Sowing Date and Seeding Rate Affect Bioactive Compound Contents of Chickpea Grains
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
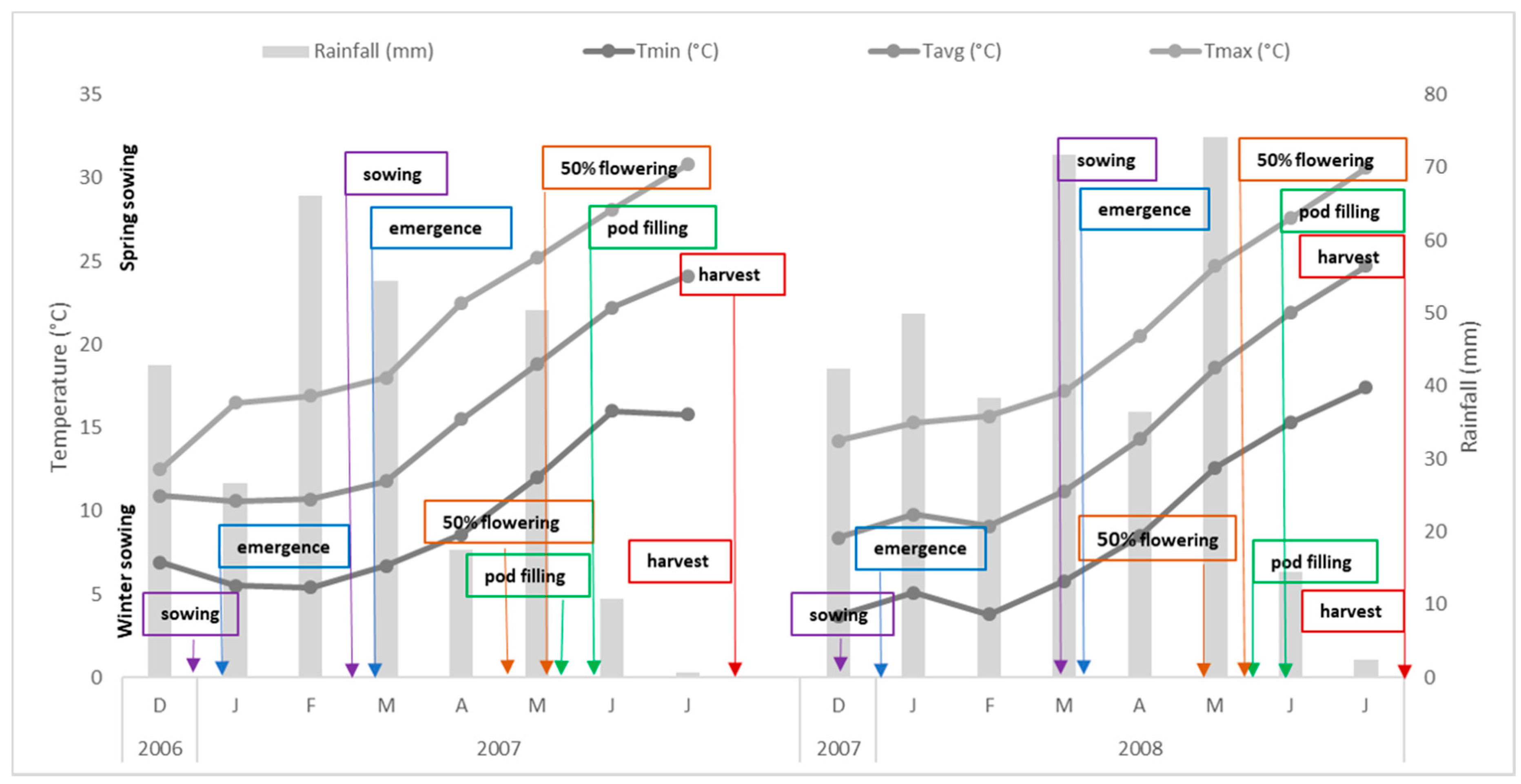
2.1. Experimental Set up and Plant Growing Conditions
2.2. Harvesting and Sample Preparation
2.3. Analytical Procedures
2.4. Statistical Design and Analysis
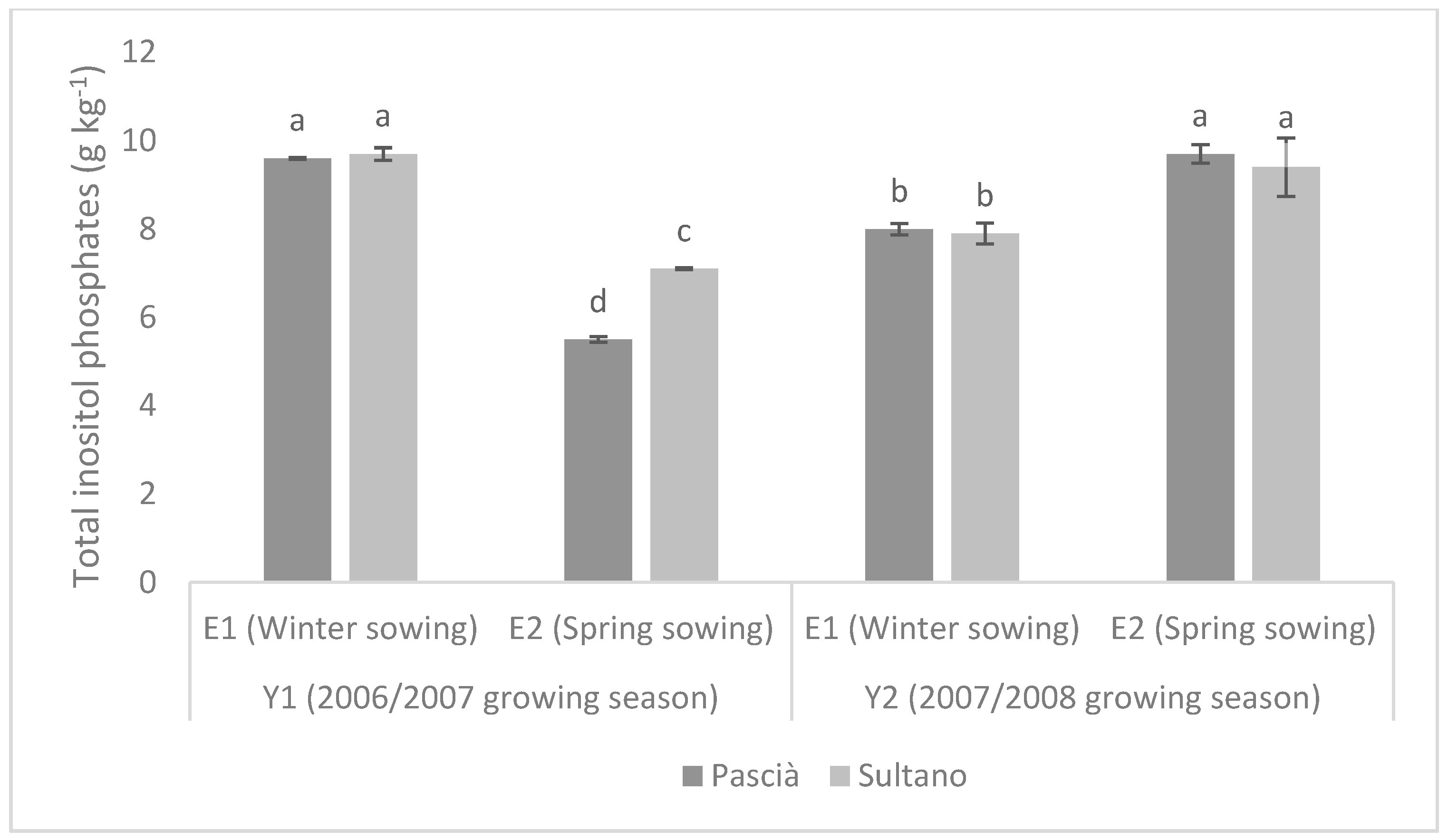
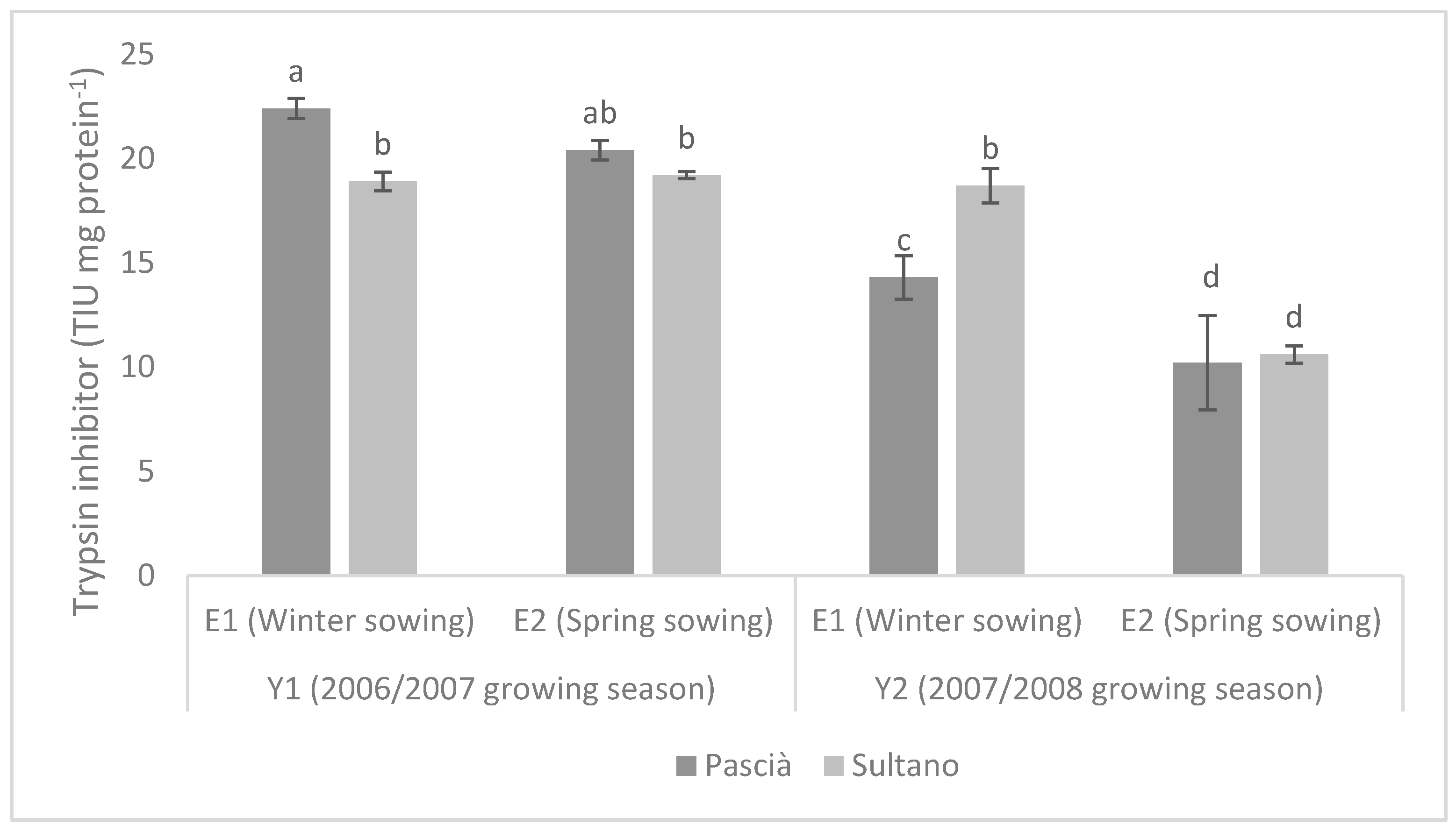
3. Results
3.1. Trial 1
3.2. Trial 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Layadi, R. Maintaining a Supply of Non-GM Feed—A Strategic Issue for European Regional Agriculture. In Genetically Modified and Non-Genetically Modified Food Supply Chains: Co-Existence and Traceability; Bertheau, Y., Ed.; Wiley-Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2012; pp. 543–562. ISBN 9-7814-4433-7785. [Google Scholar]
- Knights, E.J.; Açikgöz, N.; Warketing, T.; Bejiga, G.; Yadav, S.S.; Sandhu, J.S. Area, Production and Distribution. In Chichpea Breeding and Management; Yadav, S.S., Redden, R.J., Chen, W., Sharma, B., Eds.; CABI International: Cambridge, UK, 2006; pp. 167–178. [Google Scholar]
- Venkidasamy, B.; Selvaraj, D.; Nile, A.S.; Ramalingam, S.; Kai, G.; Nile, S.H. Indian pulses: A review on nutritional, functional and biochemical properties with future perspectives. Trends Food Sci. Technol. 2019, 88, 228–242. [Google Scholar] [CrossRef]
- Gholipoor, M.; Soltani, A. Future Climate Impacts on Chickpea in Iran and ICARDA. Res. J. Environ. Sci. 2009, 3, 16–28. [Google Scholar] [CrossRef]
- Segnalini, M.; Bernabucci, U.; Vitali, A.; Nardone, A.; Lacetera, N. Temperature humidity index scenarios in the Mediterranean basin. Int. J. Biometeorol. 2013, 57, 451–458. [Google Scholar] [CrossRef] [PubMed]
- White, J.W.; Kimball, B.A.; Wall, G.W.; Ottman, M.J.; Hunt, L.A. Responses of time of anthesis and maturity to sowing dates and infrared warming in spring wheat. Field Crop. Res. 2011, 124, 213–222. [Google Scholar] [CrossRef]
