Behavioral Characteristics of Largefin Longbarbel Catfish Hemibagrus macropterus: Effects of Sex and Body Size on Aggression and Shelter Selection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Design and Methods
2.2.1. Body Size Effects Experiment
2.2.2. Sex Effects Experiment
2.2.3. Shelter Selection Experiment
2.3. Data Collection
2.4. Behavioral Quantification and Data Analysis
3. Results
3.1. Behavioral Characteristics of H. macropterus
3.1.1. Daily Behavior
3.1.2. Territorial Behavior
3.1.3. Aggressive Behavior
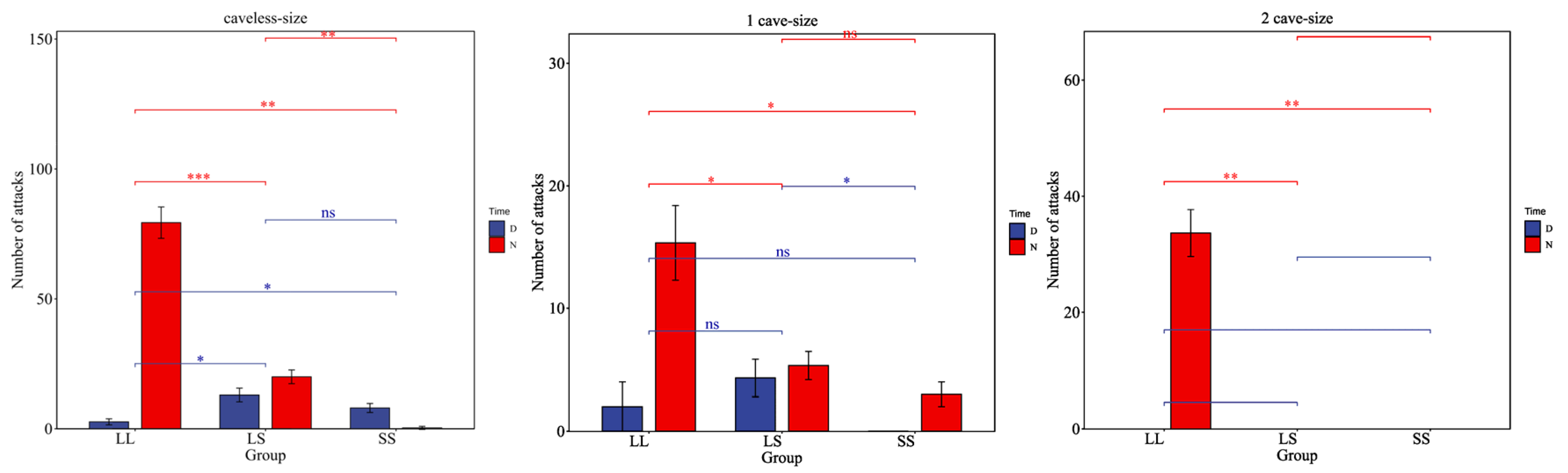
3.2. The Impact of Size on Aggressive Behavior of H. macropterus
3.3. The Impact of Sex on the Aggressive Behavior of H. macropterus
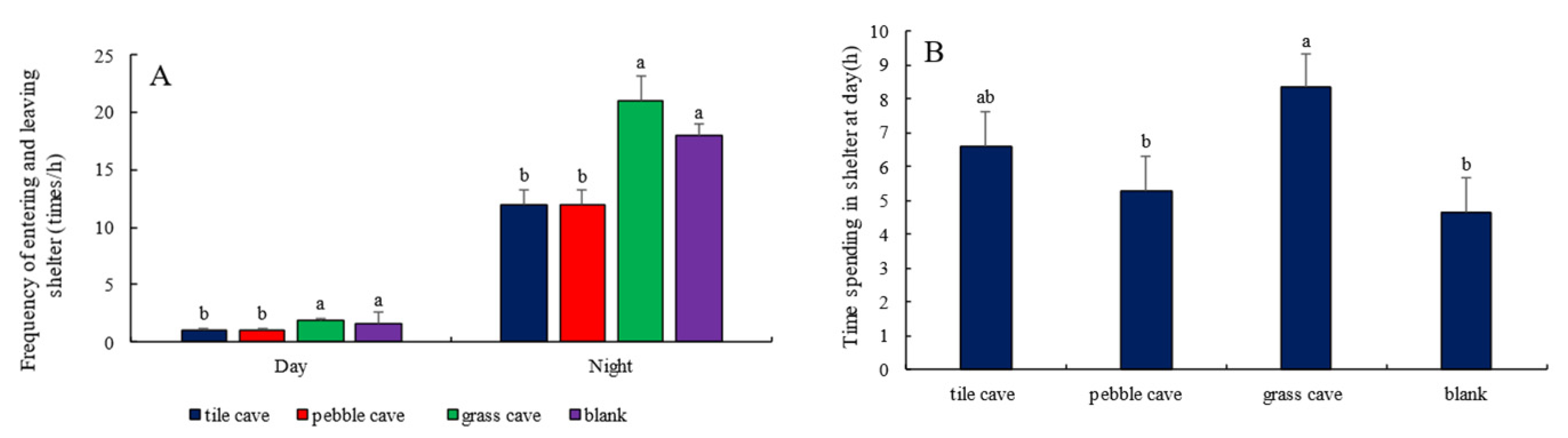
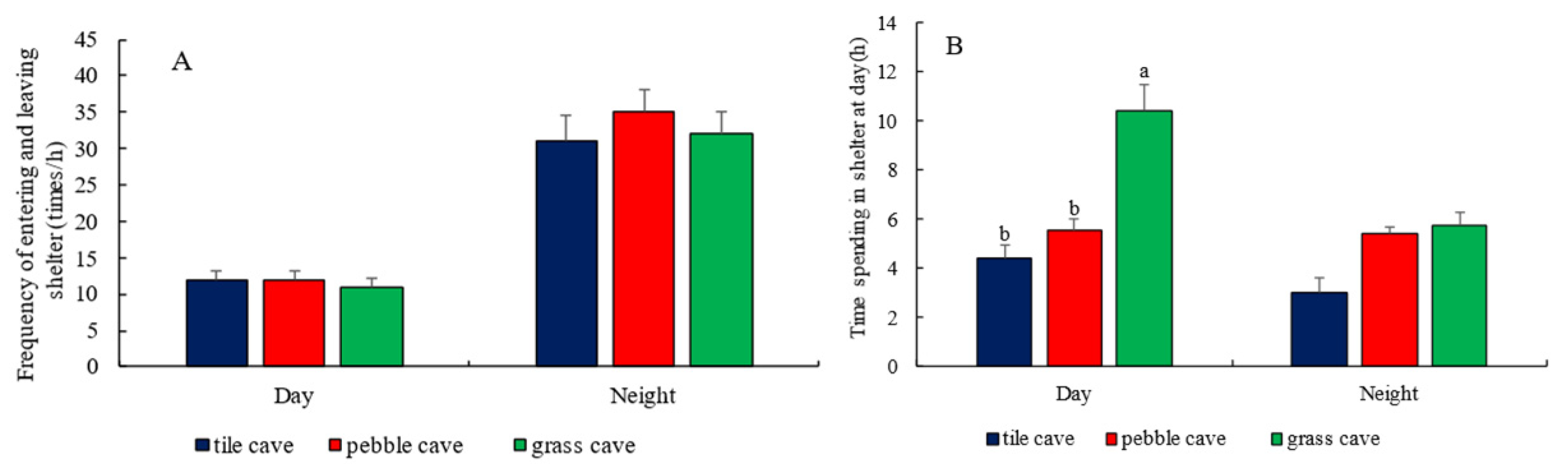
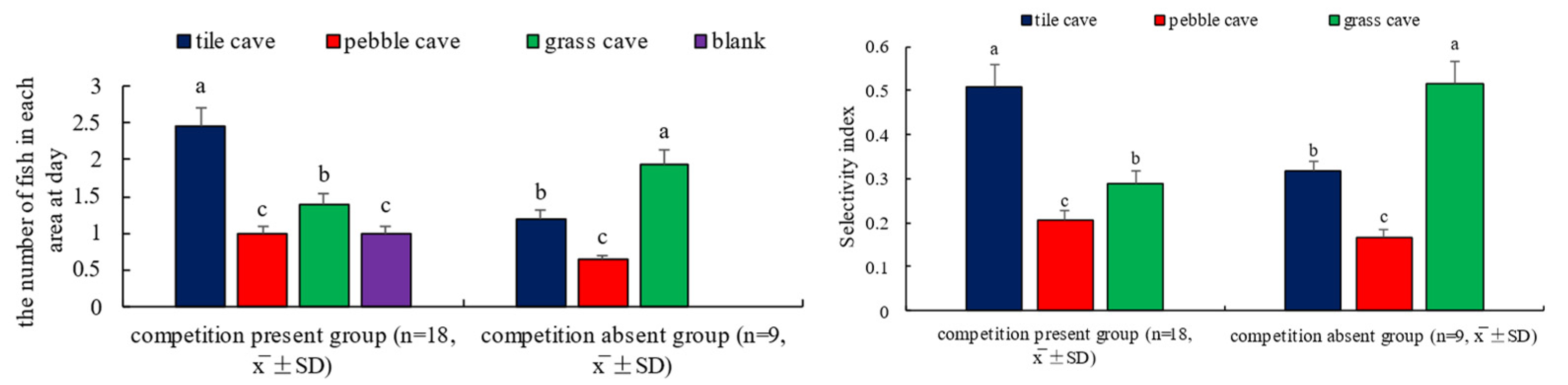
3.4. Selection of Shelter by H. macropterus
4. Discussion
4.1. Diurnal Rest and Nocturnal Activity, and Aggressive Behavior in H. macropterus
4.2. Influence of Individual Size on Aggressive Behavior
4.3. Influence of Sex on Aggressive Behavior
4.4. Shelter Selection by H. macropterus
4.5. Relationship Between the Number of Shelters and Attack Frequency
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Rong, K.; Qin, L.R.; Li, W.W.; Duan, M.; Zhang, T.L.; Liu, J.S. Research progress of aquatic animal behavior and its application in fisheries. Acta Hydrobiol. Sin. 2021, 45, 1171–1180. [Google Scholar]
- Zhang, P.D.; Zhang, X.M.; Li, J. Research advances in behavioral ecology of penaeid shrimp II. Effects of environmental factors on behavior of penaeid shrimps. Chin. J. Appl. Ecol. 2006, 17, 340–344. (In Chinese) [Google Scholar]
- Curtis, D.L.; Jensen, E.K.; McGaw, I.J. Behavioral influences on the physiological responses of Cancer gracilis, the graceful crab, during hyposaline exposure. Biol. Bull. 2007, 212, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Arnott, G.; Elwood, R. Probing aggressive motivation in a cichlid fish. Biol. Lett. 2009, 5, 762–764. [Google Scholar] [CrossRef]
- Miki, T.; Nakatsukasa, H.; Takahashi, N.; Murata, O.; Ishibashi, Y. Aggressive behaviour and cannibalism in greater amberjack, (Seriola dumerili): Effects of stocking density, feeding conditions and size differences. Aquac. Res. 2011, 42, 1339–1349. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Miki, T.; Sawada, Y.; Kurata, M. Effects of feeding conditions and size differences on aggressive behaviour and cannibalism in the Pacific bluefin tuna(Thunnus orientalis) (Temminck and Schlegel) larvae. Aquac. Res. 2013, 45, 45–53. