Effects of Developmental Programming Caused by Maternal Nutrient Intake on Postnatal Performance of Beef Heifers and Their Calves
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Management during Cow Gestation (Maternal Nutrition Treatment) and Lactation
2.2. Heifer Management during Rearing
2.2.1. Heifer Growth during Rearing
2.2.2. Heifer Reproductive Performance during Rearing
2.2.3. Heifer Metabolic and Endocrine Profiles during Rearing
2.3. Heifer Management during Gestation and First Lactation
2.4. Statistical Analyses
3. Results
3.1. Heifer Growth during Rearing
3.2. Heifer Reproductive Performance during Rearing
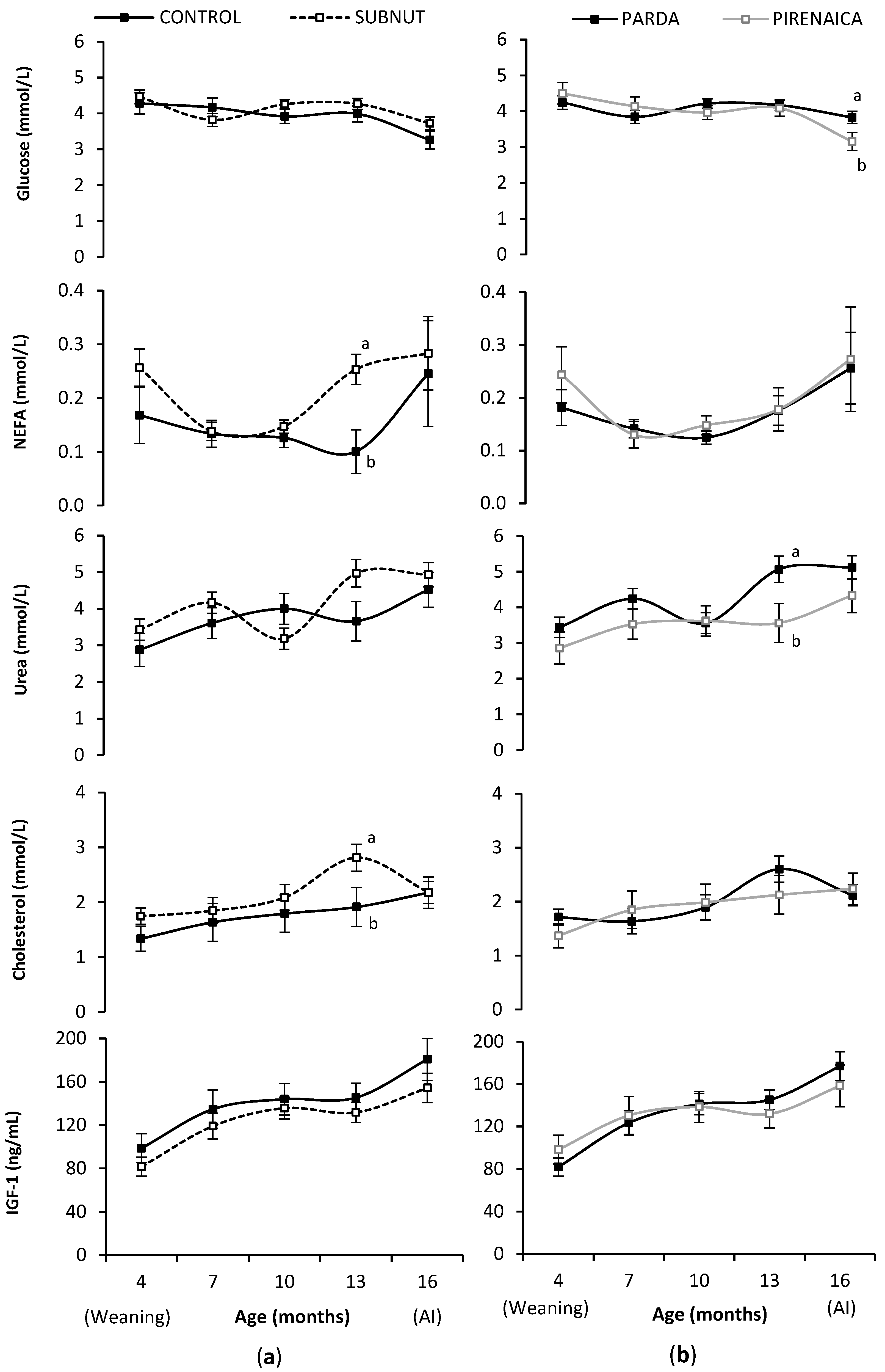
3.3. Heifer Metabolic and Endocrine Profiles during Rearing
3.4. Heifer Performance during Gestation and First Lactation
4. Discussion
4.1. Growth of Heifers and Their Calves
4.2. Heifer Reproductive Performance
4.3. Heifer Metabolic and Endocrine Profiles
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Velazquez, M.A. Impact of maternal malnutrition during the periconceptional period on mammalian preimplantation embryo development. Domest. Anim. Endocrinol. 2015, 51, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.C.; Alves, B.R.; Williams, G.L. Neuroendocrine signaling pathways and the nutritional control of puberty in heifers. Anim. Reprod. 2018, 15, 868–878. [Google Scholar] [CrossRef]
- Fleming, T.P.; Velazquez, M.A.; Eckert, J.J.; Lucas, E.S.; Watkins, A.J. Nutrition of females during the peri-conceptional period and effects on foetal programming and health of offspring. Anim. Reprod. Sci. 2012, 130, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Chavatte-Palmer, P.; Velazquez, M.A.; Jammes, H.; Duranthon, V. Review: Epigenetics, developmental programming and nutrition in herbivores. Animal 2018, 12, s363–s371. [Google Scholar] [CrossRef] [Green Version]
- Barker, D.J.; Martyn, C.N.; Osmond, C.; Hales, C.N.; Fall, C.H. Growth in utero and serum cholesterol concentrations in adult life. Br. Med. J. 1993, 307, 1524–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bell, A.W.; Greenwood, P.L. Prenatal origins of postnatal variation in growth, development and productivity of ruminants. Anim. Prod. Sci. 2016, 56, 1217–1232. [Google Scholar] [CrossRef]
- Greenwood, P.L.; Thompson, A.N.; Ford, S.P. Postnatal consequences of the maternal environment and of growth during prenatal life for productivity of ruminants. In Managing the Prenatal Environment to Enhance Livestock Productivity; Greenwood, P., Bell, A., Vercoe, P., Viljoen, G., Eds.; Springer: Dordrecht, The Netherlands, 2009. [Google Scholar]
- Kenyon, P.R.; Blair, H.T. Foetal programming in sheep—Effects on production. Small Rumin. Res. 2014, 118, 16–30. [Google Scholar] [CrossRef]
