Evaluation of Brassica Vegetables as Potential Feed for Ruminants
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples of Cabbage Wastes
2.2. Animals and Feeding
2.3. Experimental Design
2.3.1. Experiment 1: In Vitro Ruminal Fermentation and Intestinal Digestibility of Discarded Vegetables
2.3.2. Experiment 2: In Vitro Fermentation and In Situ Degradability of Diets Containing Increasing Amounts of Brussels Sprouts
2.4. Chemical Analyses
2.5. Calculations and Statistical Analyses
3. Results and Discussion
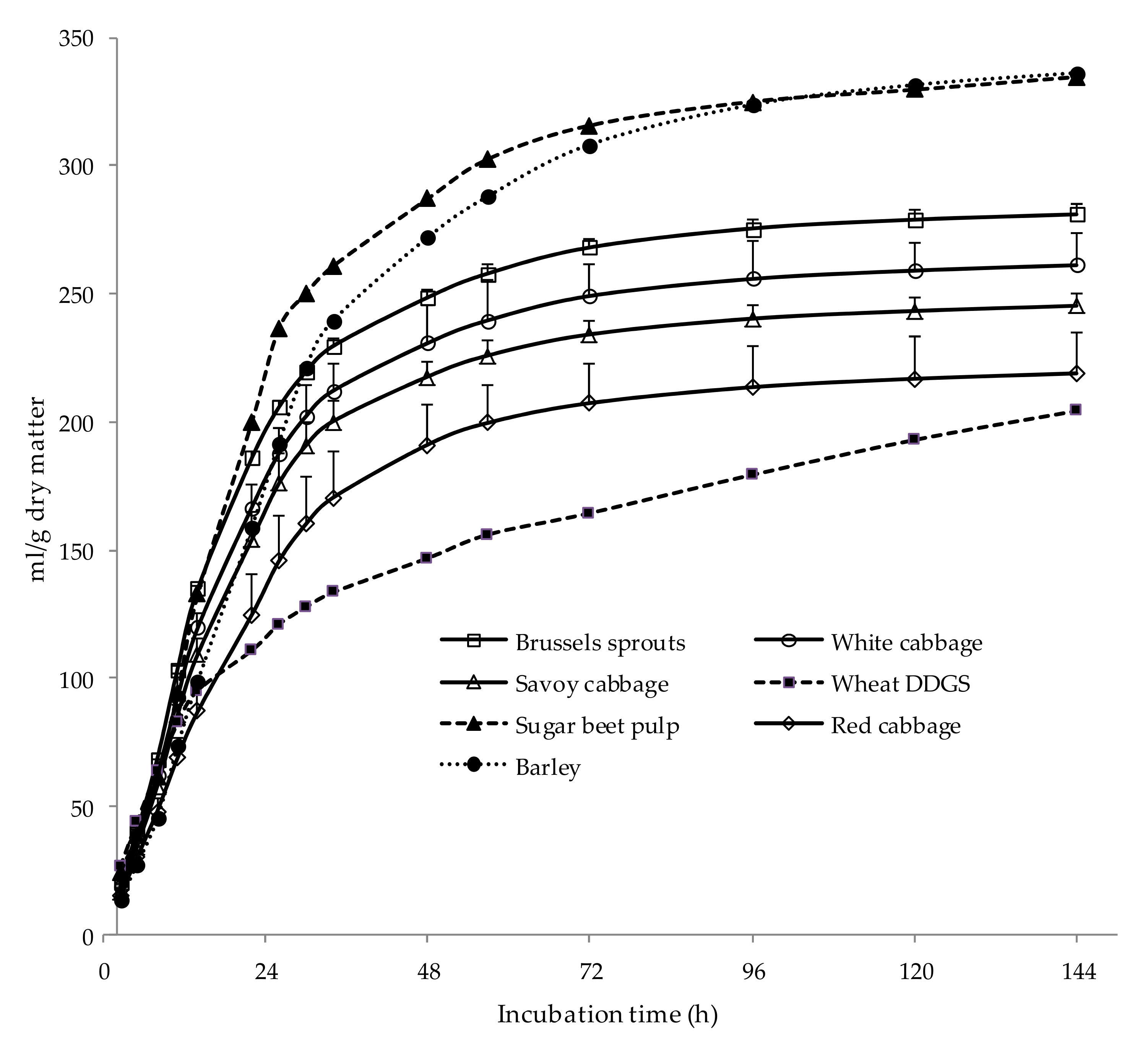
3.1. Experiment 1: Chemical Composition, In Vitro Ruminal Fermentation, and In Vitro Intestinal Digestibility of Vegetables
3.2. Experiment 2: In Vitro Fermentation and In Situ Degradability of Diets Containing Increasing Amount of Brussels Sprouts
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. Global Food Losses and Food Waste-Extent, Causes and Prevention; FAO: Rome, Italy, 2011. [Google Scholar]
- Rivin, J.; Miller, Z.; Matel, O. Using Food Waste as Livestock Feed. University of Wisconsin System Board of Regents and University of Wisconsin-Extension, Cooperative Extension: United States, 2012. Available online: https://outagamie.extension.wisc.edu/files/2012/10/Using-Food-Waste-as-Livestock-Feed.pdf (accessed on 19 July 2019).
- Pignata, G.; Nicola, S. Profitability, marketing, and vegetable loss and waste. In Good Agricultural Practices for greenhouse vegetable production in the South. East. European countries—Principles for sustainable intensification of smallholder farms; Baudoin, W., Nersisyan, A., Shamilov, A., Hodder, A., Gutierrez, D., de Pascale, S., Nicola, S., Gruda, N., Urban, L., Tanny, J., Eds.; FAO: Roma, Italy, 2017; pp. 245–267. [Google Scholar]
- Nkosi, B.D.; Meeske, R.; Ratsaka, M.M.; Langa, T.; Motiang, M.D.; Groenewald, I.B. Effects of dietary inclusion of discarded cabbage (Brassica oleracea var. capitata) on the growth performance of South African Dorper lambs. S. Afr. J. Anim. Sci. 2016, 46, 35–41. [Google Scholar] [CrossRef][Green Version]
- Bakshi, M.P.S.; Wadhwa, M.; Makkar, H. Waste to worth: Vegetable wastes as animal feed. Cab. Rev. 2016, 11, 1–26. [Google Scholar] [CrossRef]