- Wang, J.; Araus, J.L.; Wan, J. Breeding to Optimize Agriculture in a Changing World. Crop. J. 2015, 3, 169–173. [Google Scholar] [CrossRef]
- Danieli, P.P.; Primi, R.; Ronchi, B.; Ruggeri, R.; Rossini, F.; Del Puglia, S.; Cereti, C.F. The potential role of spineless safflower (Carthamus tinctorius L. var. inermis) as fodder crop in central Italy. Ital. J. Agron. 2011, 6, 4. [Google Scholar] [CrossRef]
- Leport, L.; Turner, N.C.; French, R.J.; Barr, M.D.; Duda, R.; Davies, S.L.; Tennant, D.; Siddique, K.H.M. Physiological responses of chickpea genotypes to terminal drought in a Mediterranean-type environment. Eur. J. Agron. 1999, 11, 279–291. [Google Scholar] [CrossRef]
- Day, L. Proteins from land plants-Potential resources for human nutrition and food security. Trends Food Sci. Technol. 2013, 32, 25–42. [Google Scholar] [CrossRef]
- Mustafa, A.F.; Thacker, P.A.; McKinnon, J.J.; Christensen, D.A.; Racz, V.J. Nutritional value of feed grade chickpeas for ruminants and pigs. J. Sci. Food Agric. 2000, 80, 1581–1588. [Google Scholar] [CrossRef]
- Soetan, K.O.; Oyewole, O.E. The need for adequate processing to reduce the anti- nutritional factors in plants used as human foods and animal feeds: A review. Afr. J. Food Sci. 2009, 3, 223–232. [Google Scholar]
- Enneking, D.; Wink, M. Towards the elimination of anti-nutritional factors in grain legumes. In Linking Research and Marketing Opportunities for Pulses in the 21st Century: Proceedings of the Third International Food Legumes Research Conference; Kelley, T.G., Parthasarathy Rao, P., Grisko-Kelley, H., Knight, R., Eds.; Current Plant Science and Biotechnology in Agriculture; Springer Science + Business Media Dordrecht: Dordrecht, The Netherlands, 2000; Volume 34, pp. 671–683. [Google Scholar]