- Evans, A.; Mossa, F.; Walsh, S.; Scheetz, D.; Jimenez-Krassel, F.; Ireland, J.; Smith, G.; Ireland, J. Effects of maternal environment during gestation on ovarian folliculogenesis and consequences for fertility in bovine offspring. Reprod. Domest. Anim. 2012, 47, 31–37. [Google Scholar] [CrossRef]
- Rae, M.T.; Kyle, C.E.; Miller, D.W.; Hammond, A.J.; Brooks, A.N.; Rhind, S.M. The effects of undernutrition, in utero, on reproductive function in adult male and female sheep. Anim. Reprod. Sci. 2002, 72, 63–71. [Google Scholar] [CrossRef]
- Long, N.M.; Prado-Cooper, M.J.; Krehbiel, C.R.; Wettemann, R.P. Effects of nutrient restriction of bovine dams during early gestation on postnatal growth and regulation of plasma glucose. J. Anim. Sci. 2010, 88, 3262–3268. [Google Scholar] [CrossRef] [Green Version]
- Cushman, R.A.; McNeel, A.K.; Freetly, H.C. The impact of cow nutrient status during the second and third trimesters on age at puberty, antral follicle count, and fertility of daughters. Livest. Sci. 2014, 162, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.; Stanton, J.-A.L.; Quirke, L.; Juengel, J.L. Gestational nutrition 1: Alterations to gestational nutrition can increase indicators of fertility in sheep. Reproduction 2019, 157, 199–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micke, G.C.; Sullivan, T.M.; Gatford, K.L.; Owens, J.A.; Perry, V.E.A. Nutrient intake in the bovine during early and mid-gestation causes sex-specific changes in progeny plasma IGF-I, liveweight, height and carcass traits. Anim. Reprod. Sci. 2010, 121, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Noya, A.; Serrano-Pérez, B.; Villalba, D.; Casasús, I.; Molina, E.; López-Helguera, I.; Sanz, A. Effects of maternal subnutrition during early pregnancy on cow hematological profiles and offspring physiology and vitality in two beef breeds. Anim. Sci. J. 2019, 90, 857–869. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Rodríguez, J.; Palacio, J.; Sanz, A. Metabolic and luteal function in winter-calving Spanish beef cows as affected by calf management and breed. J. Anim. Physiol. Anim. Nutr. 2010, 94, 385–394. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, J.; Sanz, A.; Ferrer, J.; Ripoll, G.; Casasús, I. First calving performance and physiological profiles of 2 yr old beef heifers according to their prebreeding growth. Can. J. Anim. Sci. 2017, 97, 488–498. [Google Scholar] [CrossRef]
- Noya, A.; Casasús, I.; Ferrer, J.; Sanz, A. Long-term effects of maternal subnutrition in early pregnancy on the cow-calf performance, immunological and physiological profiles during the next lactation. Animals 2019, 9, 936. [Google Scholar] [CrossRef] [Green Version]
- European Union. Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2010, 276, 33–79. [Google Scholar]
- Lowman, B.G.; Scott, N.A.; Somerville, S.H. Condition Scoring of Cattle, Revised Edition; East of Scotland College of Agriculture, Animal Production, Advisory and Development Department: Edinburgh, UK, 1976; Volume 6. [Google Scholar]