- Duncan, A.J.; Milne, J.A. Rumen microbial degradation of allyl cyanide as a possible explanation for the tolerance of sheep to brassica-derived glucosinolates. J. Sci. Food Agric. 1992, 58, 15–19. [Google Scholar] [CrossRef]
- Mekasha, Y.; Tegegne, A.; Yami, A.; Umunna, N.N. Evaluation of non-conventional agro-industrial by-products as supplementary feeds for ruminants: In vitro and metabolism study with sheep. Small Rumin. Res. 2002, 44, 25–35. [Google Scholar] [CrossRef]
- Ngu, N.T.; Ledin, I. Effects of feeding wastes from Brassica species on growth of goats and pesticide/insecticide residues in goat meat. Asian-Aust. J. Anim. Sci 2005, 18, 197–202. [Google Scholar] [CrossRef]
- Wadhwa, M.; Kaushal, S.; Bakshi, M.P.S. Nutritive evaluation of vegetable wastes as complete feed for goat bucks. Small Rum. Res. 2006, 64, 279–284. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M.P.S. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of Other Value-Added Products; Makkar, H.P.S., Ed.; FAO: Rome, Italy, 2013. [Google Scholar]
- Mahgoub, O.; Kadim, I.T.; Eltahir, Y.; Al-Lawatia, S.; Al-Ismaili, A.M. Nutritional Value of Vegetable Wastes as Livestock Feed. Sultan Qaboos Univ. J. Sci. 2018, 23, 78–84. [Google Scholar] [CrossRef]
- Marino, C.T.; Hector, B.; Rodrigues, P.M.; Borgatti, L.O.; Meyer, P.M.; Alves da Silva, E.J.; Ørskov, E.R. Characterization of vegetables and fruits potential as ruminant feed by in vitro gas production technique. Livest. Res. Rural Dev. 2010, 22, 168. [Google Scholar]
- Kazemi, M.; Tornaghan, A.E.; Abadi, E.I.K.; Tazik, S.A.; Tohidi, R. Red (Brassica oleracea var. capitata) and White (Brassica oleracea var. botrytis) Cabbage Leaves Nutritional Value as Forage Feed: Comparison Study of In Vitro Gas Production and Determination of Chemical Composition. Direct. Res. J. Agric. Food Sci. 2016, 4, 176–181. [Google Scholar]
- Neugart, S.; Baldermann, S.; Hanschen, F.S.; Klopsch, R.; Wiesner-Reinhold, M.; Schreiner, M. The intrinsic quality of brassicaceous vegetables: How secondary plant metabolites are affected by genetic, environmental, and agronomic factors. Sci. Hortic. 2018, 233, 460–478. [Google Scholar] [CrossRef]
- Taljaard, T.L. Cabbage poisoning in ruminants. J. S. Afr. Vet. Assoc. 1993, 64, 96–100. [Google Scholar] [PubMed]
- Goering, M.K.; Van Soest, P.J. Forage Fiber Analysis (Apparatus, Reagents, Procedures and Some Applications). In Agricultural Handbook; Agriculture Handbook No 379; Agricultural Research Services: Washington, DC, USA, 1970. [Google Scholar]
