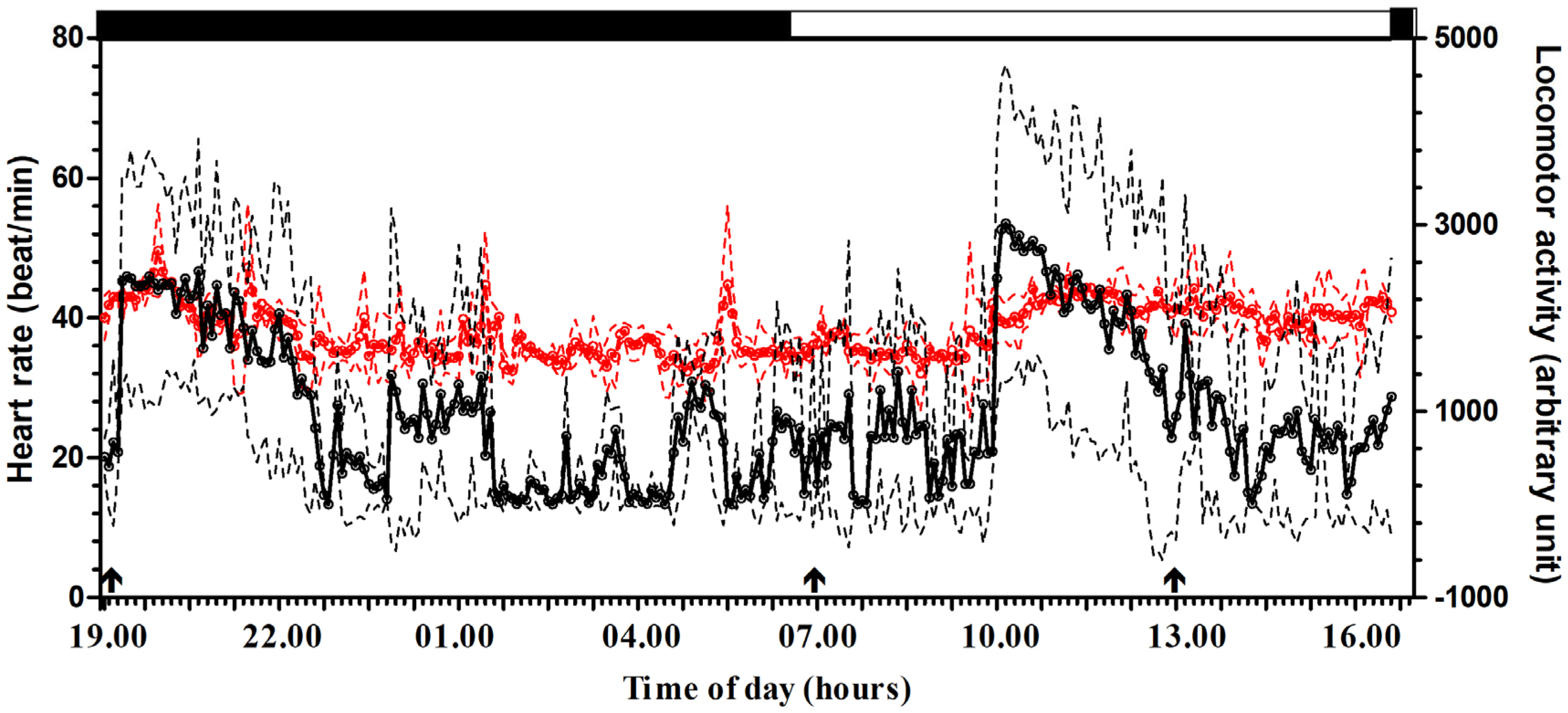
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TLA | Total locomotor activity |
| HR | Heart rate |
| PLF | Precision livestock farming |
References
- Bukhari, S.S.U.H.; Parkes, R.S.V.; Sneddon, L.U.; McElligott, A.G. The behavior and welfare of neglected species: Some examples from fish and mammals. Peer J. 2024, 12, e17149. [Google Scholar] [CrossRef] [PubMed]
- Lesimple, C. Indicators of Horse Welfare: State-of-the-Art. Animals 2020, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Banhazi, T.M.; Lehr, H.; Black, J.L.; Crabtree, H.; Schofield, P.; Tscharke, M.; Berckmans, D. Precision Livestock Farming: An International Review of Scientific and Commercial Aspects. Int. J. Agric. Biol. Eng. 2012, 5, 1. [Google Scholar] [CrossRef]
- Hanrahan, L.; Geoghegan, A.; O’Donovan, M.; Griffith, V.; Ruelle, E.; Wallace, M.; Shalloo, L. PastureBase Ireland: A Grassland Decision Support System and National Database. Comput. Electron. Agric. 2017, 136, 193–201. [Google Scholar] [CrossRef]
- Kuehne, G.; Llewellyn, R.; Pannell, D.J.; Wilkinson, R.; Dolling, P.; Ouzman, J.; Ewing, M. Predicting Farmer Uptake of New Agricultural Practices: A Tool for Research, Extension and Policy. Agric. Syst. 2017, 156, 115–125. [Google Scholar] [CrossRef]
- Borman, M.; Louhaichi, M.; Johnson, D.; Krueger, W. Combining Photographic and Precision Farming Methods to Measure Geese Grazing Impacts on Grass Seed Production. In Seed Production Research at Oregon State University USDA-ARS Cooperating; Oregon State University: Corvallis, OR, USA, 2001; pp. 63–65. [Google Scholar]
- Ruiz-Garcia, L.; Lunadei, L. The Role of RFID in Agriculture: Applications, Limitations and Challenges. Comput. Electron. Agric. 2011, 79, 42–50. [Google Scholar] [CrossRef]
- Cappai, M.G.; Rubiu, N.G.; Nieddu, G.; Bitti, M.P.L.; Pinna, W. Analysis of Fieldwork Activities during Milk Production Recording in Dairy Ewes by Means of Individual Ear Tag (ET) Alone or plus RFID Based Electronic Identification (EID). Comput. Electron. Agric. 2018, 144, 324–328. [Google Scholar] [CrossRef]
- Alvarenga, F.A.P.; Borges, I.; Palkovič, L.; Rodina, J.; Oddy, V.H.; Dobos, R.C. Using a Three-Axis Accelerometer to Identify and Classify Sheep Behaviour at Pasture. Appl. Anim. Behav. Sci. 2016, 181, 91–99. [Google Scholar] [CrossRef]