- Martín-Pedrosa, M.; Varela, A.; Guillamon, E.; Cabellos, B.; Burbano, C.; Gomez-Fernandez, J.; De Mercado, E.; Gomez-Izquierdo, E.; Cuadrado, C.; Muzquiz, M. Biochemical characterization of legume seeds as ingredients in animal feed. Span. J. Agric. Res. 2016, 14, 1–14. [Google Scholar] [CrossRef]
- Bampidis, V.A.; Christodoulou, V. Chickpeas (Cicer arietinum L.) in animal nutrition: A review. Anim. Feed Sci. Technol. 2011, 168, 1–20. [Google Scholar] [CrossRef]
- Verma, A.K.; Kumar, S.; Das, M.; Dwivedi, P.D. A comprehensive review of legume allergy. Clin. Rev. Allergy Immunol. 2013, 45, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Muzquiz, M.; Varela, A.; Burbano, C.; Cuadrado, C.; Guillamón, E.; Pedrosa, M.M. Bioactive compounds in legumes: Pronutritive and antinutritive actions. implications for nutrition and health. Phytochem. Rev. 2012, 11, 227–244. [Google Scholar] [CrossRef]
- Khattab, R.Y.; Arntfield, S.D. Nutritional quality of legume seeds as affected by some physical treatments 2. Antinutritional factors. LWT-Food Sci. Technol. 2009, 42, 1113–1118. [Google Scholar] [CrossRef]
- Milán-Carrillo, J.; Reyes-Moreno, C.; Camacho-Hernández, I.L.; Rouzaud-Sandez, O.I.L. Optimisation of extrusion process to transform hardened chickpeas (Cicer arietinum L.) into a useful product. J. Sci. Food. Agric. 2002, 1718–1728. [Google Scholar]
- El-Adawy, T.A. Nutritional composition and antinutritional factors of chickpeas (Cicer arietinum L.) undergoing different cooking methods and germination. Plant Foods Hum. Nutr. 2002, 57, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Gangola, M.P.; Jaiswal, S.; Gaur, P.M.; Båga, M. Genotype environment and their interaction influence seed quality traits in chickpea (Cicer arietinum L.). J. Food Compos. Anal. 2017, 63, 21–27. [Google Scholar] [CrossRef]
- Frimpong, A.; Sinha, A.; Tar’an, B.; Warkentin, T.D.; Gossen, B.D.; Chibbar, R.N. Genotype and growing environment influence chickpea (Cicer arietinum L.) seed composition. J. Sci. Food Agric. 2009, 89, 2052–2063. [Google Scholar] [CrossRef]