- Marcos, C.N.; de Evan, T.; Molina-Alcaide, E.; Carro, M.D. Nutritive value of tomato pomace for ruminants and its influence on in vitro methane production. Animals 2019, 9, 343. [Google Scholar] [CrossRef] [PubMed]
- Gargallo, S.; Calsamiglia, S.; Ferret, A. Technical note: A modified three-step in vitro procedure to determine intestinal digestion of proteins. J. Anim. Sci. 2006, 84, 2163–2167. [Google Scholar] [CrossRef] [PubMed]
- Belverdy, M.S.; Alamouti, A.A.; Khadem, A.A.; González, J.; Carro, M.D.; Kianmehr, M.H.; Azizi, M.H. Evaluation of a novel method for ruminal protection of soybean meal protein using different fat sources. Arch. Anim. Nutr. 2019, 73, 158–169. [Google Scholar]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- García-Martínez, R.; Ranilla, M.J.; Tejido, M.L.; Carro, M.D. Effects of disodium fumarate on in vitro rumen microbial growth, methane production and fermentation of diets differing in their forage concentrate ratio. Br. J. Nutr. 2005, 94, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.E.; Ranilla, M.J.; Tejido, M.L.; Ramos, S.; Carro, M.D. The effect of the diet fed to donor sheep on in vitro methane production and ruminal fermentation of diets of variable composition. Anim. Feed Sci. Technol. 2010, 158, 126–135. [Google Scholar] [CrossRef]
- SAS Institute. SAS/STAT® Users Guide, Version 9.3; SAS Inst. Inc.: Cary, NC, USA, 2017. [Google Scholar]
- Ranilla, M.J.; López, S.; Giráldez, F.J.; Valdés, C.; Carro, M.D. Comparative digestibility and digesta flow kinetics in two breeds of sheep. Anim. Sci. 1998, 66, 389–396. [Google Scholar] [CrossRef]
- Demeyer, D. Quantitative aspects of microbial metabolism in the rumen and hindgut. In Rumen Microbial Metabolism and Ruminant Digestion; Jouany, J.P., Ed.; INRA Editions: Paris, France, 1991; pp. 217–237. [Google Scholar]
- NRC (National Research Council). Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy of Sciences: Washington, DC, USA, 2001. [Google Scholar]
- Cone, J.W.; van Gelder, A.H. Influence of protein fermentation on gas production profiles. Anim. Feed Sci.Technol. 1999, 76, 251–264. [Google Scholar] [CrossRef]