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, Q.Y.; Fan, S.N.; Sun, N.; Li, X.D.; Zheng, Y. Aggressive behavior variation and experience effects in three families of juvenile Chinese mitten crab (Eriocheir sinensis). Behav. Process. 2019, 165, 44–50. [Google Scholar] [CrossRef]
- Sakakura, Y.; Tsukamoto, K. Onset and development of aggressive behavior in the early life stage of Japanese flounder. Fish. Sci. 2002, 68, 854–861. [Google Scholar] [CrossRef][Green Version]
- Magellan, K.; Johnson, A.; Williamson, L.; Richardson, M.; Watt, W.; Kaiser, H. Alteration of tank dimensions reduces male aggression in the swordtail. J. Appl. Ichthyol. 2012, 28, 91–94. [Google Scholar] [CrossRef]
- Stevens, K.N.; Asher, L.; Griffin, K.; Friel, M.; O’Connell, N.; Collins, L.M. A comparison of inferential analysis methods for multilevel studies: Implications for drawing conclusions in animal welfare science. Appl. Anim. Behav. Sci. 2017, 197, 101–111. [Google Scholar] [CrossRef]
- Su, X.P.; Liu, J.J.; Wang, F.; Zhang, D.; Zhu, B.; Liu, D. Effect of temperature on agonistic behavior and energy metabolism of the swimming crab (Portunus trituberculatus). Aquaculture 2020, 516, 734573. [Google Scholar] [CrossRef]
- Qiu, N.; Li, W.J.; Hou, M.M.; Wang, J.W. The selective preference of Rare Minnow (Gobiocypris rarus) to Different Habitats. Chin. J. Zool. 2021, 56, 856–864. (In Chinese) [Google Scholar]
- Guo, H.; Zhang, X.; Johnsson, J.I. Effects of size distribution on social interactions and growth of juvenile black rockfish (Sebastes schlegelii). Appl. Anim. Behav. Sci. 2017, 194, 135–142. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.G. Silurus meridionalis . Bull. Biol. 2005, 40, 12–13. (In Chinese) [Google Scholar]
- Zeng, L.Q.; Cao, Z.D.; Fu, S.J.; Peng, J.L.; Wang, Y.X. Effect of temperature on swimming performance in juvenile southern catfish (Silurus meridionalis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 153, 125–130. [Google Scholar] [CrossRef]
- Rantin, B.; Bichuette, M.E. Territoriality and agonistic behavior of subterranean Copionodontinae catfish (Siluriformes: Trichomycteridae) from Brazil. Acta Ethol. 2019, 22, 17–28. [Google Scholar] [CrossRef]