- Ruggeri, R.; Primi, R.; Danieli, P.P.; Ronchi, B.; Rossini, F. Effects of seeding date and seeding rate on yield, proximate composition and total tannins content of two Kabuli chickpea cultivars. Ital. J. Agron. 2017, 12, 201–207. [Google Scholar] [CrossRef]
- D’Antuono, F.; Rossini, F. Yield potential and ecophysiological traits of the Altamurano linseed (Linum usitatissimum L.), a landrace of southern Italy. Genet. Resour. Crop. Evolut. 2006, 53, 65–75. [Google Scholar] [CrossRef]
- Nikolopoulou, D.; Grigorakis, K.; Stasini, M.; Alexis, M.; Iliadis, K. Effects of cultivation area and year on proximate composition and antinutrients in three different kabuli-type chickpea (Cicer arientinum) varieties. Eur. Food Res. Technol. 2006, 223, 737–741. [Google Scholar] [CrossRef]
- Muzquiz, M.; Rey, C.; Cuadrado, C.; Fenwick, G.R. Effect of germination on the oligosaccharide content of lupin species. J. Chromatogr. A 1992, 607, 349–352. [Google Scholar] [CrossRef]
- Kakade, M.L.; Rackis, J.J.; McGhee, J.E.; Puski, G. Determination of trypsin inhibitor activity of soy product: A collaborative analysis of an improved procedure. Am. Assoc. Cereal Chem. 1974, 51, 376–382. [Google Scholar]
- Cuadrado, C.; Ayet, G.; Robredo, L.M.; Tabera, J.; Villa, R.; Pedrosa, M.M.; Burbano, C.; Muzquiz, M. Effect of natural fermentation on the content of inositol phosphates in lentils. In Zeitschrift für Lebensmittel-Untersuchung und Forschung; Springer: Berlin/Heidelberg, Germany, 1996; Volume 203, pp. 268–271. [Google Scholar]
- Venables, W.N.; Smith, D.M. An Introduction to R Graphics; York University: Toronto, ON, Canada, 2010; Volume 3. [Google Scholar]
- Amuti, K.S.; Pollard, C.J. Soluble carbohydrates of dry and developing seeds. Phytochemistry 1977, 16, 529–532. [Google Scholar] [CrossRef]