- Noya, A.; Casasús, I.; Rodríguez-Sánchez, J.A.; Ferrer, J.; Sanz, A. A negative energy balance during the peri-implantational period reduces dam IGF-1 but does not alter progesterone or pregnancy-specific protein B (PSPB) or fertility in suckled cows. Domest. Anim. Endocrinol. 2019, in press. [Google Scholar] [CrossRef]
- Murray, R.D.; Cartwright, T.A.; Downham, D.Y.; Murray, M.A. Some maternal factors associated with dystocia in Belgian Blue cattle. Anim. Sci. 1999, 69, 105–113. [Google Scholar] [CrossRef]
- Martin, J.L.; Vonnahme, K.A.; Adams, D.C.; Lardy, G.P.; Funston, R.N. Effects of dam nutrition on growth and reproductive performance of heifer calves. J. Anim. Sci. 2007, 85, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Casasús, I.; Sanz, A.; Villalba, D.; Ferrer, R.; Revilla, R. Factors affecting animal performance during the grazing season in a mountain cattle production system. J. Anim. Sci. 2002, 80, 1638–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cushman, R.A.; Allan, M.F.; Kuehn, L.A.; Snelling, W.M.; Cupp, A.S.; Freetly, H.C. Evaluation of antral follicle count and ovarian morphology in crossbred beef cows: Investigation of influence of stage of the estrous cycle, age, and birth weight. J. Anim. Sci. 2009, 87, 1971–1980. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.; Pugliesi, G.; Sponchiado, M.; Cardoso, B.O.; Gomes, N.S.; Mello, B.P.; Celeghini, E.C.C.; Binelli, M. Supplementation with long-acting progesterone in early diestrus in beef cattle: I. effect of artificial insemination on onset of luteolysis. Domest. Anim. Endocrinol. 2019, 67, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, P.L.; Cafe, L.M. Prenatal and pre-weaning growth and nutrition of cattle: Long-term consequences for beef production. Animal 2007, 1, 1283–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freetly, H.C.; Ferrell, C.L.; Jenkins, T.G. Timing of realimentation of mature cows that were feed-restricted during pregnancy influences calf birth weights and growth rates. J. Anim. Sci. 2000, 78, 2790–2796. [Google Scholar] [CrossRef] [Green Version]
- Cano, G.; Blanco, M.; Casasús, I.; Cortés-Lacruz, X.; Villalba, D. Comparison of B-splines and non-linear functions to describe growth patterns and predict mature weight of female beef cattle. Anim. Prod. Sci. 2016, 56, 1787–1796. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Sánchez, J.; Sanz, A.; Ferrer, J.; Casasús, I. Influence of postweaning feeding management of beef heifers on performance and physiological profiles through rearing and first lactation. Domest. Anim. Endocrinol. 2018, 65, 24–37. [Google Scholar] [CrossRef] [Green Version]
- Freetly, H.C.; Kuehn, L.A.; Cundiff, L.V. Growth curves of crossbred cows sired by Hereford, Angus, Belgian Blue, Brahman, Boran, and Tuli bulls, and the fraction of mature body weight and height at puberty. J. Anim. Sci. 2011, 89, 2373–2379. [Google Scholar] [CrossRef] [Green Version]