- Mansbridge, N.; Mitsch, J.; Bollard, N.; Ellis, K.; Miguel-Pacheco, G.G.; Dottorini, T.; Kaler, J. Feature Selection and Comparison of Machine Learning Algorithms in Classification of Grazing and Rumination Behaviour in Sheep. Sensors 2018, 18, 3532. [Google Scholar] [CrossRef]
- Plaza Martín, J.; Sánchez Martín, N.; Palacios Riocerezo, C.; Sánchez García, M.; Abecia, J.A.; Criado Nicolás, M.; Nieto de la Losa, J. GPS, LiDAR and VNIR Data to Monitor the Spatial Behavior of Grazing Sheep. J. Anim. Behav. Biometeorol. 2022, 10, 2214. [Google Scholar] [CrossRef]
- Wang, K.; Xuan, C.; Wu, P.; Liu, F.; Fan, X. Feeding Intake Estimation in Sheep Based on Ingestive Chewing Sounds. Comput. Electron. Agric. 2022, 194, 106698. [Google Scholar] [CrossRef]
- Anderson, D.; Estell, R.; Holechek, J.; Ivey, S.; Smith, G. Virtual Herding for Flexible Livestock Management—A Review. Rangel. J. 2014, 36, 205. [Google Scholar] [CrossRef]
- Xu, B.; Wang, W.; Falzon, G.; Kwan, P.; Guo, L.; Sun, Z.; Li, C. Livestock Classification and Counting in Quadcopter Aerial Images Using Mask R-CNN. Int. J. Remote Sens. 2020, 41, 8121–8142. [Google Scholar] [CrossRef]
- Fuchs, B.; Soerheim, K.; Chincarini, M.; Brunberg, E.; Stubsjøen, S.M.; Bratbergsengen, K.; Hvasshovd, S.-O.; Zimmermann, B.; Lande, U.; Grøva, L. Heart Rate Sensor Validation and Seasonal and Diurnal Variation of Body Temperature and Heart Rate in Domestic Sheep. Vet. Anim. Sci. 2019, 8, 100075. [Google Scholar] [CrossRef]
- Piccione, G.; Giannetto, C. State of the Art on Daily Rhythms of Physiology and Behaviour in Horses. Biol. Rhythm. Res. 2011, 42, 67–88. [Google Scholar] [CrossRef]
- Temple, D.; Courboulay, V.; Manteca, X.; Velarde, A.; Dalmau, A. The Welfare of Growing Pigs in Five Different Production Systems: Assessment of Feeding and Housing. Animal 2012, 6, 656–667. [Google Scholar] [CrossRef]
- Berckmans, D. Automatic On-Line Monitoring of Animals by Precision Livestock Farming. In Livestock Production and Society; Geers, R., Madec, F., Eds.; Brill|Wageningen Academic: Wageningen, The Netherlands, 2006; pp. 287–294. ISBN 978-90-8686-567-3. [Google Scholar]