- Obendorf, R.L.; Zimmerman, A.D.; Zhang, Q.; Castillo, A.; Kosina, S.M.; Bryant, E.G.; Sensenig, E.M.; Wu, J.; Schnebly, S.R. Accumulation of soluble carbohydrates during seed development and maturation of low-raffinose, low-stachyose soybean. Crop Sci. 2009, 49, 329–341. [Google Scholar] [CrossRef]
- Keller, F.; Pharr, D.M. Metabolism of carbohydrates in sinks and sources: Galactosyl-sucrose oligosaccharides. In Photoassimilate Distribution in Plants and Crops. Source-Sink Relationships; Zamski, E., Schaffer, A.A., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1996; pp. 157–183. [Google Scholar]
- Obendorf, R.L.; Gorecki, R.J. Soluble carbohydrates in legume seeds. Seed Sci. Res. 2012, 22, 219–242. [Google Scholar] [CrossRef]
- Zhawar, V.K.; Kaur, N.; Gupta, A.K. Phytic acid and raffinose series oligosaccharides metabolism in developing chickpea seeds. Physiol. Mol. Biol. Plant. 2011, 17, 355–362. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Login, A.; Rejowski, A.; Socha, A.; Zalewski, K. Influence of water deficit on the accumulation of sugars in developing field bean (Vicia faba var. minor) seeds. Seed Sci. Technol. 2000, 28, 93–100. [Google Scholar]
- Fleming, S.E. A Study of Relationships between Flatus Potential and Carbohydrate Distribution in Legume Seeds. J. Food Sci. 1981, 46, 794–798. [Google Scholar] [CrossRef]
- Sandberg, A.S.; Andersson, H.; Carlsson, N.G.; Sandström, B. Degradation of soybean oligosaccharides in the stomach and small intestine of human ileostomy subjects. In Proceedings of the International Conference on Bioavailability: Nutritional, Chemical and Food Processing Implications of Nutrient Availability, Karlsruhe, Germany, 9–12 May 1993; Volume 93, pp. 197–201. [Google Scholar]
- Karr-Lilienthal, L.K.; Kadzere, C.T.; Grieshop, C.M.; Fahey, G.C. Chemical and nutritional properties of soybean carbohydrates as related to nonruminants: A review. Livest. Prod. Sci. 2005, 97, 1–12. [Google Scholar] [CrossRef]