- Corah, L.R.; Dunn, T.G.; Kaltenbach, C.C. Influence of prepartum nutrition on the reproductive performance of beef females and the performance of their progeny. J. Anim. Sci. 1975, 41, 819–824. [Google Scholar] [CrossRef] [Green Version]
- Broekmans, F.J.M.; de Ziegler, D.; Howles, C.M.; Gougeon, A.; Trew, G.; Olivennes, F. The antral follicle count: Practical recommendations for better standardization. Fertil. Steril. 2010, 94, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.M.; Micke, G.C.; Greer, R.M.; Irving-Rodgers, H.F.; Rodgers, R.J.; Perry, V.E.A. Dietary manipulation of Bos indicus heifers during gestation affects the reproductive development of their heifer calves. Reprod. Fertil. Dev. 2009, 21, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.G.; Lemenager, R.P.; Fellner, V.; Stewart, K.R. Effect of dried distiller’s grains plus solubles in postpartum diets of beef cows on reproductive performance of dam and heifer progeny. J. Anim. Sci. 2017, 95, 4543–4553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mossa, F.; Carter, F.; Walsh, S.W.; Kenny, D.; Smith, G.W.; Ireland, J.L.H.; Hildebrandt, T.B.; Lonergan, P.; Ireland, J.; Evans, A.C.O. Maternal undernutrition in cows impairs ovarian and cardiovascular systems in their offspring. Biol. Reprod. 2013, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lea, R.G.; Andrade, L.P.; Rae, M.T.; Hannah, L.T.; Kyle, C.E.; Murray, J.F.; Rhind, S.M.; Miller, D.W. Effects of maternal undernutrition during early pregnancy on apoptosis regulators in the ovine fetal ovary. Reproduction 2006, 131, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weller, M.M.D.C.A.; Fortes, M.R.S.; Marcondes, M.I.; Rotta, P.P.; Gionbeli, T.R.S.; Valadares Filho, S.C.; Campos, M.M.; Silva, F.F.; Silva, W.; Moore, S.; et al. Effect of maternal nutrition and days of gestation on pituitary gland and gonadal gene expression in cattle. J. Dairy Sci. 2016, 99, 3056–3071. [Google Scholar] [CrossRef] [Green Version]
- Mossa, F.; Walsh, S.W.; Butler, S.T.; Berry, D.P.; Carter, F.; Lonergan, P.; Smith, G.W.; Ireland, J.J.; Evans, A.C.O. Low numbers of ovarian follicles ≥3 mm in diameter are associated with low fertility in dairy cows. J. Dairy Sci. 2012, 95, 2355–2361. [Google Scholar] [CrossRef] [Green Version]
- Rhind, S.; Rae, M.; Brooks, A. Effects of nutrition and environmental factors on the fetal programming of the reproductive axis. Reproduction 2001, 122, 205–214. [Google Scholar] [CrossRef]
- Wilkins, J.F.; Fry, R.C.; Hearnshaw, H.; Cafe, L.M.; Greenwood, P.L. Ovarian activity in heifers at 30 months of age following high or low growth in utero or from birth to weaning. In Proceedings of the 26th Biennial Conference of Australian Society of Animal Production, Perth, Australia, 10–14 July 2006. Short Comunication 17. [Google Scholar]