- Wallace, R.J.; Cotta, M.A. The Rumen Microbial Ecosystem; Hobson, P.N., Stewart, C.S., Eds.; Elsevier Applied Science: London, UK, 1988. [Google Scholar]
- AFRC (Agricultural Food Research Council). Energy and Protein Requirements of Ruminants; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Tamminga, S.; van Straalen, W.M.; Subnel, A.P.J.; Meijer, R.G.M.; Steg, A.; Wever, C.J.G.; Blok, M.C. The Dutch protein evaluation system: The DVE/OEB-system. Livest. Prod. Sci. 1994, 40, 139–155. [Google Scholar] [CrossRef]
- Madsen, J.; Hvelplund, T.; Weisbjerg, M.R.; Bertilsson, J.; Olsson, I.; Sporndly, R.; Harstad, O.M.; Volden, H.; Tuori, M.; Var-vikko, T.; et al. The AAT/PBV protein evaluation system for ruminants—a revision. Nor. J. Agric. Sci. Suppl. 1995, 19, 1–37. [Google Scholar]
- Van Soest, P.J. Nutritional Ecology of the Ruminant, 2nd ed.; Cornell University Press: Ithaca, NY, USA, 1994. [Google Scholar]
- Carro, M.D.; Lebzien, P.; Rohr, K. Effects of pore size of nylon bags and dilution rate on fermentation parameters in a semi-continuous artificial rumen. Small Rumin. Res. 1995, 15, 113–119. [Google Scholar] [CrossRef]
| Item | Cabbages | SEM 2 | p | Reference Feeds | |||||
|---|---|---|---|---|---|---|---|---|---|
| Brussels Sprouts | White Cabbage | Savoy Cabbage | Red Cabbage | Barley | Sugar Beet Pulp | Wheat DDGS | |||
| Dry matter (%) | 16.3 b | 5.64 a | 7.14 a | 6.60 a | 0.388 | <0.001 | 89.9 | 89.1 | 92.2 |
| Organic matter | 92.2 b | 84.7 a | 86.4 a | 89.1 a | 0.44 | <0.001 | 97.3 | 94.9 | 95.5 |
| Crude protein (CP) | 24.8 b | 19.5 a | 20.7 a | 19.8 a | 0.59 | <0.001 | 12.4 | 9.44 | 32.9 |
| Ether extract | 2.90 a | 3.57 ab | 4.36 a | 4.54 a | 0.220 | 0.002 | 3.16 | 0.80 | 4.61 |
| Sugars | 41.4 | 35.3 | 34.2 | 27.2 | 3.35 | 0.095 | 3.69 | 13.5 | 6.97 |
| Neutral detergent fiber (NDF) | 17.5 a | 23.5 ab | 28 b | 25.4 b | 1.54 | 0.007 | 22.7 | 48 | 29.5 |
| Acid detergent fiber | 10.3 a | 15.4 ab | 17.3 b | 17.6 b | 0.751 | <0.001 | 5.23 | 24.2 | 11.2 |
| Lignin | 0.25 | 1.53 | 1.97 | 2.43 | 0.574 | 0.119 | 1.22 | 2.16 | 3.33 |
| Hemicellulose | 7.24 | 8.15 | 10.7 | 8.87 | 0.845 | 0.087 | 19.6 | 23.9 | 18.3 |
| Lignin (% of NDF) | 1.43 | 6.20 | 7.01 | 9.40 | 1.914 | 0.092 | 5.37 | 4.49 | 11.2 |
| NDICP 1 (% CP) | 4.89 | 15.8 | 17.8 | 13.9 | 3.26 | 0.091 | 14.5 | 55 | 28.9 |