- Król, J.; Długoński, A.; Błażejewski, M.; Hliwa, P. Effect of size sorting on growth, cannibalism, and survival in Eurasian perch Perca fluviatilis L. post-larvae. Aquac. Int. 2019, 27, 945–955. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, W.; Li, G. Isolation and characterisation of collagens from the skin of largefin longbarbel catfish (Mystus macropterus). Food Chem. 2009, 115, 826–831. [Google Scholar] [CrossRef]
- Xiang, X.; Ye, Y.T.; Zhou, X.H.; Lin, S.M.; Luo, L.; Wang, Y.H. Digestive ability and nutritive value of Hemibagrus macropterus. J. Fish. China 2003, 27, 371–376. (In Chinese) [Google Scholar]
- Li, X.; Zhu, Y.; Yang, D.; Wu, X.; Li, X.; Zhu, T.; Meng, Z.; Shi, Z.; Zhang, Y. Research progress and prospect of artificial propagation of Mystus macropterus. Fish. Sci. Technol. Inf. 2023, 50, 128–132. (In Chinese) [Google Scholar]
- Shang, Y.C. Behavioral Ecology; Peking University Press: Beijing, China, 1998; pp. 105–110. [Google Scholar]
- Xi, J.; Zheng, Z.L.; Mu, Z.B.; Liu, F.; Liu, X.Y.; Shen, J.; Liu, H.P.; Zhou, Y. Study on the hiding behavior of the Glyptosternum maculatum larvae and juveniles. Acta Hydrobiol. Sin. 2021, 45, 1129–1137. (In Chinese) [Google Scholar]
- Fang, H. Selection of Shelter for Crayfish (Procambarus clarkii) and the Effect of Shelter on Crayfish; Nanjing University: Nanjing, China, 2019. (In Chinese) [Google Scholar]
- Wang, L.Q.; Cheng, Y.S. On the feeding habits and growth for larval of Clarias lazera under pond nursery. J. Fish. China 1990, 14, 105–113. (In Chinese) [Google Scholar]
- Zhou, G.; Xie, C.X.; Xiong, C.X. Preliminary research on day-and-night rhythm and daily feeds intake rate for snakehead fish fry. J. Huazhong Agric. Univ. 1996, 1, 64–67. (In Chinese) [Google Scholar]
- Trajano, E.; Mugue, N.; Krejca, J.; Vidthayanon, C.; Smart, D.; Borowsky, R. Habitat, distribution, ecology and behavior of cave batilorids from Thailand (Teleostei: Cypriniformes). Ichthyol. Explor. Freshw. 2002, 13, 169–184. [Google Scholar]
- Sapolsky, R.M. The influence of social hierarchy on primate health. Science 2005, 308, 648–652. [Google Scholar] [CrossRef]
- Miyai, C.A.; Sanches, F.H.C.; Costa, T.M.; Colpo, K.D.; Volpato, G.L.; Barreto, R.E. The correlation between subordinate fish eye colour and received attacks: A negative social feedback mechanism for the reduction of aggression during the formation of dominance hierarchies. Zoology 2011, 114, 335–339. [Google Scholar] [CrossRef]