- Mills, D.; Clarke, A. Housing, Management and Welfare. In The Welfare of Horses; Springer: Dordrecht, The Netherlands, 2007; pp. 77–97. ISBN 978-1-4020-6142-4. [Google Scholar]
- Arnold, W.; Ruf, T.; Kuntz, R. Seasonal Adjustment of Energy Budget in a Large Wild Mammal, the Przewalski Horse (Equus Ferus Przewalskii) II. Energy Expenditure. J. Exp. Biol. 2006, 209, 4566–4573. [Google Scholar] [CrossRef]
- Chaplin, S.J.; Gretgrix, L. Effect of Housing Conditions on Activity and Lying Behaviour of Horses. Animal 2010, 4, 792–795. [Google Scholar] [CrossRef]
- Bertolucci, C.; Giannetto, C.; Fazio, F.; Piccione, G. Seasonal Variations in Daily Rhythms of Activity in Athletic Horses. Animal 2008, 2, 1055–1060. [Google Scholar] [CrossRef]
- Kovács, L.; Jurkovich, V.; Bakony, M.; Szenci, O.; Póti, P.; Tőzsér, J. Welfare Implication of Measuring Heart Rate and Heart Rate Variability in Dairy Cattle: Literature Review and Conclusions for Future Research. Anim. Int. J. Anim. Biosci. 2013, 8, 316–330. [Google Scholar] [CrossRef]
- Laske, T.G.; Evans, A.L.; Arnemo, J.M.; Iles, T.L.; Ditmer, M.A.; Fröbert, O.; Garshelis, D.L.; Iaizzo, P.A. Development and Utilization of Implantable Cardiac Monitors in Free-Ranging American Black and Eurasian Brown Bears: System Evolution and Lessons Learned. Anim. Biotelemetry 2018, 6, 13. [Google Scholar] [CrossRef]
- Madliger, C.; Love, O.; Hultine, K.; Cooke, S. The Conservation Physiology Toolbox: Status and Opportunities. Conserv. Physiol. 2018, 6, coy029. [Google Scholar] [CrossRef] [PubMed]
- McCraty, R.; Mike, A.; Tomasino, D.; Bradley, R. The Coherent Heart Heart–Brain Interactions, Psychophysiological Coherence, and the Emergence of System-Wide Order. Integral Rev. 2009, 5, 2. [Google Scholar]
- Janczarek, I.; Kędzierski, W.; Wilk, I.; Wnuk-Pawlak, E.; Rakowska, A. Comparison of Daily Heart Rate Variability in Old and Young Horses: A Preliminary Study. J. Vet. Behav. 2020, 38, 1–7. [Google Scholar] [CrossRef]
- Auer, U.; Kelemen, Z.; Engl, V.; Jenner, F. Activity Time Budgets—A Potential Tool to Monitor Equine Welfare? Animals 2021, 11, 850. [Google Scholar] [CrossRef]
- Van Erp-van der Kooij, E.; Rutter, S.M. Using precision farming to improve animal welfare. CABI Rev. 2020, 15, 51. [Google Scholar] [CrossRef]