| Item | Cabbages | SEM 3 | p | Reference Feeds | |||||
|---|---|---|---|---|---|---|---|---|---|
| Brussels Sprouts | White Cabbage | Savoy Cabbage | Red Cabbage | Barley | Sugar Beet Pulp | Wheat DDGS | |||
| Gas production parameters 1 | |||||||||
| A (mL/g) | 275 c | 219 a | 229 ab | 233 b | 3.27 | <0.001 | 352 | 329 | 185 |
| c (%/h) | 5.55 c | 4.51 b | 4.75 b | 3.71 a | 0.17 | <0.001 | 5 | 5.21 | 4.15 |
| Lag (h) | 2.59 | 2.44 | 3.03 | 2.73 | 0.187 | 0.169 | 2.85 | 3.83 | 0 |
| AGPR (mL/h) | 9.11 c | 6.17 ab | 6.49 b | 5.47 a | 0.239 | <0.001 | 10.5 | 9.52 | 5.55 |
| DMED (%) | 58.4 b | 51.7 a | 49.1 a | 50.4 a | 0.69 | <0.001 | 45 | 45.2 | 38.4 |
| Fermentation parameters 2 | |||||||||
| Gas (mL) | 39.5 c | 34.2 a | 35 a | 36.8 b | 0.51 | <0.001 | 49.9 | 43.9 | 26.3 |
| pH | 6.52 a | 6.61 bc | 6.65 c | 6.60 b | 0.011 | <0.001 | 6.60 | 6.56 | 6.73 |
| Total volatile fatty acids (VFA; µmol) | 1632 | 1636 | 1612 | 1673 | 19.6 | 0.186 | 1452 | 1700 | 1311 |
| Individual VFA (mol/100 mol) | |||||||||
| Acetate (Ac) | 57.5 a | 62.6 b | 62.5 b | 62.9 b | 0.25 | <0.001 | 56.6 | 65.5 | 53.4 |
| Propionate (Pr) | 28.2 b | 24.5 a | 24.3 a | 24.1 a | 0.25 | <0.001 | 22.2 | 25.2 | 33.3 |
| Butyrate | 9.06 b | 8.18 a | 8.04 a | 8.31 a | 0.104 | <0.001 | 17.5 | 6.93 | 6.34 |
| Minor VFA | 5.23 b | 4.73 a | 5.15 b | 4.65 a | 0.106 | <0.001 | 3.70 | 2.31 | 6.96 |
| Ac/Pr (mol/mol) | 2.04 a | 2.55 b | 2.57 b | 2.60 b | 0.031 | <0.001 | 2.55 | 2.60 | 1.61 |
| NH3-N (mg/L) | 288 c | 223 a | 257 b | 218 a | 5.4 | <0.001 | 156 | 86.8 | 223 |
| Item | Brussels Sprouts | White Cabbage | Savoy Cabbage | Red Cabbage | SEM 1 | p |
|---|---|---|---|---|---|---|
| DM rumen (%) | 93.1 b | 91.9 b | 89.5 ab | 86.9 a | 1.03 | 0.001 |
| CP rumen (%) | 91.6 a | 94.4 b | 92.7 ab | 91.8 a | 0.77 | 0.042 |
| DM intestinal (%) | 73.5 c | 45.7 b | 44.2 b | 35.3 a | 2.57 | <0.001 |
| CP intestinal (%) | 90.2 c | 71.2 ab | 74.1 b | 61.4 a | 3.24 | 0.008 |
| Item | Diet | |||
|---|---|---|---|---|
| Control | BS8 | BS16 | BS24 | |
| Diet ingredients (g/100 g fresh matter) | ||||
| Alfalfa hay | 40.0 | 40.0 | 40.0 | 40.0 |
| Concentrate | 60.0 | 60.0 | 60.0 | 60.0 |
| Concentrate ingredients (g/100 g fresh matter) | ||||
| Brussel sprouts | - | 8.0 | 16.0 | 24.0 |
| Corn | 32.0 | 28.0 | 25.0 | 23.0 |
| Barley | 30.0 | 28.8 | 26.5 | 23.0 |