- Wang, C.L.; Zeng, X.C.; Shen, J.Y.; Huang, C. Behavior response of dominant and subordinate crayfish Procambarus clarkii to social context change by a larger intruder. Chin. J. Zool. 2015, 50, 555–562. (In Chinese) [Google Scholar]
- Martins, C.I.M.; Aanyu, M.; Schrama, J.W.; Verreth, J.A.J. Size distribution in African catfish (Clarias gariepinus) affects feeding behaviour but not growth. Aquaculture 2005, 250, 300–307. [Google Scholar] [CrossRef]
- Salas-Leiton, E.; Anguís, V.; Rodríguez-Rúa, A.; Cañavate, J.P. Stocking homogeneous size groups does not improve growth performance of Senegalese sole (Solea senegalensis, Kaup 1858) juveniles: Individual growth related to fish size. Aquac. Eng. 2010, 43, 108–113. [Google Scholar] [CrossRef]
- Enquist, M.; Leimar, O. Evolution of fighting behaviour: Decision rules and assessment of relative strength. J. Theor. Biol. 1983, 102, 387–410. [Google Scholar] [CrossRef]
- Johnsson, J.I.; Nöbbelin, F.; Bohlin, T. Territorial competition among wild brown trout fry: Effects of ownership and body size. J. Fish Biol. 1999, 54, 469–472. [Google Scholar] [CrossRef]
- Johnsson, J.I.; Winberg, S.; Sloman, K.A. Social interactions. In Behaviour and Physiology of Fish, Fish Physiology 24; Sloman, K., Wilson, R., Balshine, S., Eds.; Academic Press: Cambridge, MA, USA, 2006; pp. 151–196. [Google Scholar]
- Vehanen, T.; Mäki-Petäys, A.; Aspi, J.; Muotka, T. Intercohort competition causes spatial segregation in brown trout in artificial streams. J. Fish Biol. 1999, 55, 35–46. [Google Scholar] [CrossRef]
- Colella, D.J.; Paijmans, K.C.; Wong, M.Y.L. Size, sex and social experience: Experimental tests of multiple factors mediating contest behaviour in a rockpool fish. Ethology 2019, 125, 369–379. [Google Scholar] [CrossRef]
- Evans, D.L.; Shehadi-Moacdieh, M. Body size and prior residency in staged encounters between female prawns, Palaemon elegans Rathke (Decapoda: Palaemonidae). Anim. Behav. 1988, 36, 452–455. [Google Scholar] [CrossRef]
- Briffa, M.; Dallaway, D. Inter-sexual contests in the hermit crab Pagurus bernhardus: Females fight harder but males win more encounters. Behav. Ecol. Sociobiol. 2007, 61, 1781–1787. [Google Scholar] [CrossRef]
- Arnott, G.; Elwood, R.W. Sex differences in aggressive behaviour in convict cichlids. Anim. Behav. 2009, 78, 1221–1227. [Google Scholar] [CrossRef]
- Draud, M.; Macías-Ordóñez, R.; Verga, J.; Itzkowitz, M. Female and male Texas cichlids (Herichthys cyanoguttatum) do not fight by the same rules. Behav. Ecol. 2004, 15, 102–108. [Google Scholar] [CrossRef]