- Sanz, A.; Macmillan, K.; Colazo, M.G. A review of the ovarian synchronization programs based on the use of gonadotrophin releasing hormone and prostaglandin F2α for dairy and beef heifers. ITEA Inf. Tec. Econ. Ag. 2019, 115, 326–342. [Google Scholar] [CrossRef]
- Gasser, C.L. Joint Alpharma-beef species symposium: Considerations on puberty in replacement beef heifers. J. Anim. Sci. 2013, 91, 1336–1340. [Google Scholar] [CrossRef]
- Trivers, R.L.; Willard, D.E. Natural selection of parental ability to vary the sex ratio of offspring. Science 1973, 179, 90–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delesa, E.K.; Yohannes, A.; Alemayehu, M.; Samuel, T.; Yehualaeshet, T. Calves’ sex ratio in naturally and artificially bred cattle in central Ethiopia. Theriogenology 2014, 82, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.R.; Lee, J.M.; Berry, D.P. Climatic factors and secondary sex ratio in dairy cows. J. Dairy Sci. 2006, 89, 3221–3227. [Google Scholar] [CrossRef] [Green Version]
- Ithurralde, J.; Pérez-Clariget, R.; Corrales, F.; Fila, D.; López-Pérez, Á.; Marichal, M.J.; Saadoun, A.; Bielli, A. Sex-dependent effects of maternal undernutrition on growth performance, carcass characteristics and meat quality of lambs. Livest. Sci. 2019, 221, 105–114. [Google Scholar] [CrossRef]
- Maresca, S.; Lopez Valiente, S.; Rodriguez, A.M.; Long, N.M.; Pavan, E.; Quintans, G. Effect of protein restriction of bovine dams during late gestation on offspring postnatal growth, glucose-insulin metabolism and IGF-1 concentration. Livest. Sci. 2018, 212, 120–126. [Google Scholar] [CrossRef]
- Vanholder, T.; Leroy, J.L.M.R.; Soom, A.V.; Opsomer, G.; Maes, D.; Coryn, M.; Kruif, A. Effect of non-esterified fatty acids on bovine granulosa cell steroidogenesis and proliferation in vitro. Anim. Reprod. Sci. 2005, 87, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Beam, S.W.; Butler, W.R. Energy balance and ovarian follicle development prior to the first ovulation postpartum in dairy cows receiving three levels of dietary fat. Biol. Reprod. 1997, 56, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Vanholder, T.; Lmr Leroy, J.; Van Soom, A.; Maes, D.; Coryn, M.; Fiers, T.; de Kruif, A.; Opsomer, G. Effect of non-esterified fatty acids on bovine theca cell steroidogenesis and proliferation in vitro. Anim. Reprod. Sci. 2006, 92, 51–63. [Google Scholar] [CrossRef]
- Walsh, K.; O’Kiely, P.; Moloney, A.P.; Boland, T.M. Intake, digestibility, rumen fermentation and performance of beef cattle fed diets based on whole-crop wheat or barley harvested at two cutting heights relative to maize silage or ad libitum concentrates. Anim. Feed Sci. Technol. 2008, 144, 257–278. [Google Scholar] [CrossRef]