| Wheat | 15.0 | 15.0 | 15.0 | 15.0 |
| Soybean meal 46% | 14.0 | 11.2 | 8.5 | 6.0 |
| Wheat bran | 7.0 | 7.0 | 7.0 | 7.0 |
| Calcium soap | 1.0 | 1.0 | 1.0 | 1.0 |
| Calcium carbonate | 0.5 | 0.5 | 0.5 | 0.5 |
| Mineral/vitamin premix | 0.5 | 0.5 | 0.5 | 0.5 |
| Chemical composition of diets 1 | ||||
| Dry matter | 89.7 | 89.7 | 89.7 | 89.7 |
| Organic matter | 93.0 | 92.4 | 91.6 | 90.9 |
| Crude protein | 16.1 | 16.1 | 16.1 | 16.1 |
| Neutral detergent fiber | 31.5 | 31.6 | 31.7 | 31.6 |
| Acid detergent fiber | 15.9 | 16.1 | 16.4 | 16.7 |
| Ether extract | 4.1 | 4.1 | 4.0 | 4.0 |
| Item | Diet | SEM 2 | p | ||||
|---|---|---|---|---|---|---|---|
| Control | BS8 | BS16 | BS24 | Lineal | Quadratic | ||
| Gas production parameters 1 | |||||||
| A (mL/g DM) | 280 a | 289 ab | 290 ab | 293 b | 3.47 | 0.027 | 0.388 |
| c (%/h) | 3.90 | 4.00 | 4.00 | 3.99 | 0.061 | 0.449 | 0.330 |
| Lag (h) | 1.10 | 0.87 | 0.62 | 0.64 | 0.21 | 0.126 | 0.574 |
| AGPR (mL/h) | 7.40 | 7.98 | 8.06 | 8.09 | 0.21 | 0.053 | 0.239 |
| DMED (%) | 40.6 | 41.9 | 42.2 | 41.9 | 0.62 | 0.147 | 0.214 |
| Fermentation parameters (8 h) | |||||||
| Total volatile fatty acids (VFA; µmol) | 1284 a | 1391 ab | 1501 bc | 1561 c | 34.8 | <0.001 | 0.512 |
| Individual VFA (mol/ 100 mol) | |||||||
| Acetate (Ac) | 61.1 | 61.6 | 61.3 | 61.7 | 0.17 | 0.152 | 0.822 |
| Propionate (Pr) | 22.9 a | 22.9 a | 23.3 b | 23.6 b | 0.11 | 0.001 | 0.384 |
| Butyrate | 12.8 c | 12.5 bc | 12.3 b | 11.8 a | 0.12 | <0.001 | 0.582 |
| Minor VFA 3 | 3.11 | 3.01 | 3.04 | 2.95 | 0.061 | 0.141 | 0.929 |
| Ac/Pr (mol/mol) | 2.69 b | 2.71 b | 2.64 ab | 2.63 a | 0.022 | 0.019 | 0.454 |
| NH3-N (mg/L) | 143 a | 148 ab | 154 b | 155 b | 2.9 | 0.033 | 0.560 |
| CH4 (mL) | 6.90 | 7.24 | 7.02 | 7.36 | 0.330 | 0.453 | 0.991 |
| CH4/VFA (mL/mmol) | 5.40 | 5.22 | 4.68 | 4.71 | 0.311 | 0.092 | 0.739 |
| AFOM (%) 4 | 31.3 a | 33.4 ab | 36.3 bc | 37.9 c | 0.84 | <0.001 | 0.474 |
| Fermentation parameters (24 h) | |||||||
| pH | 6.79 b | 6.75 ab | 6.74 a | 6.73 a | 0.01 | 0.004 | 0.394 |
| Total VFA (µmol) | 2446 a | 2650 b | 2673 b | 2684 b | 0.1 | <0.001 | 0.008 |
| Individual VFA (mol/ 100 mol) | |||||||
| Acetate (Ac) | 61.5 | 61.6 | 61.5 | 61.7 | 28.36 | 0.342 | 0.583 |
| Propionate (Pr) | 18.7 a | 19.0 ab | 19.3 b | 19.6 b | 0.12 | <0.001 | 0.788 |