- Champ, M.M.-J. Non-nutrient bioactive substances of pulses. Br. J. Nutr. 2003, 88, 307. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, D.A.; Laroche, A.; Frick, M.; Huel, R.; Puchalski, B. Cold induced expression of plant defensin and lipid transfer protein transcripts in winter wheat. Physiol. Plant. 2003, 117, 195–205. [Google Scholar] [CrossRef]
- Parisi, K.; Shafee, T.M.A.; Quimbar, P.; van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. The evolution, function and mechanisms of action for plant defensins. Semin. Cell Dev. Biol. 2019, 88, 107–118. [Google Scholar] [CrossRef] [PubMed]
- García-Carreño, F.L. Proteinase inhibitors. Trends Food Sci. Technol. 1996, 7, 197–204. [Google Scholar] [CrossRef]
- Chongo, G.; Gossen, B.D. Effect of plant age on resistance to Ascochyta rabiei in chickpea. Can. J. Plant Pathol. 2001, 23, 358–363. [Google Scholar] [CrossRef]
- Basandrai, A.K.; Basandrai, D.; Pande, S.; Sharma, M.; Thakur Sanjay, K.; Thakur, H.L. Development of ascochyta blight (Ascochyta rabiei) in chickpea as affected by host resistance and plant age. Eur. J. Plant Pathol. 2007, 119, 77–86. [Google Scholar] [CrossRef]
- Jhorar, O.P.; Mathauda, S.S.; Singh, G.; Butler, D.R.; Mavi, H.S. Relationships between climatic variables and Ascochyta blight of chickpea in Punjab, India. Agric. Forest Meteorol. 1997, 87, 171–177. [Google Scholar] [CrossRef]
- Oluwatosin, O.B. Genotype × environment influence on cowpea (Vigna unguiculata (L) Walp) antinutritional factors: 1-Trypsin inhibitors, tannins, phytic acid and haemagglutinin. J. Sci. Food Agric. 1999, 79, 265–272. [Google Scholar] [CrossRef]
- Berger, J.D.; Siddique, K.H.M.; Loss, S.P. Cool season grain legumes for Mediterranean environments: Species × environment interaction in seed quality traits and anti-nutritional factors in the genus Vicia. Aust. J. Agric. Res. 1999, 50, 389. [Google Scholar] [CrossRef]