- Kelly, A.K.; McGee, M.; Crews, D.H., Jr.; Sweeney, T.; Boland, T.M.; Kenny, D.A. Repeatability of feed efficiency, carcass ultrasound, feeding behavior, and blood metabolic variables in finishing heifers divergently selected for residual feed intake. J. Anim. Sci. 2010, 88, 3214–3225. [Google Scholar] [CrossRef]
- Chandra, G.; Aggarwal, A.; Kumar, M.; Singh, A.K. Effect of zinc and vitamin E supplementation on hormones and blood biochemicals in peri-partum Sahiwal cows. J. Trace Elem. Med. Biol. 2018, 50, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, T.; Chimonyo, M.; Okoh, A.; Muchenje, V.; Dzama, K.; Raats, J. Assessing the nutritional status of beef cattle: Current practices and future prospects. Afr. J. Biotechnol. 2007, 6, 2727–2734. [Google Scholar]
- Gross, J.J.; Kessler, E.C.; Albrecht, C.; Bruckmaier, R.M. Response of the cholesterol metabolism to a negative energy balance in dairy cows depends on the lactational stage. PLoS ONE 2015, 10, e0121956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manthey, A.K.; Anderson, J.L.; Perry, G.A.; Keisler, D.H. Feeding distillers dried grains in replacement of forage in limit-fed dairy heifer rations: Effects on metabolic profile and onset of puberty. J. Dairy Sci. 2017, 100, 2591–2602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, J.L.; Kalscheur, K.F.; Clapper, J.A.; Perry, G.A.; Keisler, D.H.; Garcia, A.D.; Schingoethe, D.J. Feeding fat from distillers dried grains with solubles to dairy heifers: II. Effects on metabolic profile. J. Dairy Sci. 2015, 98, 5709–5719. [Google Scholar] [CrossRef] [Green Version]
- Yelich, J.V.; Wettemann, R.P.; Dolezal, H.G.; Lusby, K.S.; Bishop, D.K.; Spicer, L.J. Effects of growth rate on carcass composition and lipid partitioning at puberty and growth hormone, insulin-like growth factor I, insulin, and metabolites before puberty in beef heifers. J. Anim. Sci. 1995, 73, 2390–2405. [Google Scholar] [CrossRef] [Green Version]
- Kerr, D.E.; Manns, J.G.; Laarveld, B.; Fehr, M.I. Profiles of serum IGF-I concentrations in calves from birth to eighteen months of age and in cows throughout the lactation cycle. Can. J. Anim. Sci. 1991, 71, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Cooke, R.F.; Bohnert, D.W.; Francisco, C.L.; Marques, R.S.; Mueller, C.J.; Keisler, D.H. Effects of bovine somatotropin administration on growth, physiological, and reproductive responses of replacement beef heifers. J. Anim. Sci. 2013, 91, 2894–2901. [Google Scholar] [CrossRef] [Green Version]

| Chemical Composition | Total Mixed Ration † | Concentrate ‡ | Meadow Hay § | Barley Straw ф |
|---|---|---|---|---|
| DM (g/kg) | 908 | 907 | 886 | 902 |
| CP (g/kg DM) | 124 | 152 | 154 | 40 |
| NDF (g/kg DM) | 466 | 262 | 569 | 796 |
| ADF (g/kg DM) | 253 | 62 | 320 | 456 |
| ADL (g/kg DM) | 40 | 8 | 58 | 58 |
| Ash (g/kg DM) | 113 | 60 | 98 | 65 |
| ME (MJ/kg DM) | 11.0 | 14.4 | 9.8 | 7.5 |