| Butyrate | 15.5 d | 14.9 b | 14.7b | 14.1 a | 0.14 | <0.001 | 0.931 |
| Minor VFA 3 | 4.27 a | 4.53 ab | 4.53 ab | 4.57 b | 0.092 | 0.048 | 0.217 |
| Ac/Pr (mol/mol) | 3.31 c | 3.28 bc | 3.20 ab | 3.16 a | 0.0143 | 0.003 | 0.962 |
| NH3-N (mg/L) | 189 | 203 | 207 | 209 | 6.1 | 0.090 | 0.506 |
| CH4 (mL) | 14.9 | 15.1 | 14.8 | 15.2 | 0.41 | 0.804 | 0.846 |
| CH4/VFA (mL/mmol) | 6.10 b | 5.69 ab | 5.53 a | 5.65 a | 0.140 | 0.039 | 0.095 |
| AFOM (%) 4 | 60.5 a | 64.0 b | 65.1 b | 65.4 b | 0.66 | 0.001 | 0.007 |
| Item 1 | Diet | SEM 2 | p | ||||
|---|---|---|---|---|---|---|---|
| Control | BS8 | BS16 | BS24 | Lineal | Quadratic | ||
| Dry matter | |||||||
| a (%) | 33.6 a | 34.7 a | 37.9 b | 40.5 c | 0.33 | <0.001 | 0.269 |
| b (%) | 45.6 b | 44.6 b | 42.5 a | 40.9 a | 0.34 | 0.001 | 0.628 |
| a + b (%) | 79.2 | 79.4 | 80.4 | 81.4 | 0.59 | 0.141 | 0.705 |
| c (h−1) | 0.262 | 0.240 | 0.206 | 0.238 | 0.0102 | 0.227 | 0.180 |
| ED (%) | 72.7 a | 72.7 a | 73.2 ab | 75.1 b | 0.67 | 0.042 | 0.192 |
| Crude protein | |||||||
| a (%) | 35.7 a | 47.2 c | 45.8 b | 49.1 d | 0.23 | <0.001 | <0.001 |
| b (%) | 55.9 c | 44.5 b | 45.7 b | 42.2 a | 0.34 | <0.001 | 0.001 |
| a + b (%) | 91.6 | 91.7 | 91.5 | 91.3 | 0.23 | 0.551 | 0.728 |
| c (h−1) | 0.167 a | 0.176 ab | 0.227 bc | 0.242 c | 0.0091 | 0.008 | 0.871 |
| ED (%) | 80.2 a | 83.1 b | 84.3 b | 85.0 b | 0.77 | 0.004 | 0.211 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Evan, T.; Vintimilla, A.; Marcos, C.N.; Ranilla, M.J.; Carro, M.D. Evaluation of Brassica Vegetables as Potential Feed for Ruminants. Animals 2019, 9, 588. https://doi.org/10.3390/ani9090588
de Evan T, Vintimilla A, Marcos CN, Ranilla MJ, Carro MD. Evaluation of Brassica Vegetables as Potential Feed for Ruminants. Animals. 2019; 9(9):588. https://doi.org/10.3390/ani9090588
Chicago/Turabian Stylede Evan, Trinidad, Andrea Vintimilla, Carlos N. Marcos, María José Ranilla, and María Dolores Carro. 2019. "Evaluation of Brassica Vegetables as Potential Feed for Ruminants" Animals 9, no. 9: 588. https://doi.org/10.3390/ani9090588
APA Stylede Evan, T., Vintimilla, A., Marcos, C. N., Ranilla, M. J., & Carro, M. D. (2019). Evaluation of Brassica Vegetables as Potential Feed for Ruminants. Animals, 9(9), 588. https://doi.org/10.3390/ani9090588