- Pesenti Rossi, G.; Dalla Costa, E.; Barbieri, S.; Minero, M.; Canali, E. A systematic review on the application of precision livestock farming technologies to detect lying, rest and sleep behavior in dairy calves. Front. Vet. Sci. 2024, 11, 1477731. [Google Scholar] [CrossRef]
- Niccolai, L.J.; Devineau, O.; Thiel, A.; Zimmermann, B.; Evans, L.A. Connecting the dots: Relationship between heart rate and overall dynamic body acceleration in free-ranging cattle. Conserv. Physiol. 2024, 12, coae085. [Google Scholar] [CrossRef]
- Nelson, W.; Tong, Y.L.; Lee, J.K.; Halberg, F. Methods for Cosinor-Rhythmometry. Chronobiologia 1979, 6, 305–323. [Google Scholar]
- Farsi, H.; Harti, D.; Achaâban, M.R.; Piro, M.; Ouassat, M.; Challet, E.; Pévet, P.; El Allali, K. Validation of locomotion scoring as a new an inexpensie technique to record ircadian locomotor activity in large mammals. Helyon 2018, 4, e00980. [Google Scholar] [CrossRef]
- Refinetti, R. Non-Stationary Time Series and the Robustness of Circadian Rhythms. J. Theor. Biol. 2004, 227, 571–581. [Google Scholar] [CrossRef]
- Giannetto, C.; Cerutti, R.; Scaglione, M.; Fazio, F.; Aragona, F.; Arfuso, F.; Zumbo, A.; Piccione, G. Simultaneous Recording of Subcutaneous Temperature and Total Locomotor Activity in Bos Taurus and Bos Indicus Raised in a Subtropical Region of Argentina. Trop. Anim. Health Prod. 2022, 54, 371. [Google Scholar] [CrossRef] [PubMed]
- Harewood, E.J.; McGowan, C.M. Behavioral and Physiological Responses to Stabling in Naive Horses. J. Equine Vet. Sci. 2005, 25, 164–170. [Google Scholar] [CrossRef]
- Piccione, G.; Messina, V.; Bazzano, M.; Giannetto, C.; Fazio, F. Heart Rate, Net Cost of Transport, and Metabolic Power in Horse Subjected to Different Physical Exercises. J. Equine Vet. Sci. 2013, 33, 586–589. [Google Scholar] [CrossRef]
- Giannetto, C.; Fazio, F.; Assenza, A.; Alberghina, D.; Panzera, M.; Piccione, G. Intrasubject and intersubject variabilities in the daily rhythm of total locomotor activity in horses. J. Vet. Behav. 2016, 12, 42–48. [Google Scholar] [CrossRef]
- Piccione, G.; Assenza, A.; Attanzio, G.; Fazio, F.; Caola, G. Chronophysiology of arterial blood pressure and heart rate in athletic horses. Slov. Vet. Res. 2001, 38, 243–248. [Google Scholar]
