Cold-Induced DHRS4 Promotes Thermogenesis via Enhanced Fatty Acid β-Oxidation in Porcine Subcutaneous Adipocytes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Data Analysis of snRNA-Seq
2.3. Isolation of SVF and Differentiation of Preadipocytes
2.4. Oil Red O Staining
2.5. Construction of DHRS4 Cell Line
2.6. Overexpression of DHRS4 in SVF Cells
2.7. RNA Isolation and RT-qPCR
2.8. Western Blotting
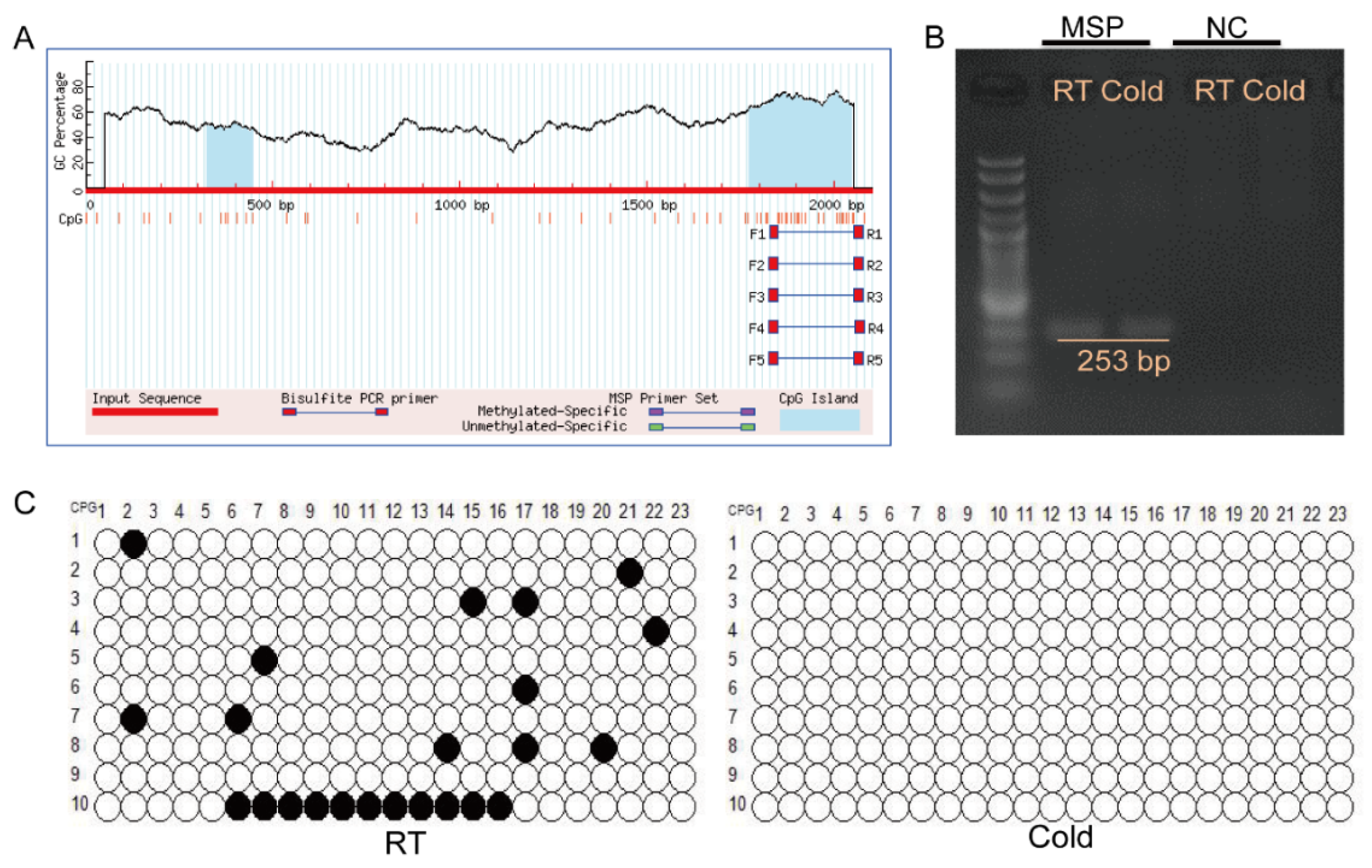
2.9. Analysis of DNA Methylation Levels in the Promoter Region of DHRS4
2.10. Determination of Free Fatty Acids
2.11. Statistical Analysis
3. Results
3.1. Subclusters of Adipocytes at Single-Cell Resolution
3.2. Upregulation of DHRS4 Expression Was Observed in the Cold-Exposed Group
3.3. DHRS4 Enhances Thermogenesis in ISP4# Cells by Upregulating Fatty Acid β-Oxidation
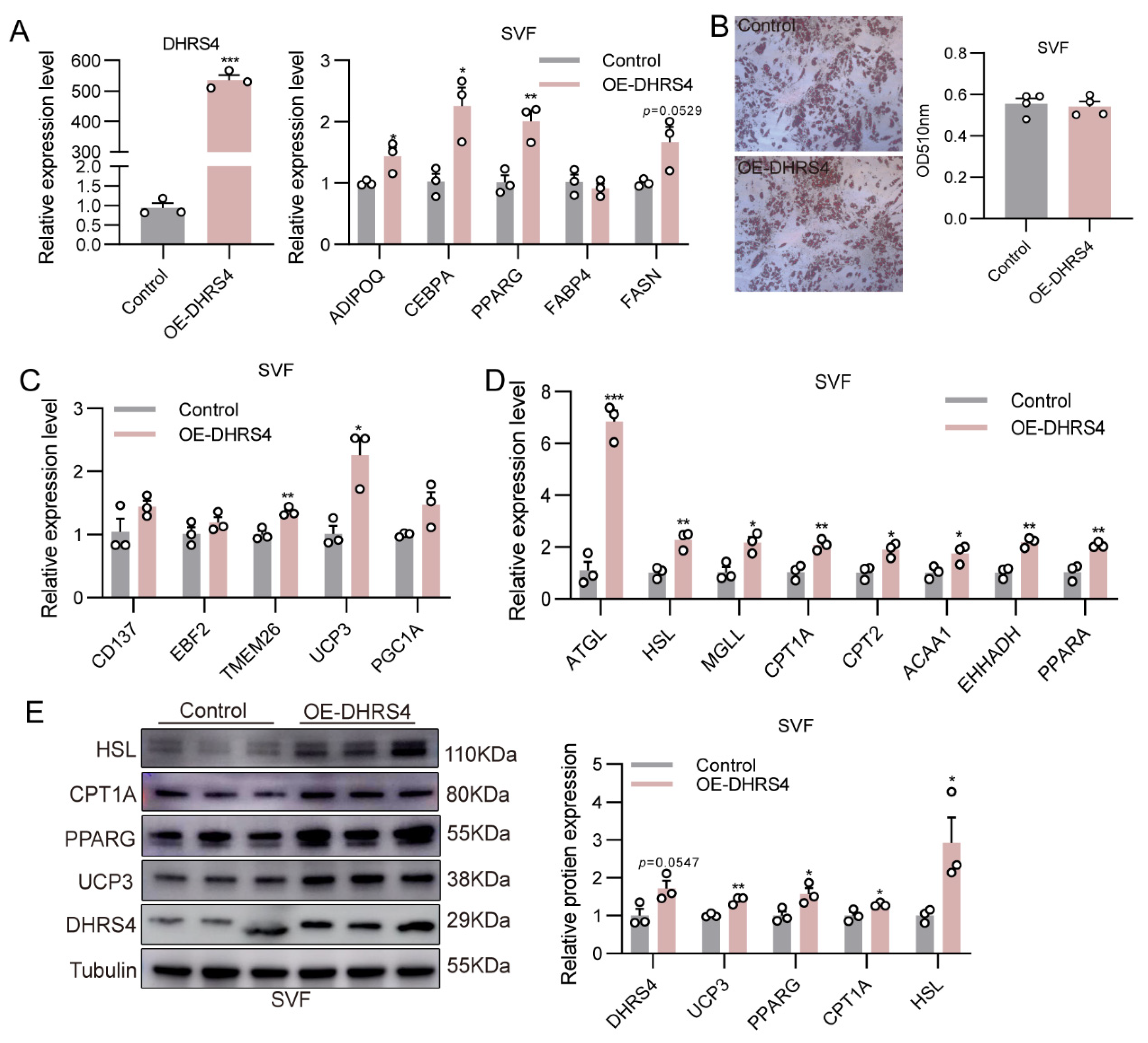
3.4. DHRS4 Promotes Thermogenesis in SVF via Fatty Acid β-Oxidation
3.5. Cold Exposure Activates the Methylation of the DHRS4 Promoter Region
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Cereijo, R.; Villarroya, J.; Giralt, M. Brown adipose tissue as a secretory organ. Nat. Rev. Endocrinol. 2017, 13, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Chouchani, E.T.; Kajimura, S. Metabolic adaptation and maladaptation in adipose tissue. Nat. Metab. 2019, 1, 189–200. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Song, Z.; Xiaoli, A.M.; Yang, F. Regulation and Metabolic Significance of De Novo Lipogenesis in Adipose Tissues. Nutrients 2018, 10, 1383. [Google Scholar] [CrossRef] [PubMed]
- Jeon, Y.G.; Kim, Y.Y.; Lee, G.; Kim, J.B. Physiological and pathological roles of lipogenesis. Nat. Metab. 2023, 5, 735–759. [Google Scholar] [CrossRef]