- Reddon, A.R.; Voisin, M.R.; Menon, N.; Marsh-Rollo, S.E.; Wong, M.Y.L.; Balshine, S. Rules of engagement for resource contests in a social fish. Anim. Behav. 2011, 82, 93–99. [Google Scholar] [CrossRef]
- Wong, M.Y.L.; Buston, P.M.; Munday, P.L.; Jones, G.P. Monogamy when there is potential for polygyny: Tests of multiple hypotheses in a group-living fish. Behav. Ecol. 2008, 19, 353–361. [Google Scholar] [CrossRef]
- Ward, G.; FitzGerald, G.J. Effects of sex ratio on male behaviour and reproductive success in a field population of threes pine sticklebacks (Gasterosteus aculeatus) (Pisces: Gasterosteidae). J. Zool. 1988, 215, 597–610. [Google Scholar] [CrossRef]
- Trivers, R.L. Parental investment and sexual selection. In Sexual Selection and the Descent of Man; Campbell, B., Ed.; Aldine: Chicago, IL, USA, 1972; pp. 136–179. [Google Scholar]
- Emlen, S.T.; Oring, L.W. Ecology, sexual selection, and the evolution of mating systems. Science 1977, 197, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Neat, F.C.; Huntingford, F.A.; Beveridge, M.M.C. Fighting and assessment in male cichlid fish: The effects of asymmetries in gonadal state and body size. Anim. Behav. 1998, 55, 883. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, X.J. Effect of body size and temperature on the metabolism of bagrid catfish, Mystus macropterus. J. Southwest China Norm. Univ. (Nat. Sci. Ed.) 2006, 31, 138–142. (In Chinese) [Google Scholar]
- Martin, S.D.; Harris, B.A.; Collums, J.R.; Bonett, R.M. Life between predators and a small space: Substrate selection of an interstitial space-dwelling stream salamander. J. Zool. 2012, 287, 205–214. [Google Scholar] [CrossRef]
- Medeiros, P.R.; Souza, A.T.; Ilarri, M.I. Habitat use and behavioural ecology of the juveniles of two sympatric damselfishes (Actinopterygii: Pomacentridae) in the south western Atlantic Ocean. J. Fish Biol. 2010, 77, 1599–1615. [Google Scholar] [CrossRef]
- Simmons, C.M.; Szedlmayer, S.T. Territoriality, reproductive behavior, and parental care in gray triggerfish, Balistes capriscus, from the northern gulf of Mexico. Bull. Mar. Sci. 2012, 88, 197–209. [Google Scholar] [CrossRef]
- Liu, Q. Procambarus clarkii Response to Two Kinds of Environmental Heterogeneity and the Selection of Shelter; Nanjing University: Nanjing, China, 2017. (In Chinese) [Google Scholar]