| Item | Maternal Nutrition | Breed | RSD | p-Value | |||
|---|---|---|---|---|---|---|---|
| CONTROL | SUBNUT | Parda | Pirenaica | Maternal Nutrition | Breed | ||
| Heifer performance | |||||||
| LW at weaning (kg) | 152 a | 133 b | 147 | 138 | 10.3 | 0.020 | 0.270 |
| LW at AI (kg) | 415 | 400 | 420 a | 395 b | 20.5 | 0.199 | 0.036 |
| ADG during rearing (kg/d) | 0.741 | 0.792 | 0.823 a | 0.710 b | 0.0757 | 0.148 | 0.002 |
| Age at AI (months) | 16.0 | 15.7 | 15.6 | 16.1 | 0.42 | 0.248 | 0.056 |
| Height at withers | |||||||
| At 4 months (weaning, cm) | 95 | 92 | 95 | 92 | 2.9 | 0.156 | 0.126 |
| At 12 months (cm) | 115 | 113 | 117 a | 112 b | 3.3 | 0.208 | 0.010 |
| At 16 months (cm) | 121 | 120 | 124 a | 118 b | 2.8 | 0.435 | 0.001 |
| Heart girth | |||||||
| At 4 months (weaning, cm) | 119 | 115 | 118 | 115 | 5.3 | 0.060 | 0.223 |
| At 12 months (cm) | 162 | 158 | 163 a | 157 b | 5.5 | 0.110 | 0.027 |
| At 16 months (cm) | 175 | 173 | 178 a | 170 b | 5.9 | 0.416 | 0.006 |
| External pelvic area | |||||||
| At 4 months (weaning, dm2) | 9.6 | 8.7 | 8.8 | 9.5 | 1.07 | 0.146 | 0.242 |
| At 12 months (dm2) | 18.3 | 17.5 | 18.7 a | 17.1 b | 1.42 | 0.343 | 0.043 |
| At 16 months (dm2) | 21.9 | 21.0 | 22.6 a | 20.3 b | 1.31 | 0.208 | 0.002 |
| Items | Maternal Nutrition | Breed | RSD | p-Value | |||
|---|---|---|---|---|---|---|---|
| CONTROL | SUBNUT | Parda | Pirenaica | Maternal Nutrition | Breed | ||
| Age at puberty (months) | 12.0 | 12.1 | 11.6 | 12.6 | 1.58 | 0.905 | 0.169 |
| LW at puberty (kg) | 341 | 336 | 350 | 327 | 23.8 | 0.659 | 0.076 |
| Mature LW at puberty (%) † | 59 | 58 | 61 | 56 | 4.8 | 0.723 | 0.055 |
| Puberty reached by 12 months (%) ‡ | 63 | 50 | 63 | 60 | - | 0.210 | 0.272 |
| Puberty reached by 16 months (%) | 94 | 89 | 95 | 87 | - | 0.409 | 0.333 |
| Fertility to a single AI (%) | 78.6 | 81.3 | 82.4 | 76.9 | - | 0.343 | 0.328 |
| Items | Maternal Nutrition | Breed | RSD | p-Value | |||
|---|---|---|---|---|---|---|---|
| CONTROL | SUBNUT | Parda | Pirenaica | Maternal Nutrition | Breed | ||
| Small follicles (<5 mm) | |||||||
| At 9.5 months (n) | 8 | 9 | 10 | 7 | 4.4 | 0.365 | 0.217 |
| At 13 months (n) | 10 | 10 | 9 | 11 | 4.1 | 0.964 | 0.432 |
| At 15.5 months (n) | 16 a | 11b | 13 | 14 | 4.5 | 0.011 | 0.418 |
| Medium follicles (5 < x < 10 mm) | |||||||
| At 9.5 months (n) | 0.8 b | 2.5 a | 1.8 | 1.4 | 1.45 | 0.019 | 0.524 |
| At 13 months (n) | 0.9 | 1.9 | 2.1 | 0.7 | 1.79 | 0.234 | 0.100 |
| At 15.5 months (n) | 1.4 | 0.8 | 0.9 | 1.3 | 1.40 | 0.364 | 0.637 |
| Large follicles (>10 mm) | |||||||
| At 9.5 months (n) | 0.8 | 0.4 | 0.8 a | 0.4 b | 0.49 | 0.108 | 0.044 |
| At 13 months (n) | 0.9 a | 0.4 b | 0.5 | 0.8 | 0.57 | 0.041 | 0.367 |
| At 15.5 months (n) | 0.4 b | 0.9 a | 0.9 | 0.4 | 0.51 | 0.032 | 0.056 |
| Dominant follicle diameter | |||||||
| At 9.5 months (mm) | 11.2 | 9.5 | 10.9 | 9.8 | 1.69 | 0.054 | 0.227 |