- Cho, C.H.; Patel, S.; Rajbhandari, P. Adipose tissue lipid metabolism: Lipolysis. Curr. Opin. Genet. Dev. 2023, 83, 102114. [Google Scholar] [CrossRef]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J. The Biochemistry and Physiology of Mitochondrial Fatty Acid β-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernández-Fernández, C.; Mouriño-Bayolo, D. Mitochondrial β-oxidation of saturated fatty acids in humans. Mitochondrion 2019, 46, 73–90. [Google Scholar] [CrossRef]
- Sepa-Kishi, D.M.; Jani, S.; Da Eira, D.; Ceddia, R.B. Cold acclimation enhances UCP1 content, lipolysis, and triacylglycerol resynthesis, but not mitochondrial uncoupling and fat oxidation, in rat white adipocytes. Am. J. Physiol. Cell Physiol. 2019, 316, C365–C376. [Google Scholar] [CrossRef]
- Sadler, D.G.; Treas, L.; Sikes, J.D.; Porter, C. A modest change in housing temperature alters whole body energy expenditure and adipocyte thermogenic capacity in mice. Am. J. Physiol. Endocrinol. Metab. 2022, 323, E517–E528. [Google Scholar] [CrossRef] [PubMed]
- Valdivia, L.F.G.; Castro, É.; Eichler, R.; Moreno, M.F.; de Sousa, É.; Jardim, G.F.R.; Peixoto, Á.S.; Moraes, M.N.; Castrucci, A.M.L.; Nedergaard, J.; et al. Cold acclimation and pioglitazone combined increase thermogenic capacity of brown and white adipose tissues but this does not translate into higher energy expenditure in mice. Am. J. Physiol. Endocrinol. Metab. 2023, 324, E358–E373. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Qimuge, N.R.; Qin, J.; Cai, R.; Li, X.; Chu, G.Y.; Pang, W.J.; Yang, G.S. Acute and chronic cold exposure differentially affects the browning of porcine white adipose tissue. Animal 2018, 12, 1435–1441. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Wang, L.; Ling, D.; Nong, Q.; Xie, J.; Zhu, X.; Shan, T. Cold Exposure Induces Depot-Specific Alterations in Fatty Acid Composition and Transcriptional Profile in Adipose Tissues of Pigs. Front. Endocrinol. 2022, 13, 827523. [Google Scholar] [CrossRef]