- Zhou, Z.; Bar, I.; Sambasivam, P.T.; Ford, R. Determination of the Key Resistance Gene Analogs Involved in Ascochyta rabiei Recognition in Chickpea. Front. Plant Sci. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
| Cultivar (C) | Growing Season (Y) | Sowing Date (E) | Interactions (p-Level) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | S | p | Y1 | Y2 | p | E1 | E2 | p | C × Y | C × E | Y × E | C × Y × E | |
| Sucrose | 19.4 | 15.1 | ** | 18.2 | 16.4 | ns | 17.5 | 17.0 | ns | ** | ns | ns | ns. |
| Raffinose | 5.0 | 5.6 | ** | 4.8 | 5.8 | ** | 5.2 | 5.4 | ns | ns | ** | ** | ns |
| Stachyose | 18.9 | 17.2 | ns | 17.8 | 18.4 | ns | 17.7 | 18.4 | ns | ns | ns | ns | ns |
| Maltose | 2.5 | 2.8 | ns | 2.5 | 2.7 | ns | 3.0 | 2.3 | ** | ** | ns | ns | * |
| Galactinol | 2.5 | 2.5 | ns | 2.6 | 2.4 | ns | 2.7 | 2.3 | ns | * | ns | ns | ns |
| Ciceritol | 29.4 | 35.5 | ** | 35.7 | 29.1 | ** | 32.8 | 32.1 | * | ** | ** | * | ns |
| TSGs | 77.7 | 78.8 | ns | 81.7 | 74.8 | ** | 79.0 | 77.5 | ns | ns | * | ns | ns |
| IP3 | 0.1 | 0.1 | ns | 0.1 | 0.1 | ns | 0.1 | 0.1 | ns | ns | ns | ns | ns |
| IP4 | 0.3 | 0.2 | ns | 0.3 | 0.2 | ns | 0.2 | 0.2 | ns | ns | ns | ns | ns |
| IP5 | 1.0 | 1.0 | ns | 1.2 | 0.9 | ** | 1.1 | 1.0 | ns | ns | ns | ** | ns |
| IP6 | 6.8 | 7.1 | ns | 6.4 | 7.5 | ** | 7.4 | 6.6 | ** | * | ns | ** | * |
| TIPs | 8.2 | 8.5 | ns | 8.0 | 8.8 | ns | 8.8 | 7.9 | ** | * | ns | ** | * |
| TI | 16.8 | 16.9 | ns | 20.2 | 13.4 | ** | 18.6 | 15.1 | ** | ns | ns | * | * |
| Cultivar (C) | p | Sowing Date (S) | p | Seeding Rate (R) | p | Interactions (p-Level) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | S | E1 | E2 | L | H | C × S | C × R | S × R | C × S × R | ||||
| Sucrose | 16.2 | 13.8 | ns | 14.9 | 15.1 | ns | 16.4 | 13.6 | ** | ns | ns | ns | ns |
| Raffinose | 5.0 | 5.4 | ns | 5.0 | 5.5 | ** | 5.8 | 4.7 | ** | ns | ns | ns | ns |
| Stachyose | 18.2 | 16.5 | ns | 16.9 | 17.8 | ns | 18.3 | 16.5 | ** | ns | ns | ns | ** |