| At 13 months (mm) | 11.1 | 10.2 | 10.9 | 10.5 | 3.19 | 0.544 | 0.807 |
| At 15.5 months (mm) | 10.5 | 11.4 | 12.4 a | 9.5 b | 2.31 | 0.451 | 0.017 |
| Corpus luteum | |||||||
| Heifers with CL at 13 months (%) | 88 | 72 | 84 | 73 | - | 0.191 | 0.246 |
| CL diameter at 13 months (mm) | 19.2 | 17.9 | 18.6 | 18.5 | 4.25 | 0.601 | 0.968 |
| Heifers with CL at 15.5 months (%) | 94 | 83 | 95 | 80 | - | 0.282 | 0.186 |
| CL diameter at 15.5 months (mm) | 13.2 | 17.2 | 16.6 | 13.8 | 4.13 | 0.119 | 0.265 |
| Ovary diameter | |||||||
| At 9.5 months (mm) | 14.0 | 14.4 | 15.5 a | 12.9 b | 1.41 | 0.639 | 0.009 |
| At 13 months (mm) | 18.6 | 17.5 | 18.3 | 17.7 | 2.02 | 0.325 | 0.608 |
| At 15.5 months (mm) | 17.8 | 18.8 | 19.9 a | 16.7 b | 1.73 | 0.292 | 0.003 |
| Items | Maternal Nutrition | Breed | RSD | p-Value | |||
|---|---|---|---|---|---|---|---|
| CONTROL | SUBNUT | Parda | Pirenaica | Maternal Nutrition | Breed | ||
| Heifer performance | |||||||
| Gestation ADG (kg/day) | 0.334 | 0.283 | 0.298 | 0.319 | 0.0969 | 0.275 | 0.645 |
| LW at calving (kg) | 520 | 491 | 516 | 494 | 33.0 | 0.103 | 0.204 |
| BCS at calving | 3.0 | 3.0 | 2.8 b | 3.2 a | 0.16 | 0.425 | 0.001 |
| Age at calving (months) | 26.4 | 26.3 | 26.1 | 26.6 | 1.52 | 0.844 | 0.584 |
| Calving assistance (%) | 26.7 | 16.7 | 25.0 | 18.2 | - | 0.304 | 0.338 |
| LW at weaning (kg) | 469 | 452 | 478 | 443 | 42.0 | 0.445 | 0.124 |
| Lactation ADG (kg/day) | −0.519 | –0.349 | –0.373 | –0.494 | 0.2318 | 0.168 | 0.323 |
| Calf performance | |||||||
| Male/female calf ratio | 8/7 | 3/9 | 8/8 | 3/8 | - | 0.109 | 0.163 |
| LW at birth (kg) | 35 | 34 | 36 | 33 | 3.7 | 0.321 | 0.134 |
| LW at weaning (kg) | 111 | 105 | 122 a | 94 b | 19.4 | 0.505 | 0.012 |
| Lactation ADG (kg/day) | 0.720 | 0.680 | 0.814 a | 0.587 b | 0.1918 | 0.684 | 0.031 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noya, A.; Casasús, I.; Ferrer, J.; Sanz, A. Effects of Developmental Programming Caused by Maternal Nutrient Intake on Postnatal Performance of Beef Heifers and Their Calves. Animals 2019, 9, 1072. https://doi.org/10.3390/ani9121072
Noya A, Casasús I, Ferrer J, Sanz A. Effects of Developmental Programming Caused by Maternal Nutrient Intake on Postnatal Performance of Beef Heifers and Their Calves. Animals. 2019; 9(12):1072. https://doi.org/10.3390/ani9121072
Chicago/Turabian StyleNoya, Agustí, Isabel Casasús, Javier Ferrer, and Albina Sanz. 2019. "Effects of Developmental Programming Caused by Maternal Nutrient Intake on Postnatal Performance of Beef Heifers and Their Calves" Animals 9, no. 12: 1072. https://doi.org/10.3390/ani9121072
APA StyleNoya, A., Casasús, I., Ferrer, J., & Sanz, A. (2019). Effects of Developmental Programming Caused by Maternal Nutrient Intake on Postnatal Performance of Beef Heifers and Their Calves. Animals, 9(12), 1072. https://doi.org/10.3390/ani9121072