- Huang, D.Y.; Ichikawa, Y. Purification and characterization of a novel cytosolic NADP(H)-dependent retinol oxidoreductase from rabbit liver. Biochim. Biophys. Acta 1997, 1338, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Li, R.; Song, X.; Liu, G.; Li, Y.; Chang, X.; Li, C.; Huang, D. Identification of a novel isoform of DHRS4 protein with a nuclear localization signal. Gene 2012, 494, 161–167. [Google Scholar] [CrossRef]
- Matsunaga, T.; Endo, S.; Maeda, S.; Ishikura, S.; Tajima, K.; Tanaka, N.; Nakamura, K.T.; Imamura, Y.; Hara, A. Characterization of human DHRS4: An inducible short-chain dehydrogenase/reductase enzyme with 3beta-hydroxysteroid dehydrogenase activity. Arch. Biochem. Biophys. 2008, 477, 339–347. [Google Scholar] [CrossRef]
- Endo, S.; Maeda, S.; Matsunaga, T.; Dhagat, U.; El-Kabbani, O.; Tanaka, N.; Nakamura, K.T.; Tajima, K.; Hara, A. Molecular determinants for the stereospecific reduction of 3-ketosteroids and reactivity towards all-trans-retinal of a short-chain dehydrogenase/reductase (DHRS4). Arch. Biochem. Biophys. 2009, 481, 183–190. [Google Scholar] [CrossRef]
- Xiao, L.; Zhang, Y.; Luo, Q.; Guo, C.; Chen, Z.; Lai, C. DHRS4-AS1 regulate gastric cancer apoptosis and cell proliferation by destabilizing DHX9 and inhibited the association between DHX9 and ILF3. Cancer Cell Int. 2023, 23, 304. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, K.; Zou, X.; Hua, Z.; Wang, H.; Bian, W.; Wang, H.; Chen, F.; Dai, T. LncRNA DHRS4-AS1 ameliorates hepatocellular carcinoma by suppressing proliferation and promoting apoptosis via miR-522-3p/SOCS5 axis. Bioengineered 2021, 12, 10862–10877. [Google Scholar] [CrossRef]