| Maltose | 3.2 | 2.3 | ns | 3.1 | 2.4 | ** | 2.7 | 2.7 | ns | ns | ns | ns | ns |
| Galactinol | 2.7 | 2.1 | ns | 2.6 | 2.1 | ** | 2.4 | 2.4 | ns | ns | ns | ns | ns |
| Ciceritol | 26.8 | 30.4 | ** | 29.5 | 27.7 | ** | 29.1 | 28.0 | ns | ns | ns | ns | ns |
| TSGs | 72.2 | 70.4 | ns | 72.0 | 70.7 | ns | 74.8 | 67.9 | ** | ns | ns | ns | ns |
| IP3 | 0.1 | 0.1 | ns | 0.1 | 0.1 | ns | 0.1 | 0.1 | ns | ns | ns | ns | ns |
| IP4 | 0.2 | 0.2 | ns | 0.2 | 0.2 | ns | 0.2 | 0.2 | ns | ns | ns | ns | ns |
| IP5 | 1.0 | 0.9 | ns | 0.8 | 1.0 | ** | 0.9 | 0.9 | ns | ns | ns | ns | ns |
| IP6 | 7.6 | 7.3 | ns | 6.8 | 8.0 | ** | 6.8 | 8.0 | ns | ns | ns | ns | ns |
| TIPs | 8.9 | 8.5 | ns | 8.0 | 9.3 | ** | 8.8 | 8.6 | ns | ns | ns | ns | ns |
| TI | 13.3 | 15.2 | ** | 16.1 | 12.3 | ** | 13.4 | 15.0 | ns | ns | ns | ** | ns |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primi, R.; Ruggeri, R.; Ronchi, B.; Bernabucci, U.; Rossini, F.; Martin-Pedrosa, M.; Danieli, P.P. Sowing Date and Seeding Rate Affect Bioactive Compound Contents of Chickpea Grains. Animals 2019, 9, 571. https://doi.org/10.3390/ani9080571
Primi R, Ruggeri R, Ronchi B, Bernabucci U, Rossini F, Martin-Pedrosa M, Danieli PP. Sowing Date and Seeding Rate Affect Bioactive Compound Contents of Chickpea Grains. Animals. 2019; 9(8):571. https://doi.org/10.3390/ani9080571
Chicago/Turabian StylePrimi, Riccardo, Roberto Ruggeri, Bruno Ronchi, Umberto Bernabucci, Francesco Rossini, Mercedes Martin-Pedrosa, and Pier Paolo Danieli. 2019. "Sowing Date and Seeding Rate Affect Bioactive Compound Contents of Chickpea Grains" Animals 9, no. 8: 571. https://doi.org/10.3390/ani9080571
APA StylePrimi, R., Ruggeri, R., Ronchi, B., Bernabucci, U., Rossini, F., Martin-Pedrosa, M., & Danieli, P. P. (2019). Sowing Date and Seeding Rate Affect Bioactive Compound Contents of Chickpea Grains. Animals, 9(8), 571. https://doi.org/10.3390/ani9080571