- Chen, T.; Guo, J.Y.; Tang, J.Q.; Huang, C. The differences of growth and living strategies between burrow and corner habitat in Procambarus clarkii. J. Nanjing Univ. (Nat. Sci.) 2011, 47, 635–641. (In Chinese) [Google Scholar]
- Wang, X.T.; Cheng, C.; Huang, C. Relationship between corner space and living conditions in red swamp crawfish (Procambarus clarkii). Fish. Sci. 2010, 29, 348–351. (In Chinese) [Google Scholar]



| Weight (g) | Length (cm) | Number of Fish | |
|---|---|---|---|
| Experiment 1—Size | 164.25 ± 20.29 (large) 45.67 ± 7.67 (small) | 27.25 ± 1.44 (large) 16.42 ± 1.32 (small) | 18 |
| Experiment 2—Sex | 133.69 ± 22.92 (female) 134.83 ± 9.61 (male) | 25.48 ± 1.87 (female) 26.27 ± 0.25 (male) | 18 |
| Experiment 3—The shelter | 74.23 ± 0.90 | 19.17 ± 0.58 | 30 |
| Types of Behavior | Name of Behavior | Specific Description of Behavioral Characteristics |
|---|---|---|
| Daily Behavior | Probe | Repeatedly extends its head or part of its body from a hidden cave; swims out a short distance (within the designated hiding area) and quickly returns to the cave; probes towards the water surface. This behavior occurs after the fish is placed in the tank for the first time. |
| Explore | Following probing behavior, the fish leaves the cave, swims further distances (crossing partition lines), explores other caves, and investigates the upper water column (swimming in higher water levels). | |
| Patrol | The fish swims out to inspect the surroundings of the cave and then returns. | |
| Cruise | The fish swims around the tank without being fixed to any particular spot. | |
| Rollover | The fish swims with its side facing upwards (commonly observed when entering pebble caves or resting at drainage pipes). | |
| Predation | When prey fish swims near the cave entrance, the H. macropterus strikes quickly and then returns to the cave. | |
| Thigmotactic | The fish seeks contact with objects (such as drainage pipes or the exterior of tile caves) and remains in contact for an extended period. | |
| Territorial Behavior | Invade | An intruder forces the resident fish out of its territory and occupies that territory. |
| Drive | The resident fish exhibits active aggression towards the intruder, attempting to drive it away from its territory when the intruder approaches. This includes rapidly charging at the intruder and continuing the pursuit for a certain distance after the intruder attempts to escape. | |