- Xu, S.; Zheng, X.; Wu, H.; You, X.; Wu, J.; Zhou, H.; Wu, J.; Liu, Q.; Ye, R.Q. Feasibility study of lncRNA DHRS4-AS1 sponge miR-222-3p in the diagnosis of thyroid cancer. Endokrynol. Pol. 2024, 75, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, G.; Zhang, Z.; Wang, Z.; Ren, M.; Wang, X.; Li, H.; Yu, Y.; Liu, J.; Cai, L.; et al. The downregulated long noncoding RNA DHRS4-AS1 is protumoral and associated with the prognosis of clear cell renal cell carcinoma. OncoTargets Ther. 2018, 11, 5631–5646. [Google Scholar] [CrossRef]
- Cheng, Y.M.; Hong, P.C.; Song, M.M.; Zhu, H.N.; Qin, J.; Zhang, Z.D.; Chen, H.; Ma, X.Z.; Tian, M.Y.; Zhu, W.Y.; et al. An immortal porcine preadipocyte cell strain for efficient production of cell-cultured fat. Commun. Biol. 2023, 6, 1202. [Google Scholar] [CrossRef]
- Sun, W.; Dong, H.; Becker, A.S.; Dapito, D.H.; Modica, S.; Grandl, G.; Opitz, L.; Efthymiou, V.; Straub, L.G.; Sarker, G.; et al. Cold-induced epigenetic programming of the sperm enhances brown adipose tissue activity in the offspring. Nat. Med. 2018, 24, 1372–1383. [Google Scholar] [CrossRef]
- Taylor, B.C.; Steinthal, L.H.; Dias, M.; Yalamanchili, H.K.; Ochsner, S.A.; Zapata, G.E.; Mehta, N.R.; McKenna, N.J.; Young, N.L.; Nuotio-Antar, A.M. Histone proteoform analysis reveals epigenetic changes in adult mouse brown adipose tissue in response to cold stress. Epigenet. Chromatin 2024, 17, 12. [Google Scholar] [CrossRef]
- Weiner, J.; Rohde, K.; Krause, K.; Zieger, K.; Klöting, N.; Kralisch, S.; Kovacs, P.; Stumvoll, M.; Blüher, M.; Böttcher, Y.; et al. Brown adipose tissue (BAT) specific vaspin expression is increased after obesogenic diets and cold exposure and linked to acute changes in DNA-methylation. Mol. Metab. 2017, 6, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Ahn, J.; Suh, Y.; Ziouzenkova, O.; Lee, J.W.; Lee, K. Retinol Binding Protein 7 Promotes Adipogenesis in vitro and Regulates Expression of Genes Involved in Retinol Metabolism. Front. Cell Dev. Biol. 2022, 10, 876031. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, Y.; Wu, G.; Zheng, J.; Lu, H.; Shi, X.; Yang, G. Ectopic expression of RBP4 impairs the insulin pathway and inguinal fat deposition in mice. J. Physiol. Biochem. 2014, 70, 479–486. [Google Scholar] [CrossRef]
- Cheng, J.; Song, Z.Y.; Pu, L.; Yang, H.; Zheng, J.M.; Zhang, Z.Y.; Shi, X.E.; Yang, G.S. Retinol binding protein 4 affects the adipogenesis of porcine preadipocytes through insulin signaling pathways. Biochem. Cell Biol. 2013, 91, 236–243. [Google Scholar] [CrossRef]
- Wu, Y.; Huang, T.; Li, X.; Shen, C.; Ren, H.; Wang, H.; Wu, T.; Fu, X.; Deng, S.; Feng, Z.; et al. Retinol dehydrogenase 10 reduction mediated retinol metabolism disorder promotes diabetic cardiomyopathy in male mice. Nat. Commun. 2023, 14, 1181. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Ye, Z.; Li, M.; Wei, W.; Chen, J.; Zhang, L. Cold-Induced DHRS4 Promotes Thermogenesis via Enhanced Fatty Acid β-Oxidation in Porcine Subcutaneous Adipocytes. Animals 2025, 15, 1190. https://doi.org/10.3390/ani15091190
Ma X, Ye Z, Li M, Wei W, Chen J, Zhang L. Cold-Induced DHRS4 Promotes Thermogenesis via Enhanced Fatty Acid β-Oxidation in Porcine Subcutaneous Adipocytes. Animals. 2025; 15(9):1190. https://doi.org/10.3390/ani15091190
Chicago/Turabian StyleMa, Xiangfei, Zijian Ye, Mengting Li, Wei Wei, Jie Chen, and Lifan Zhang. 2025. "Cold-Induced DHRS4 Promotes Thermogenesis via Enhanced Fatty Acid β-Oxidation in Porcine Subcutaneous Adipocytes" Animals 15, no. 9: 1190. https://doi.org/10.3390/ani15091190
APA StyleMa, X., Ye, Z., Li, M., Wei, W., Chen, J., & Zhang, L. (2025). Cold-Induced DHRS4 Promotes Thermogenesis via Enhanced Fatty Acid β-Oxidation in Porcine Subcutaneous Adipocytes. Animals, 15(9), 1190. https://doi.org/10.3390/ani15091190