- Büttner, D.; Wollnik, F. Spontaneous short-term fluctuations in the daily pattern of heart rate, body temperature and locomotor activity in the laboratory rat. Lab. Anim. 1982, 16, 319–326. [Google Scholar] [CrossRef]
- Bojsen, B.H.; Witthøfft, H.; Styrishave, B.; Andersen, O. In Situ Studies on Heart Rate and Locomotor Activity in the Freshwater Crayfish, Astacus Astacus (L.) in Relation to Natural Fluctuations in Temperature and Light Intensity. Freshw. Biol. 1998, 39, 455–465. [Google Scholar] [CrossRef]
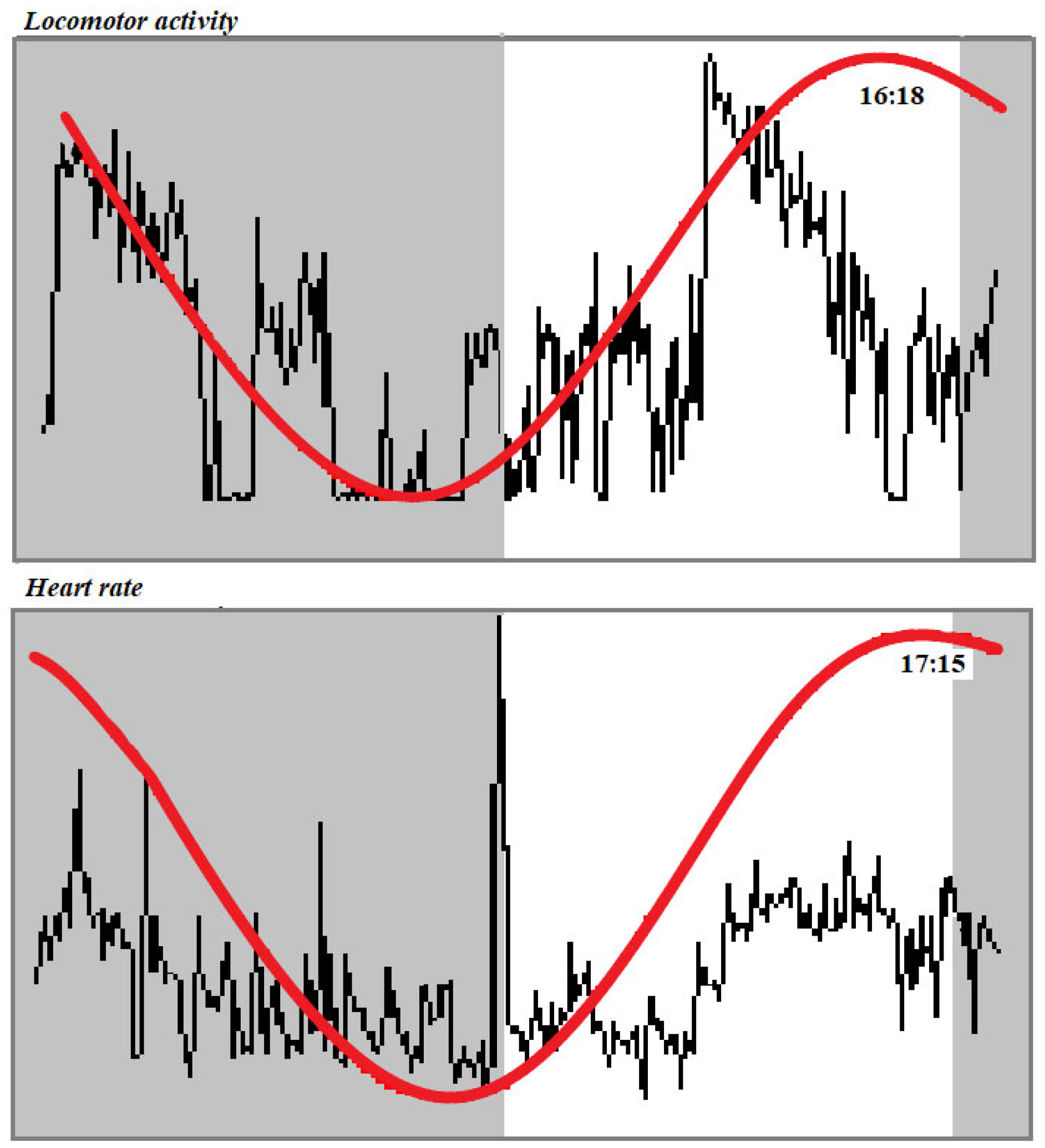

| Mesor | Amplitude | Acrophase (h·min) | Robustness (%) | |
|---|---|---|---|---|
| Total locomotor activity (arbitrary unit) | 1018.60 ± 689.13 | 543.38 ± 407.25 | 17.05 ± 1.15 | 17.95 ± 10.53 |
| Heart rate (beats/min) | 38.33 ± 0.43 | 3.65 ± 1.37 | 16.40 ± 0.30 | 37.05 ± 0.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragona, F.; Rizzo, M.; Arrigo, F.; Arfuso, F.; Fazio, F.; Giudice, E.; Pugliatti, P.; Piccione, G.; Giannetto, C. Pilot Study: Simultaneous Daily Recording of Total Locomotor Activity and Heart Rate in Horses for Application in Precision Livestock Farming. Animals 2025, 15, 1189. https://doi.org/10.3390/ani15091189
Aragona F, Rizzo M, Arrigo F, Arfuso F, Fazio F, Giudice E, Pugliatti P, Piccione G, Giannetto C. Pilot Study: Simultaneous Daily Recording of Total Locomotor Activity and Heart Rate in Horses for Application in Precision Livestock Farming. Animals. 2025; 15(9):1189. https://doi.org/10.3390/ani15091189
Chicago/Turabian StyleAragona, Francesca, Maria Rizzo, Federica Arrigo, Francesca Arfuso, Francesco Fazio, Elisabetta Giudice, Pietro Pugliatti, Giuseppe Piccione, and Claudia Giannetto. 2025. "Pilot Study: Simultaneous Daily Recording of Total Locomotor Activity and Heart Rate in Horses for Application in Precision Livestock Farming" Animals 15, no. 9: 1189. https://doi.org/10.3390/ani15091189
APA StyleAragona, F., Rizzo, M., Arrigo, F., Arfuso, F., Fazio, F., Giudice, E., Pugliatti, P., Piccione, G., & Giannetto, C. (2025). Pilot Study: Simultaneous Daily Recording of Total Locomotor Activity and Heart Rate in Horses for Application in Precision Livestock Farming. Animals, 15(9), 1189. https://doi.org/10.3390/ani15091189