| Defend | The resident fish actively attacks and pursues any nearby intruders before returning to its original position. | |
| Guard | The resident fish frequently enters and exits the cave, patrols within the vicinity, and swims a short distance away before returning to the cave. | |
| Deter | After being driven away, the intruder attempts to approach and re-enter the cave but quickly retreats upon observing the resident, who does not demonstrate aggressive behavior but has established a deterrent effect on the intruder. | |
| Aggressive Behavior | Chase | Occurs when Fish A approaches Fish B, resulting in Fish A persistently getting closer while Fish B continuously evades or flees; typically happens outside of territorial boundaries. |
| Reverse Chase | Fish B rapidly turns around to pursue Fish A while Fish A is chasing. | |
| Crash | Two fish collide head-on. | |
| Bite | Use of the mouth to make contact with the head or body of another fish. | |
| Active Attack | The fish suddenly accelerates when swimming near a stationary or slowly moving fish, exhibiting chase-like behavior. | |
| Elude | The fish escapes when under attack. | |
| Avoid Each Other | After encountering each other, both fish quickly turn and swim away. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhu, Y.; Chen, S.; Zhu, T.; Wu, X.; Li, X. Behavioral Characteristics of Largefin Longbarbel Catfish Hemibagrus macropterus: Effects of Sex and Body Size on Aggression and Shelter Selection. Animals 2025, 15, 1192. https://doi.org/10.3390/ani15091192
Li X, Zhu Y, Chen S, Zhu T, Wu X, Li X. Behavioral Characteristics of Largefin Longbarbel Catfish Hemibagrus macropterus: Effects of Sex and Body Size on Aggression and Shelter Selection. Animals. 2025; 15(9):1192. https://doi.org/10.3390/ani15091192
Chicago/Turabian StyleLi, Xiaoli, Yongjiu Zhu, Siqi Chen, Tingbing Zhu, Xingbing Wu, and Xuemei Li. 2025. "Behavioral Characteristics of Largefin Longbarbel Catfish Hemibagrus macropterus: Effects of Sex and Body Size on Aggression and Shelter Selection" Animals 15, no. 9: 1192. https://doi.org/10.3390/ani15091192
APA StyleLi, X., Zhu, Y., Chen, S., Zhu, T., Wu, X., & Li, X. (2025). Behavioral Characteristics of Largefin Longbarbel Catfish Hemibagrus macropterus: Effects of Sex and Body Size on Aggression and Shelter Selection. Animals, 15(9), 1192. https://doi.org/10.3390/ani15091192





